Abstract
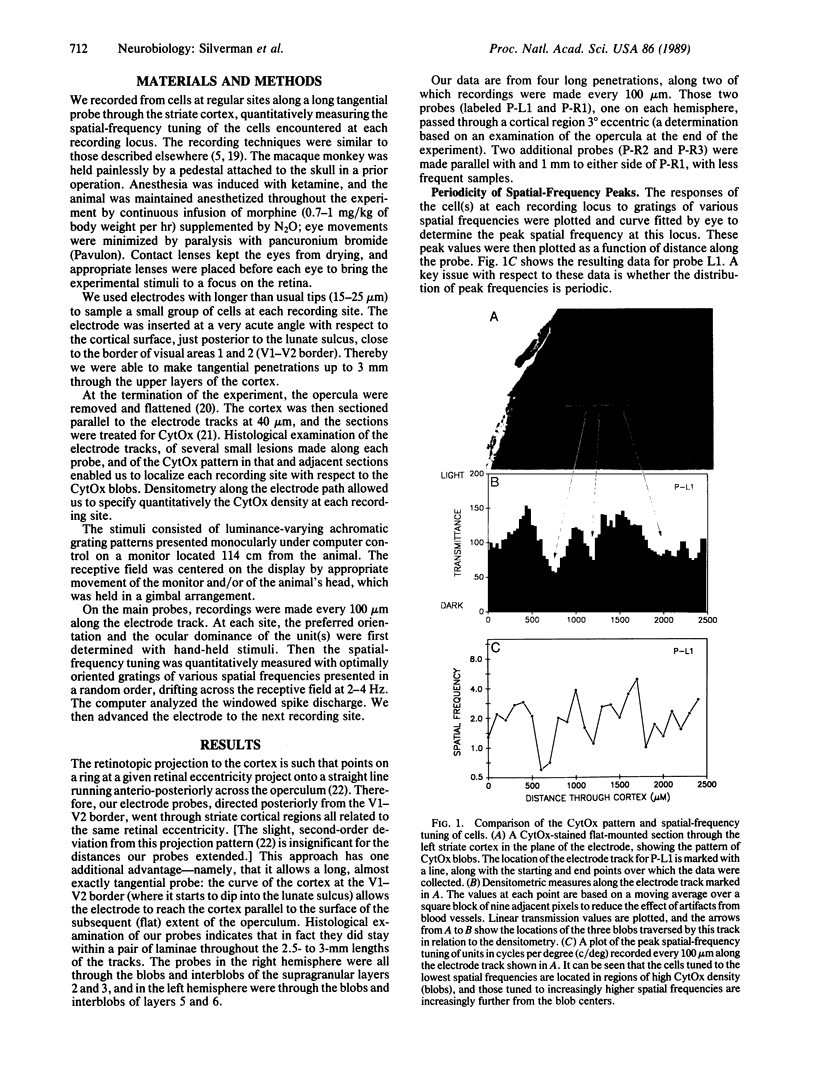
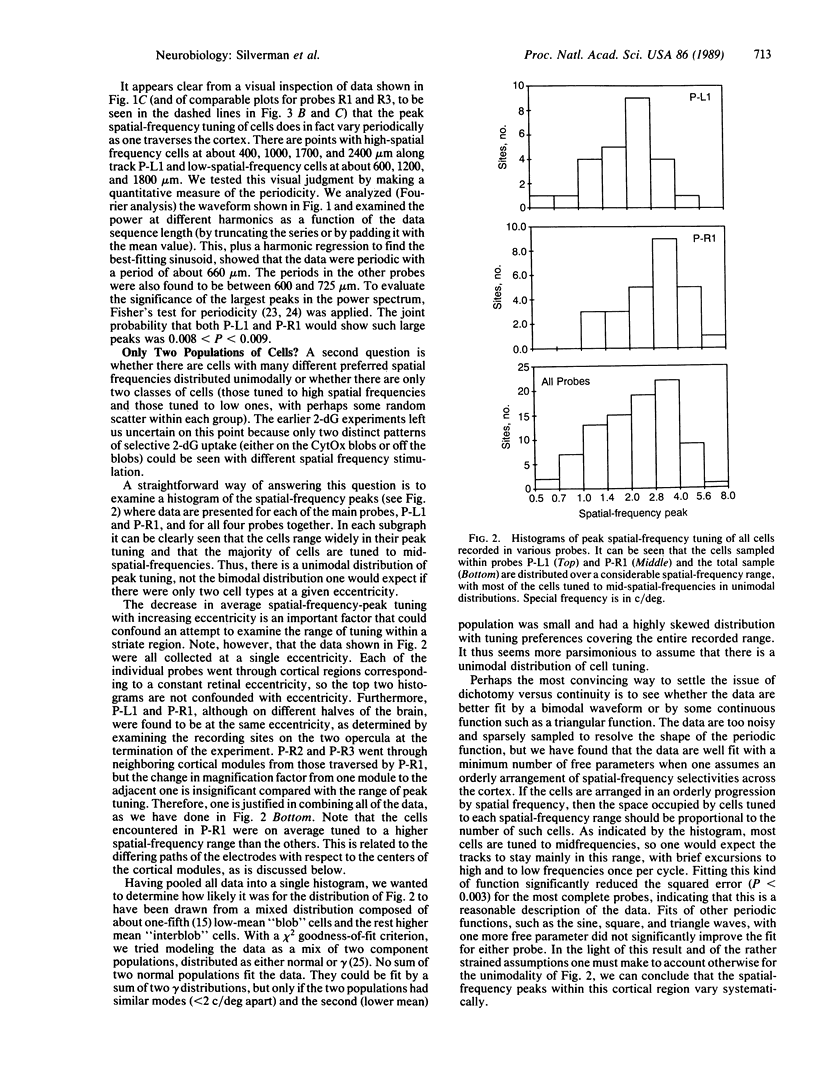
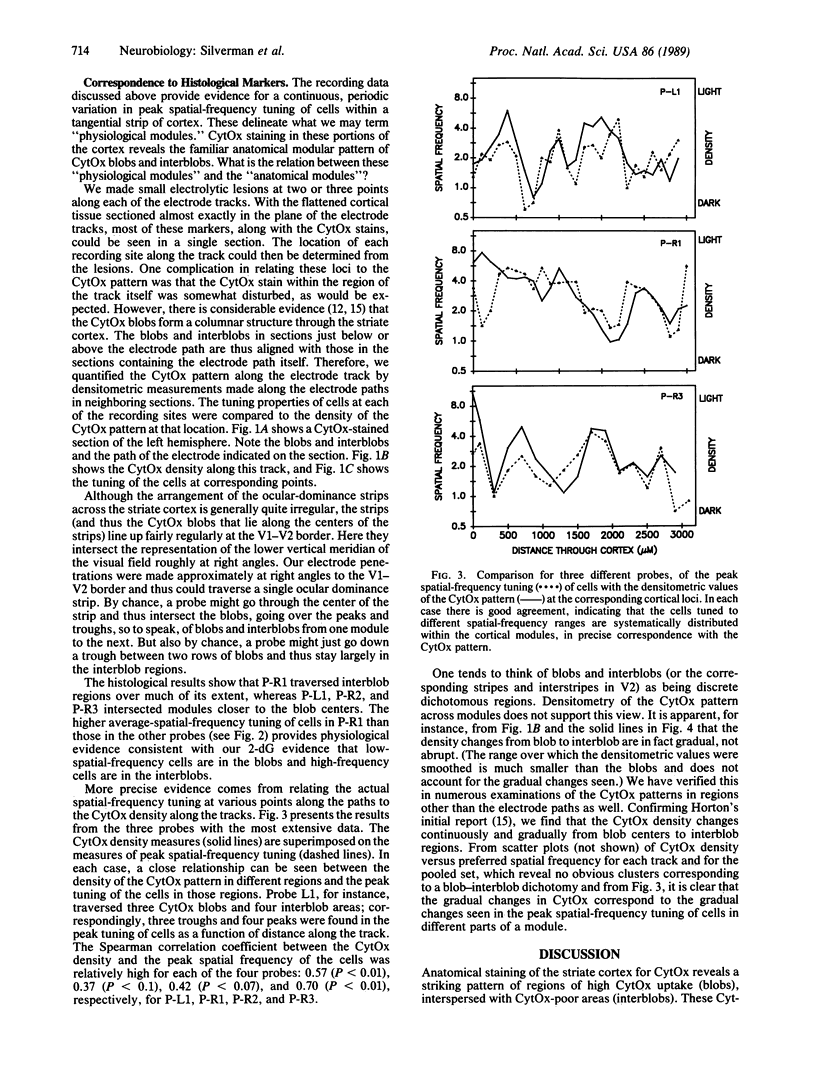
We measured the spatial-frequency tuning of cells at regular intervals along tangential probes through the monkey striate cortex and correlated the recording sites with the cortical cytochrome oxidase (CytOx) patterns to address three questions with regard to the cortical spatial-frequency organization. (i) Is there a periodic anatomical arrangement of cells tuned to different spatial-frequency ranges? We found there is, because the spatial-frequency tuning of cells along tangential probes changed systematically, varying from a low frequency to a middle range to high frequencies and back again repeatedly over distances of about 0.6-0.7 mm. (ii) Are there just two populations of cells, low-frequency and high-frequency units, at a given eccentricity (perhaps corresponding to the magno- and parvocellular geniculate pathways) or is there a continuum of spatial-frequency peaks? We found a continuum of peak tuning. Most cells are tuned to intermediate spatial frequencies and form a unimodal rather than a bimodal distribution of cell peaks. Furthermore, the cells with different peak frequencies were found to be continuously and smoothly distributed across a module. (iii) What is the relation between the physiological spatial-frequency organization and the regions of high CytOx concentration ("blobs")? We found a systematic correlation between the topographical variation in spatial-frequency tuning and the modular CytOx pattern, which also varied continuously in density. Low-frequency cells are at the center of the blobs, and cells tuned to increasingly higher spatial frequencies are at increasing radial distances.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore C., Campbell F. W. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. J Physiol. 1969 Jul;203(1):237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Robson J. G. Application of Fourier analysis to the visibility of gratings. J Physiol. 1968 Aug;197(3):551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R. L., Albrecht D. G., Thorell L. G. Spatial frequency selectivity of cells in macaque visual cortex. Vision Res. 1982;22(5):545–559. doi: 10.1016/0042-6989(82)90113-4. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Yund E. W., Hepler N. The orientation and direction selectivity of cells in macaque visual cortex. Vision Res. 1982;22(5):531–544. doi: 10.1016/0042-6989(82)90112-2. [DOI] [PubMed] [Google Scholar]
- DeYoe E. A., Van Essen D. C. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature. 1985 Sep 5;317(6032):58–61. doi: 10.1038/317058a0. [DOI] [PubMed] [Google Scholar]
- Field D. J. Relations between the statistics of natural images and the response properties of cortical cells. J Opt Soc Am A. 1987 Dec;4(12):2379–2394. doi: 10.1364/josaa.4.002379. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. C. Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex. Philos Trans R Soc Lond B Biol Sci. 1984 Jan 17;304(1119):199–253. doi: 10.1098/rstb.1984.0021. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hubel D. H. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981 Aug 20;292(5825):762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J Comp Neurol. 1974 Dec 1;158(3):267–293. doi: 10.1002/cne.901580304. [DOI] [PubMed] [Google Scholar]
- Humphrey A. L., Hendrickson A. E. Background and stimulus-induced patterns of high metabolic activity in the visual cortex (area 17) of the squirrel and macaque monkey. J Neurosci. 1983 Feb;3(2):345–358. doi: 10.1523/JNEUROSCI.03-02-00345.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. H. Pattern detection and the two-dimensional fourier transform: flickering checkerboards and chromatic mechanisms. Vision Res. 1976;16(3):277–287. doi: 10.1016/0042-6989(76)90111-5. [DOI] [PubMed] [Google Scholar]
- Klein S. A., Levi D. M. Hyperacuity thresholds of 1 sec: theoretical predictions and empirical validation. J Opt Soc Am A. 1985 Jul;2(7):1170–1190. doi: 10.1364/josaa.2.001170. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci. 1984 Jan;4(1):309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Specificity of cortico-cortical connections in monkey visual system. Nature. 1983 Aug 11;304(5926):531–534. doi: 10.1038/304531a0. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973 Jul;13(7):1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J Physiol. 1978 Oct;283:101–120. doi: 10.1113/jphysiol.1978.sp012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S., Zeki S. Segregation of pathways leading from area V2 to areas V4 and V5 of macaque monkey visual cortex. Nature. 1985 May 23;315(6017):322–325. doi: 10.1038/315322a0. [DOI] [PubMed] [Google Scholar]
- Silverman M. S., Tootell R. B. Modified technique for cytochrome oxidase histochemistry: increased staining intensity and compatibility with 2-deoxyglucose autoradiography. J Neurosci Methods. 1987 Jan;19(1):1–10. doi: 10.1016/0165-0270(87)90016-1. [DOI] [PubMed] [Google Scholar]
- Switkes E., Tootell R. B., Silverman M. S., De Valois R. Picture processing techniques applied to autoradiographic studies of visual cortex. J Neurosci Methods. 1986 Feb;15(4):269–280. doi: 10.1016/0165-0270(86)90140-8. [DOI] [PubMed] [Google Scholar]
- Tootell R. B., Hamilton S. L., Silverman M. S., Switkes E. Functional anatomy of macaque striate cortex. I. Ocular dominance, binocular interactions, and baseline conditions. J Neurosci. 1988 May;8(5):1500–1530. doi: 10.1523/JNEUROSCI.08-05-01500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell R. B., Silverman M. S., De Valois R. L., Jacobs G. H. Functional organization of the second cortical visual area in primates. Science. 1983 May 13;220(4598):737–739. doi: 10.1126/science.6301017. [DOI] [PubMed] [Google Scholar]
- Tootell R. B., Silverman M. S., Hamilton S. L., De Valois R. L., Switkes E. Functional anatomy of macaque striate cortex. III. Color. J Neurosci. 1988 May;8(5):1569–1593. doi: 10.1523/JNEUROSCI.08-05-01569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell R. B., Silverman M. S., Hamilton S. L., Switkes E., De Valois R. L. Functional anatomy of macaque striate cortex. V. Spatial frequency. J Neurosci. 1988 May;8(5):1610–1624. doi: 10.1523/JNEUROSCI.08-05-01610.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell R. B., Silverman M. S., Switkes E., De Valois R. L. Deoxyglucose analysis of retinotopic organization in primate striate cortex. Science. 1982 Nov 26;218(4575):902–904. doi: 10.1126/science.7134981. [DOI] [PubMed] [Google Scholar]
- Tootell R. B., Silverman M. S. Two methods for flat-mounting cortical tissue. J Neurosci Methods. 1985 Nov-Dec;15(3):177–190. doi: 10.1016/0165-0270(85)90097-4. [DOI] [PubMed] [Google Scholar]
- van der Horst G. J., Bouman M. A. Spatiotemporal chromaticity discrimination. J Opt Soc Am. 1969 Nov;59(11):1482–1488. doi: 10.1364/josa.59.001482. [DOI] [PubMed] [Google Scholar]