Abstract
Objectives: To examine whether antibiotics are indicated in treating uncomplicated acute sinusitis and, if so, whether newer and more expensive antibiotics with broad spectra of antimicrobial activity are more effective than amoxycillin or folate inhibitors.
Design: Meta-analysis of randomised trials.
Setting: Outpatient clinics.
Subjects: 2717 patients with acute sinusitis or acute exacerbation of chronic sinusitis from 27 trials.
Interventions: Any antibiotic versus placebo; amoxycillin or folate inhibitors versus newer, more expensive antibiotics.
Main outcome measurements: Clinical failures and cures.
Results: Compared with placebo, antibiotics decreased the incidence of clinical failures by half (risk ratio 0.54 (95% confidence interval 0.37 to 0.79)). Risk of clinical failure among 1553 randomised patients was not meaningfully decreased with more expensive antibiotics as compared with amoxycillin (risk ratio 0.86 (0.62 to 1.19); risk difference 0.9 fewer failures per 100 patients (1.4 more failures to 3.1 fewer failures per 100 patients)). The results were similar for other antibiotics versus folate inhibitors (risk ratio 1.01 (0.52 to 1.97)), but data were sparse (n=410) and of low quality.
Conclusions: Amoxycillin and folate inhibitors are essentially as effective as more expensive antibiotics for the initial treatment of uncomplicated acute sinusitis. Small differences in efficacy may exist, but are unlikely to be clinically important.
Key messages
A major question in managing acute sinusitis is whether antibiotics should be used, and if so which drugs should be chosen
In a comprehensive meta-analysis we evaluated evidence from randomised controlled trials comparing, firstly, antibiotics against placebo and, secondly, amoxycillin and folate inhibitors against newer, more expensive antibiotics
Antibiotics were significantly more efficacious than placebo in achieving cure of clinical symptoms, but over two thirds of placebo patients showed spontaneous resolution or improvement of symptoms
Amoxycillin and folate inhibitors had overall similar efficacy compared with newer antibiotics
The current evidence does not justify the use of expensive, broad spectrum antibiotics in the community for treating uncomplicated acute sinusitis
Introduction
Acute sinusitis is a common infection. It is usually treated with antibiotics, often in conjunction with decongestants. A wide variety of antibiotics are used, but there is little information to allow doctors to determine the best initial choice of antibiotic, in particular whether any of the newer broad spectrum drugs are significantly more effective than older, less expensive drugs such as amoxycillin or co-trimoxazole (trimethoprim plus sulfamethoxazole). The usual pathogens in this infection are Streptococcus pneumoniae and Haemophilus influenzae, with a lesser contribution of Moraxella catarrhalis and other species.1 These species are generally but not uniformly susceptible to amoxycillin and co-trimoxazole. If newer, more expensive antibiotics are more effective then their use would be warranted, but, if not, they should be reserved for specific circumstances. Avoiding unnecessary use of newer, broad spectrum antibiotics is important because of costs but also because of concern about the rising rate of antimicrobial resistance.
A recent meta-analysis considered 12 randomised trials comparing antibiotics of different classes and four trials comparing similar class antibiotics and found no substantive differences among them in the treatment of acute sinusitis.2 However, the analysis was limited to randomised studies of adults published from 1984 to 1995. No overall comparison with the older drugs amoxycillin and co-trimoxazole was carried out, and the effects of antibiotics compared with placebo were not formally addressed. Our study focuses on both of these issues.
Methods
Study selection
Using the terms of specific antibiotic classes and “sinusitis,” we searched Medline up to May 1998 for randomised trials of acute sinusitis. We also manually searched Excerpta Medica and recent abstracts for the interscience conference on antimicrobial agents and chemotherapy (1993-7)3 and inspected references of all trials, review articles, and special issues for additional studies. No language restrictions were applied. Trials were eligible for inclusion if three criteria were fulfilled: (a) the trial compared amoxycillin or a folate inhibitor with another antibiotic, generally one with a broad spectrum of activity, including cephalosporins, penicillins with β lactamase inhibitors, tetracyclines, quinolones, and macrolides; (b) patients were randomly assigned to treatment arms; and (c) the trial evaluated acute sinusitis or an acute exacerbation of chronic sinusitis. We excluded trials that compared doses of non-antimicrobial drugs and trials of subacute or chronic sinusitis (mean duration of symptoms >3 weeks). We also examined placebo controlled studies to assess the effect of antibiotics on the natural course of acute sinusitis.
Data extraction
Data were extracted independently by two authors. Outcomes of interest were clinical cure, improvement, and failure as assessed within 48 hours of the end of treatment. Cures and failures were recorded as defined by the individual study: cure generally meant resolution of all signs and symptoms, and failure generally signified no change or worsening of signs and symptoms. We also extracted data on radiographic cure, improvement, or failure and bacteriological cure or failure as defined by each study. In our main analyses we used clinical outcomes as the end points most relevant to doctors because primary care practitioners do not routinely obtain sinus films for uncomplicated acute sinusitis and almost never perform sinus aspirates, and because there is no evidence of a correlation between radiographic or bacteriological failure and clinical outcomes. We separately assessed bacteriological and radiographic failures and patient withdrawals due to adverse drug effects.
Quality assessment
We assessed studies for the following characteristics: blinded versus unblinded design, criteria for diagnosis of sinusitis, clinical outcomes, loss of subjects to follow up, and use of decongestants. The diagnosis of sinusitis was considered “firm” if culture of sinus aspirations or radiographic evaluations (presence of air-fluid levels, mucosal thickening >6 mm, or sinus opacification) were confirmatory. Any other diagnostic criteria, including nasal swabs, were considered “subjective.” We considered outcome criteria to be well specified when symptoms or signs were assessed by patients or physicians in a way that could be replicated; criteria were specified to some extent when the signs or symptoms used to evaluate outcome were noted but not how these were evaluated; and criteria were unclear when no mention was made of how clinical outcomes were determined.
In addition to this subject-specific assessment of quality, we used the scale developed by Jadad et al to assess the methodological quality of clinical trials.4 This scale has a maximum score of 5 (highest quality) and focuses on randomisation, double blinding, and description of withdrawals.
Data synthesis and statistical analysis
We pooled the results from (a) placebo controlled studies to determine the effect of treatment with any antibiotic on the outcome of acute sinusitis, (b) studies in which amoxycillin was compared with various antibiotics except folate inhibitors to compare the outcomes of treatment, and (c) studies in which folate inhibitors were compared with other antibiotics except amoxycillin. We pooled risk ratios, risk differences, and event rates in the control group using both the Mantel-Haenszel fixed effects model5 and the DerSimonian and Laird random effects model,6 which takes into account the variability of the true treatment effect between studies. We assessed the heterogeneity between studies with χ2 tests and deemed P<0.1 to indicate significance.7
Unless stated otherwise, we report the results calculated with the random effects model, but fixed effect calculations provided similar estimates. We also report rates weighted by the inverse of their variance with random effects8 and results from a series of sensitivity analyses.
Results
Trial characteristics and quality assessment
We identified 80 randomised clinical trials of antibiotic treatment of acute sinusitis. Most were ineligible for our meta-analysis: 48 did not use the reference drugs pertinent to this analysis, three inextricably combined patients with sinusitis with those with other infections,9–11 and two inextricably combined patients with acute, chronic, and recurrent sinusitis.12,13 Of the 27 trials that qualified for our meta-analysis, six were placebo controlled (one study comparing amoxycillin also had a placebo arm),14–19 13 compared amoxycillin with other antibiotics,16,20–31 and eight compared a folate inhibitor (co-trimoxazole, trimethoprim plus sulfametopyrazine, or brodimoprim) with other antibiotics.32–39 (For details of these trials, see extra table on the BMJ website.) An additional large (n=438) and well done trial using penicillin V as the reference drug was excluded from our main analysis because penicillin V is less active in vitro than amoxycillin against H influenzae and M catarrhalis but was included in the sensitivity analysis.40 Among the included trials, sample size ranged from 14 to 323 patients (2717 patients overall). The mean ages of patients ranged from 25 to 44 years, except for two trials that evaluated paediatric patients exclusively.16,20
Eleven of the 27 trials were double blind, and six were single blind (five investigator blind). Twelve trials used “firm” methods for diagnosing acute sinusitis, and the others used clinical criteria. Eight trials required the use of decongestants and two trials allowed it; 17 did not deal with this issue by protocol. The criteria for clinical outcomes were well specified in eight of the trials, specified to some extent in 12, and unclear in seven trials. Antral punctures were done in three trials,20–30 and either antral puncture or nasal swabs in two trials,21,24 both in the amoxycillin analysis.
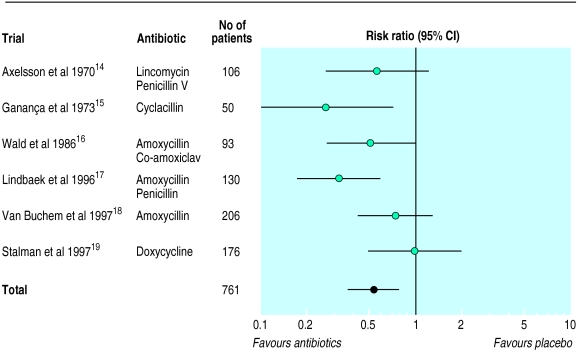
Antibiotics v placebo
In the six studies comparing any antibiotic with placebo, antibiotics were significantly more effective, reducing treatment failures by almost half (table 1, fig 1). However, symptoms improved or disappeared in 69% of patients without any antibiotic treatment (95% confidence interval 57% to 79%). Although the observed heterogeneity between trials did not reach significance, there was a suggestion that one trial that included patients simply on the basis of sinusitis-like symptoms without further diagnostic documentation had the highest rates of cure or improvement in the placebo group (85% at 10 days) and showed no benefit from antibiotics,19 whereas trials with more tightly defined patient populations and lower spontaneous improvement rates showed a clear benefit from antibiotics.
Table 1.
Meta-analysis of clinical outcomes recorded in six trials of 761 patients comparing antibiotics with placebo for treating uncomplicated acute sinusitis14-19
| Outcome | Risk ratio (95% CI) for antibiotic treatment | Outcome (95% CI) with placebo |
|---|---|---|
| Clinical cure | 1.33 (1.02 to 1.74) | 34% (21% to 51%) |
| Clinical failure | 0.54 (0.37 to 0.79) | 31% (21% to 43%) |
Figure 1.
Random effects model of risk ratios (95% confidence intervals) of clinical failure associated with antibiotic treatment of acute sinusitis compared with placebo
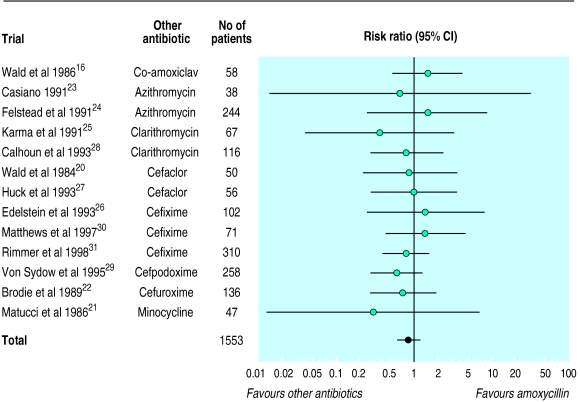
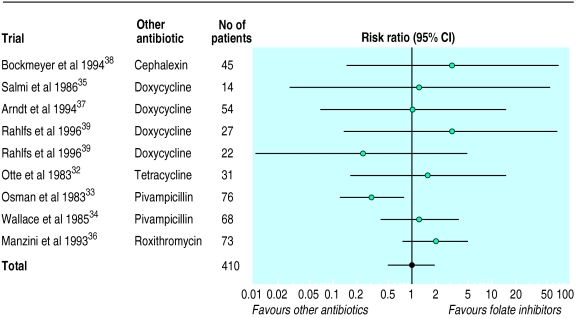
Amoxycillin and folate inhibitors v other antibiotics
Clinical outcomes
There was no statistically significant or clinically meaningful difference in rate of failure or cure between amoxycillin and other antibiotics (table 2, fig 2). Compared with other antibiotics, treatment of 100 patients with amoxycillin would lead to only 0.85 more failures. The results were similar for folate inhibitors, but the data were more limited (table 2, fig 3). Compared with other drugs, the risk differences of clinical cure with amoxycillin were 3.2% (95% confidence interval −1.5% to 7.8%) and with folate inhibitors they were 1.2% (−10% to 12.4%). The results were similar when we added a trial comparing penicillin with azithromycin to the comparisons with amoxycillin.
Table 2.
Meta-analysis of outcomes recorded in trials comparing newer, more expensive antibiotics with amoxycillin or folate inhibitors for treating uncomplicated acute sinusitis
| Outcome | No of studies | No of patients | Risk ratio (95% CI)* | Outcome (95% CI) with reference drug† |
|---|---|---|---|---|
| Newer, more expensive antibiotics v amoxycillin16 20-31 | ||||
| Clinical failure | 1316 20-31 | 1553 | 0.86 (0.62 to 1.19) | 11% (8% to 14%) |
| Clinical cure | 1116 20-29 | 1172 | 1.04 (0.98 to 1.11) | 72% (64% to 80%) |
| Radiographic failure | 420 21 25 28 | 270 | 0.89 (0.35 to 2.26) | 17% (9% to 31%) |
| Bacteriological failure | 716 20 21 25 28 30 31 | 435 | 0.68 (0.41 to 1.14) | 10% (5% to 19%) |
| Withdrawal | 1216 21-31 | 1505 | 1.01 (0.56 to 1.81) | 4% (3% to 6%) |
| Newer, more expensive antibiotics v folate inhibitors32-39 | ||||
| Clinical failure | 832-39 | 410 | 1.01 (0.52 to 1.97) | 11% (6% to 22%) |
| Clinical cure | 732-38 | 361 | 1.01 (0.88 to 1.17) | 73% (58% to 84%) |
| Radiographic failure | 332 37 38 | 132 | 1.46 (0.79 to 2.71) | 20% (7% to 44%) |
| Bacteriological failure | 332 36 38 | 122 | 1.70 (0.90 to 3.21) | 19% (9% to 37%) |
| Withdrawal | 532 35-38 | 219 | 0.47 (0.10 to 2.20) | 6% (3% to 13%) |
Risk ratio for treatment with other antibiotics. †Amoxycillin or folate inhibitor.
Figure 2.
Random effects model of risk ratios (95% confidence intervals) of clinical failure associated with treatment of acute sinusitis with more expensive antibiotics compared with amoxycillin
Figure 3.
Random effects model of risk ratios (95% confidence intervals) of clinical failure associated with treatment of acute sinusitis with more expensive antibiotics compared with folate inhibitors
There was no heterogeneity of treatment effects in the comparisons with amoxycillin. By contrast, there was some evidence of heterogeneity in the studies comparing folate inhibitors with other antibiotics (P=0.09 for clinical cure, P=0.18 for clinical failures), possibly because co-trimoxazole seemed less effective than pivampicillin plus pivmecillinam in one study.34
Sensitivity analyses showed similar results (table 3). In all of these analyses there was a tendency for an estimated 11-20% risk reduction in clinical failures with other antibiotics compared with amoxycillin that did not reach formal statistical significance. This tendency corresponded to a clinically negligible benefit (less than 1 failure averted per 100 patients). Because of sparse data, sensitivity analysis was less useful for folate inhibitors.
Table 3.
Sensitivity and subgroup analyses for clinical failures recorded in trials comparing newer, more expensive antibiotics with amoxycillin or folate inhibitors for treating uncomplicated acute sinusitis
| Subgroups of trials | Other antibiotics v amoxycillin
|
Other antibiotics v folate inhibitors
|
|||
|---|---|---|---|---|---|
| No of trials (patients) | Risk ratio (95% CI)* | No of trials (patients) | Risk ratio (95% CI)* | ||
| Patients: | |||||
| Children | 2 (108) | 1.24 (0.54 to 2.84) | 0 | not applicable | |
| Adults | 11 (1445) | 0.80 (0.56 to 1.14) | 9 (410) | 1.01 (0.52 to 1.97) | |
| Antibiotics used in comparison: | |||||
| Tetracyclines | 1 (47) | 3.39 (0.15 to 79.2) | 5 (148) | 1.17 (0.32 to 4.23) | |
| All others | 12 (1506) | 0.87 (0.63 to 1.20) | 4 (262) | 1.03 (0.35 to 3.00) | |
| Resistant pathogens excluded: | |||||
| Yes | 3 (176) | 1.00 (0.37 to 2.72) | 1 (45) | 3.41 (0.15 to 79.5) | |
| No | 10 (1377) | 0.84 (0.60 to 1.19) | 8 (365) | 0.96 (0.48 to 1.95) | |
| Diagnosis of sinusitis: | |||||
| Subjective | 4 (543) | 0.89 (0.46 to 1.71) | 8 (379) | 0.99 (0.47 to 2.08) | |
| Firm | 9 (1010) | 0.88 (0.60 to 1.28) | 1 (31) | 1.65 (0.17 to 16.3) | |
| Assessment of outcomes: | |||||
| Unclear | 3 (468) | 0.88 (0.50 to 1.55) | 4 (131) | 1.18 (0.44 to 3.13) | |
| Specified | 10 (1085) | 0.85 (0.57 to 1.26) | 5 (279) | 1.05 (0.35 to 3.10) | |
| Blinding: | |||||
| Unblinded or single blind | 8 (821) | 0.89 (0.53 to 1.50) | 7 (365) | 0.99 (0.44 to 2.23) | |
| Double blind | 5 (732) | 0.84 (0.55 to 1.27) | 2 (45) | 1.54 (0.22 to 11.0) | |
| Publication date: | |||||
| 1983-91 | 7 (640) | 1.00 (0.57 to 1.75) | 4 (189) | 0.71 (0.28 to 1.81) | |
| 1993-8 | 6 (913) | 0.82 (0.55 to 1.23) | 5 (221) | 1.77 (0.79 to 3.96) | |
| Jadad quality score: | |||||
| <3 | 6 (539) | 0.85 (0.49 to 1.48) | 7(365) | 0.99 (0.44 to 2.23) | |
| ⩾3 | 7 (1014) | 0.86 (0.58 to 1.28) | 2 (45) | 1.54 (0.22 to 11.0) | |
Risk ratios <1 mean that other antibiotics were better than the reference drugs (amoxycillin or folate inhibitors). There was no significant heterogeneity between subgroups for any of the sensitivity analyses (P>0.1).
Radiographic and bacteriological outcomes and patient withdrawals
Radiographic and bacteriological data were not available for many trials (table 2). Rates of radiographic failures within 48 hours of the end of treatment were not significantly different among patients treated with other antibiotics compared with patients treated with amoxycillin or penicillin or folate inhibitors. Likewise, rates of bacteriological failure were not significantly different, although most samples were obtained with nasal swabs and the data are therefore not reliable. There was no significant difference between different treatments in the rate of patients withdrawal.
Discussion
This meta-analysis showed that in two thirds of the cases of sinusitis, there is spontaneous improvement or cure without antibiotic treatment. Among patients with sinusitis defined by clinical criteria alone, the rate of spontaneous resolution may be even higher. Treatment with any antibiotic reduced the rate of clinical failures by half. Treatment with newer, generally more expensive, antibiotics did not seem to reduce the rate of treatment failure beyond what amoxycillin and co-trimoxazole could achieve.
Limitations of study
We compared the reference drugs amoxycillin and folate inhibitors with a heterogeneous array of antibiotics with differing antibacterial spectra. It is possible that, by grouping these drugs, we have obscured some important and systematic differences between the drug classes. There were too few studies in any single antibiotic group to allow a meaningful meta-analysis of each class. However, simple inspection of figures 2 and 3 suggests that there was no consistent superiority of any drug class over the reference drugs.
The total number of patients available for pooling in this meta-analysis was small. It is possible that a significant advantage of newer antibiotics might have been evident if more data were available. However, the chance of this advantage being large enough to be clinically important is small. Even with the most extreme values for the 95% confidence intervals, clinical failure would be averted in one of 32 patients treated with amoxycillin or one of 16 patients treated with a folate inhibitor, probably not enough to justify routine use of newer antibiotics as first line treatment. If the data were affected by publication bias, the effect presumably would be to reduce the amount of data unfavourable to the newer drugs. In that case, the advantages of the newer drugs would be even less than we found. Bias related to poor quality of the studies would also presumably act in favour of the newer drugs,41 in which case their advantages would again be reduced. Sensitivity analysis showed that, when only trials with a Jadad quality score of at least 3 were considered, the estimates for all major end points of treatment effect were unchanged for the major comparisons.
Another concern is the comparability of patients included in these trials to current patient populations. Some of the studies were conducted when the rates of antimicrobial resistance of H influenzae, M catarrhalis, and S pneumoniae were much lower. Yet sensitivity analysis showed no evidence of a difference in results between recent and older studies, or between studies that included or excluded patients infected by drug resistant organisms. We were unable to find sufficient data based on sinus puncture to allow us to evaluate the effect of resistance to the antibiotic treatment on the outcome of sinusitis.
Implications of study
We found only two studies, with a total of 113 patients, that directly compared amoxycillin and folate inhibitors.42,43 The small number of patients did not allow a meaningful comparison of the drugs: the risk ratio of failure with folate inhibitors versus amoxycillin was 0.5, but the 95% confidence interval was wide (0.08 to 3.01). Co-trimoxazole has a broader spectrum than amoxycillin, being active against amoxycillin resistant H influenzae and M catarrhalis. Its use should largely satisfy those concerned about antimicrobial resistance when prescribing treatment for community acquired acute sinusitis.
Complications of sinusitis can be serious, including brain abscess, orbital cellulitis, subdural empyema, and meningitis. We found no mention of such complications among more than 2700 patients in 27 trials. Large referral hospitals rarely report such complications.44,45 To our knowledge, there are no data to suggest that the use of newer, more expensive antibiotics would reduce the rate of these rare complications. Nevertheless, it should be emphasised that our data apply to patients with uncomplicated, community acquired, acute sinusitis. Patients with complicated sinusitis and those severely ill with sinusitis or with important underlying diseases might merit initial treatment with drugs other than amoxycillin or a folate inhibitor.
Our meta-analysis highlights the need to improve the quality of studies in outpatient antibiotic management. Because of the subjective nature of the relevant end points, double blind design is extremely important in evaluating treatments for sinusitis. Study protocols should require either radiographic findings or antral puncture and aspiration as criteria for study entry. Nasal swabs have been shown to be inaccurate indicators of the pathogens in sinusitis.46,47 Patients with chronic and subacute sinusitis should be studied separately from patients with acute sinusitis. The use of decongestants should be specified by protocol. Clinical outcomes should be defined with a detailed scoring system. Patients with infection caused by drug resistant organisms should not be excluded from the analysis; instead, particular attention should be paid to the outcome of these infections in order to determine if antibiotic resistance is an important predictor of treatment failure. Finally, the optimal duration of treatment should be addressed, as was done in one recent, well conducted study.47
Conclusions
Most clinical trials of new antibiotics compare the drugs with other newer drugs rather than with the inexpensive older drugs that we examined. There are obvious commercial reasons for this strategy: if the efficacy of a new drug were shown to be merely equivalent to that of an older drug the findings would hardly provide a useful marketing tool. There is societal value in decreasing the unnecessary use of newer, broad spectrum antibiotics to reduce the cost of care and possibly to reduce the rate of development of resistant microorganisms in the community.48–50 Even more fundamental is the need for accurate, inexpensive, and non-invasive methods to diagnose acute bacterial sinusitis.51,52 Such methods might sharply reduce the number of patients needing any antibiotic treatment given that most of the patients with acute sinusitis experienced spontaneous cure or improvement of symptoms.
Footnotes
Funding: This work was supported by grant R01 HS07782 from the Agency for Health Care Policy and Research of the US Public Health Service and grant T32 AI07389 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Conflict of interest: None.
References
- 1.Gwaltney JM. Sinusitis. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. 4th ed. Edinburgh: Churchill Livingstone; 1995. p. 585. [Google Scholar]
- 2.De Bock GH, Dekker FW, Stolk J, Springer MP, Kievit J, van Houwelinge JC. Antimicrobial treatment in acute maxillary sinusitis: a meta-analysis. J Clin Epidemiol. 1997;50:881–890. doi: 10.1016/s0895-4356(97)00117-0. [DOI] [PubMed] [Google Scholar]
- 3.American Society for Microbiology. Program and abstracts of the interscience conference on antimicrobial agents and chemotherapy. Washington, DC: American Society for Microbiology; 1993. , 1994, 1995, 1996, 1997. [Google Scholar]
- 4.Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 5.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 6.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;ii:121–145. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 7.Lau J, Ioannidis JPA, Schmid CH. Quantitative methods for systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 8.Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care. 1990;6:5–30. doi: 10.1017/s0266462300008916. [DOI] [PubMed] [Google Scholar]
- 9.Soderstrom M, Blomberg J, Christensen P, Hovelius B. Erythromycin and phenoxymethylpenicillin (penicillin V) in the treatment of respiratory tract infections as related to microbiological findings and serum C-reactive protein. Scand J Infect Dis. 1991;23:347–354. doi: 10.3109/00365549109024322. [DOI] [PubMed] [Google Scholar]
- 10.Alvart R. An open multicentre study to compare the efficacy and safety of sultamicillin with that of cefuroxime axetil in acute ear, nose and throat infections in adults. J Int Med Res. 1992;20(suppl 1):53–61A. [PubMed] [Google Scholar]
- 11.Falser N, Mittermayer H, Weuta H. Antibacterial treatment of otitis and sinusitis with ciprofloxacin and penicillin V—a comparison. Infection. 1988;16(suppl 1):S51–S54. doi: 10.1007/BF01650508. [DOI] [PubMed] [Google Scholar]
- 12.Johnson SE, Ford RD. Cephalexin dosage in general practice assessed by double-blind trial. Curr Med Res Opin. 1972;i:37–48. doi: 10.1185/03007997209111143. [DOI] [PubMed] [Google Scholar]
- 13.Podvinec M. Co-tetroxazin (Tibirox) und Doxycyclin (Vibramycin) in der Behandlung von Infekten der oberen Luftwege: eine Doppelblindstudie. Ther Umsch. 1982;39:815–820. [PubMed] [Google Scholar]
- 14.Axelsson A, Chidekel N, Grebelius N, Jensen C. Treatment of acute maxillary sinusitis: a comparison of four different methods. Acta Otolaryngol (Stockh) 1970;70:71–76. doi: 10.3109/00016487009181861. [DOI] [PubMed] [Google Scholar]
- 15.Ganança M, Trabulsi LR. The therapeutic effects of cyclacillin in acute sinusitis: in vitro and in vivo correlations in a placebo-controlled study. Curr Med Res Opin. 1973;i:362–368. doi: 10.1185/03007997309111694. [DOI] [PubMed] [Google Scholar]
- 16.Wald ER, Chiponis D, Ledesma-Medina J. Comparative effectiveness of amoxicillin and amoxicillin-clavulanate potassium in acute paranasal sinus infections in children: a double-blind, placebo-controlled trial. Pediatrics. 1986;77:795–800. [PubMed] [Google Scholar]
- 17.Lindbaek M, Hjortdahl P, Johnsen ULH. Randomised, double blind, placebo controlled trial of penicillin V and amoxycillin in treatment of acute sinus infections in adults. BMJ. 1996;313:325–329. doi: 10.1136/bmj.313.7053.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Buchem FL, Knottnerus JA, Schrijnemaekers VJJ, Peeters MF. Primary-care based randomised placebo-controlled trial of antibiotic treatment in acute maxillary sinusitis. Lancet. 1997;349:683–687. doi: 10.1016/s0140-6736(96)07585-x. [DOI] [PubMed] [Google Scholar]
- 19.Stalman W, van Essen GA, van der Graaf Y, de Melker RA. The end of antibiotic treatment in adults with acute sinusitis-like complaints in general practice? A placebo-controlled double-blind randomized doxycycline trial. Br J Gen Pract. 1997;47:794–799. [PMC free article] [PubMed] [Google Scholar]
- 20.Wald ER, Reilly JS, Casselbrant M, Ledesma-Medina J, Milmoe GJ, Bluestone CD, et al. Treatment of acute maxillary sinusitis in childhood: a comparative study of amoxicillin and cefaclor. J Pediatr. 1984;104:297–302. doi: 10.1016/s0022-3476(84)81018-5. [DOI] [PubMed] [Google Scholar]
- 21.Matucci KF, Levin WJ, Mohsen AH. Acute bacterial sinusitis. Arch Otolaryngol Head Neck Surg. 1986;112:73–76. doi: 10.1001/archotol.1986.03780010075014. [DOI] [PubMed] [Google Scholar]
- 22.Brodie DP, Knight S, Cunningham K. Comparative study of cefuroxime axetil and amoxycillin in the treatment of acute sinusitis in general practice. J Int Med Res. 1989;17:547–551. doi: 10.1177/030006058901700608. [DOI] [PubMed] [Google Scholar]
- 23.Casiano RR. Azithromycin and amoxicillin in the treatment of acute maxillary sinusitis. Am J Med. 1991;91((3A):27–30S. doi: 10.1016/0002-9343(91)90398-h. [DOI] [PubMed] [Google Scholar]
- 24.Felstead SJ, Daniel R.for the European Azithromycin Study Group. Short-course treatment of sinusitis and other upper respiratory tract infections with azithromycin: a comparison with erythromycin and amoxycillin J Int Med Res 199119363–372. [DOI] [PubMed] [Google Scholar]
- 25.Karma P, Pukander J, Penttila M, Ylikoski J, Savolainen S, Olen L, et al. The comparative efficacy and safety of clarithromycin and amoxycillin in the treatment of outpatients with acute maxillary sinusitis. J Antimicrob Chemother. 1991;27(suppl A):83–90. doi: 10.1093/jac/27.suppl_a.83. [DOI] [PubMed] [Google Scholar]
- 26.Edelstein DR, Sanford EA, Chow JM, Duerksen RL, Johnson J, Ronis M, et al. Once-a-day therapy for sinusitis: a comparison study of cefixime and amoxicillin. Laryngoscope. 1993;103:33–41. doi: 10.1288/00005537-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Huck W, Reed BD, Nielsen RW, Ferguson RT, Gray DW, Lund GK, et al. Cefaclor vs amoxicillin in the treatment of acute, recurrent, and chronic sinusitis. Arch Fam Med. 1993;ii:497–503. doi: 10.1001/archfami.2.5.497. [DOI] [PubMed] [Google Scholar]
- 28.Calhoun KH, Hokanson JA. Multicenter comparison of clarithromycin and amoxicillin in the treatment of acute maxillary sinusitis. Arch Fam Med. 1993;ii:837–840. doi: 10.1001/archfami.2.8.837. [DOI] [PubMed] [Google Scholar]
- 29.Von Sydow C, Savolainen S, Soderqvist A. Treatment of acute maxillary sinusitis—comparing cefpodoxime proxetil with amoxicillin. Scand J Infec Dis. 1995;27:229–234. doi: 10.3109/00365549509019014. [DOI] [PubMed] [Google Scholar]
- 30.Matthews BL, Suprax/Amoxicillin Clinical Sinusitis Study Team. Effectiveness and safety of cefixime and amoxicillin in adults with acute bacterial sinusitis. In: Edlestein DR, ed. Sinusitis: optimizing management strategies. A special report. Postgrad Med 1998; May: 41-9.
- 31.Rimmer D, Suprax/Amoxicillin Clinical Sinusitis Study Team. Efficacy of cefixime and amoxicillin in adults with acute sinusitis. In: Edlestein DR, ed. Sinusitis: optimizing management strategies. A special report. Postgrad Med 1998; May: 50-7.
- 32.Otte J, Viada JA, Buchi MD, Slagado O. Tratamiento de los procesos sinusales agudos del adulto con tetraciclina y una combinacion de sulfametopirazina-trimethoprim. Rev Med Chile. 1983;111:1157–1161. [PubMed] [Google Scholar]
- 33.Osman MF, Menday AP. Single-blind comparison of miraxid and co-trimoxazole in patients with sinusitis and otitis. In: Spitzy KH, Karrer K, editors. Proceedings of the 13th International Congress of Chemotherapy. Vienna: H Egermann; 1983. pp. 26–29. . (SS 4.1/1-5.) [Google Scholar]
- 34.Wallace RB, Marsh BT, Talbot DJ. A multi-centre general practice clinical evaluation of pivmecillinam plus pivampicillin (“Miraxid”) and co-trimoxazole (“Septrim”) in respiratory tract infections. Curr Med Res Opin. 1985;9:659–665. doi: 10.1185/03007998509109648. [DOI] [PubMed] [Google Scholar]
- 35.Salmi HA, Lehtomaki K, Kylmamaa T. Comparison of brodimoprim and doxycycline in acute respiratory tract infections: a double-blind clinical trial. Drugs Exp Clin Res. 1986;12:349–353. [PubMed] [Google Scholar]
- 36.Manzini M, Caroggio A. Efficacy and tolerability of brodimoprim and roxithromycin in acute sinusitis of bacterial origin in adults. J Chemother. 1993;5:521–525. [PubMed] [Google Scholar]
- 37.Arndt J, Riebenfeld D, Maier H, Weidauer H. Therapeutic efficacy and tolerability of brodimoprim in comparison with doxycycline in acute sinusitis in adults. J Chemother. 1994;6:322–327. doi: 10.1080/1120009x.1994.11741167. [DOI] [PubMed] [Google Scholar]
- 38.Bockmeyer M, Riebenfeld D, Clasen B. Controlled study of brodimoprim and cephalexin in the treament of patients with acute sinusitis in general practice. Clin Ther. 1994;16:653–661. [PubMed] [Google Scholar]
- 39.Rahlfs VW, Macchiocchi A, Monti T. Brodimoprim in upper respiratory tract infections. Clin Drug Invest. 1996;11:65–76. [Google Scholar]
- 40.Hayle R, Lingaas E, Hoivik HO, Odegard T. Efficacy and safety of azithromycin versus phenoxymethylpenicillin in the treatment of acute maxillary sinusitis. Eur J Clin Microbiol Infect Dis. 1996;15:849–853. doi: 10.1007/BF01691214. [DOI] [PubMed] [Google Scholar]
- 41.Schultz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 42.Hamory BH, Sande MA, Sydnor A, Seale DL, Gwaltney JM. Etiology and antimicrobial therapy of acute maxillary sinusitis. J Infect Dis. 1979;139:197–202. doi: 10.1093/infdis/139.2.197. [DOI] [PubMed] [Google Scholar]
- 43.Nyffeneger R, Riebenfeld D, Macciocchi A. Brodimoprim versus amoxicillin in the treatment of acute sinusitis. Clin Ther. 1991;13:589–595. [PubMed] [Google Scholar]
- 44.Skelton R, Maixner W, Isaacs D. Sinusitis-induced subdural empyema. Arch Dis Child. 1992;67:1478–1480. doi: 10.1136/adc.67.12.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson DL, Markle BM, Wiedermann BL, Hanahan L. Treatment of intracranial abscesses associated with sinusitis in children and adolescents. J Pediatr. 1988;113:15–23. doi: 10.1016/s0022-3476(88)80522-5. [DOI] [PubMed] [Google Scholar]
- 46.Evans FO, Sydnor JB, Morre WE, Moore GR, Manwaring JL, Brill AH, et al. Sinusitis of the maxillary antrum. N Engl J Med. 1975;293:735–739. doi: 10.1056/NEJM197510092931502. [DOI] [PubMed] [Google Scholar]
- 47.Williams JW, Holleman DR, Samsa GP, Simel DL. Randomized controlled trial of 3 vs. 10 days of trimethoprim/sulfamethoxazole for acute maxillary sinusitis. JAMA. 1995;273:1015–1021. [PubMed] [Google Scholar]
- 48.Arason VA, Kristinsson KG, Sigurdsson JA, Stefandottir G, Molstad S, Gudmundsson S. Do antimicrobials increase the carriage rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. BMJ. 1996;313:387–391. doi: 10.1136/bmj.313.7054.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seppala H, Klaukka T, Lehtonen R, Nenonen E, Huovinen P. Outpatient use of erythromycin: link to increased erythromycin resistance in group A streptococci. Clin Infect Dis. 1995;21:1378–1385. doi: 10.1093/clinids/21.6.1378. [DOI] [PubMed] [Google Scholar]
- 50.Nissinen A, Gronroos P, Huovinen P, Herva E, Katila ML, Klaukka T, et al. Development of beta-lactamase-mediated resistance to penicillin in middle-ear isolates of Moraxella catarrhalis in Finnish children, 1978-1993. Clin Infect Dis. 1995;21:1193–1196. doi: 10.1093/clinids/21.5.1193. [DOI] [PubMed] [Google Scholar]
- 51.Hansen JG, Schmidt H, Rosborg J, Lund E. Predicting acute maxillary sinusitis in a general practice population. BMJ. 1995;311:233–236. doi: 10.1136/bmj.311.6999.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindbaek M, Hjortdahl P, Johnsen UL. Use of symptoms, signs, and blood tests to diagnose acute sinus infections in primary care: comparison with computer tomography. Fam Med. 1996;28:183–188. [PubMed] [Google Scholar]