Abstract
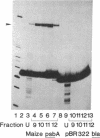
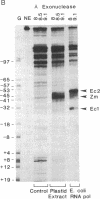
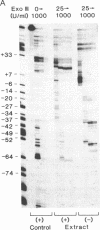
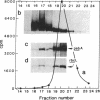
Preparations of partially purified chloroplast DNA-dependent RNA polymerase from maize and some other plants transcribe cloned chloroplast genes preferentially and much more actively from appropriately negatively supercoiled templates than from relaxed templates. We have found that the polymerase in such fractions does not bind to promoter regions of the maize chloroplast genes psbA and rbcL on small linear DNA fragments but that some protein(s) in unfractionated chloroplast extracts does bind. DEAE chromatography of the extracts has permitted the separation of a DNA-binding fraction from the bulk of the RNA polymerase activity. The binding fraction contains plastid RNA polymerase activity that is relatively independent of template topology.
Full text
PDF

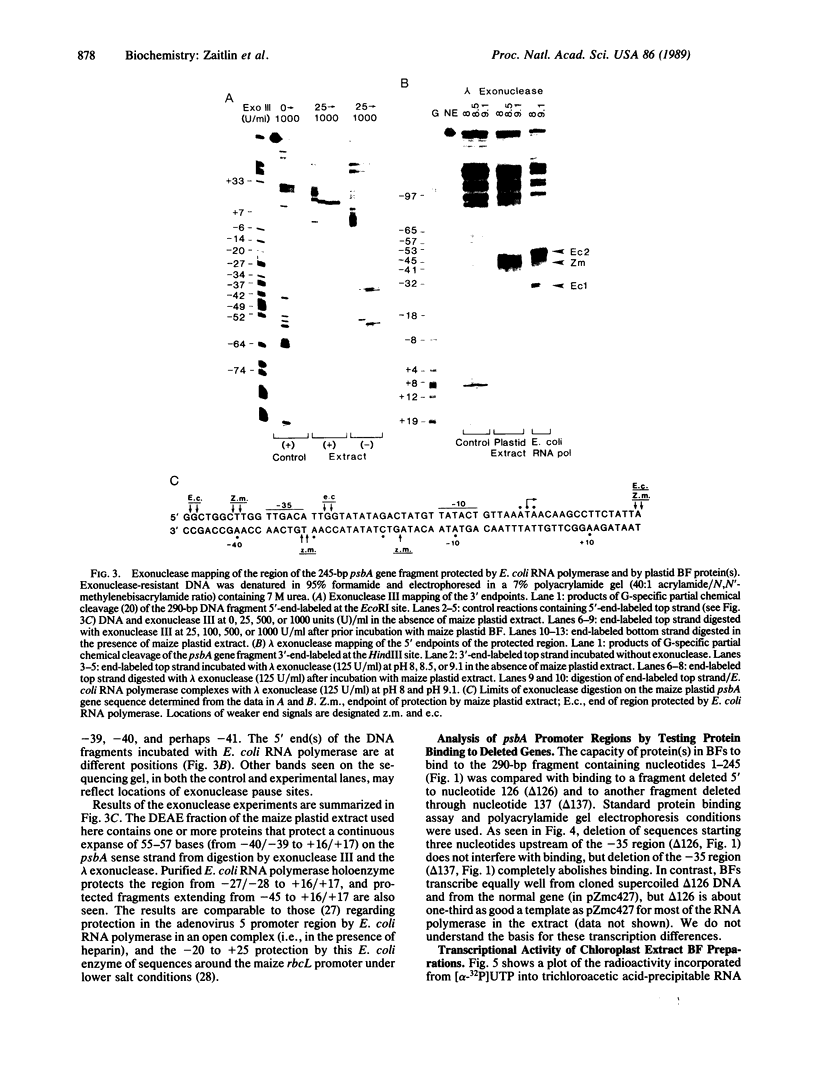
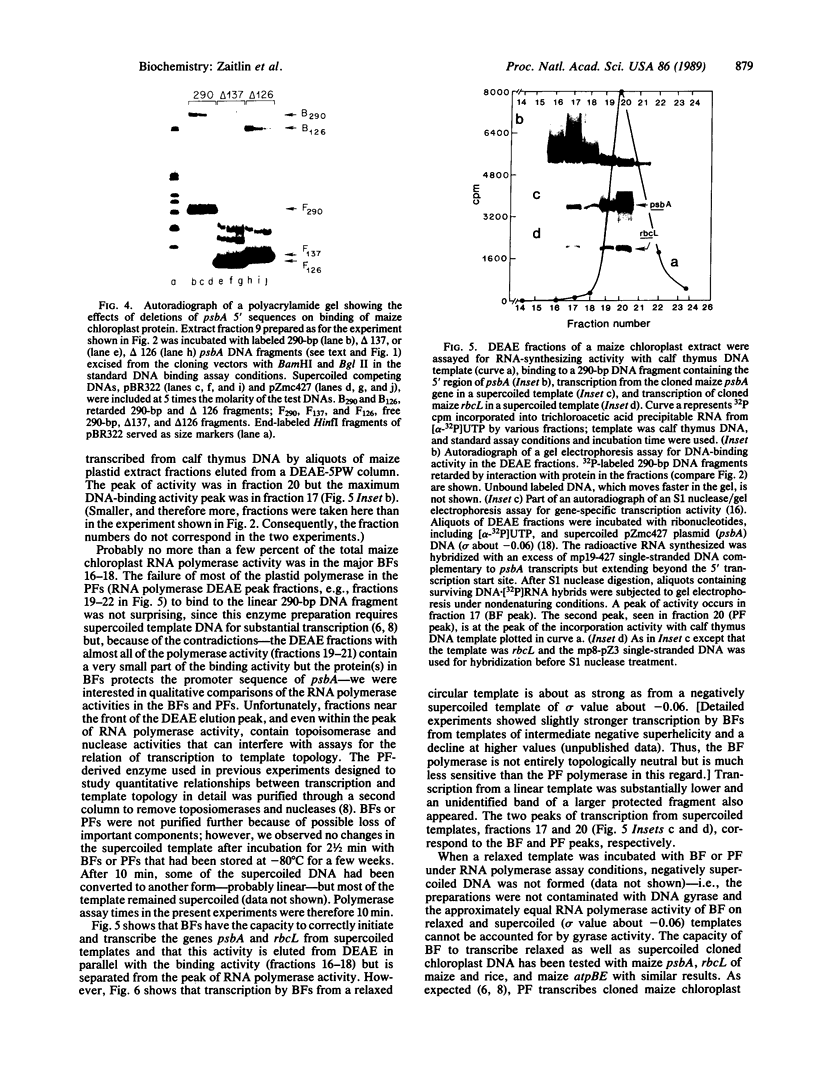

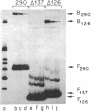
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Bogorad L. Light-induced increase in the activity of maize plastid DNA-dependent RNA polymerase. Eur J Biochem. 1976 Aug 16;67(2):615–620. doi: 10.1111/j.1432-1033.1976.tb10727.x. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Gralla J. D. All three elements of the lac ps promoter mediate its transcriptional response to DNA supercoiling. J Mol Biol. 1987 May 5;195(1):89–97. doi: 10.1016/0022-2836(87)90329-9. [DOI] [PubMed] [Google Scholar]
- Borowiec J. A., Gralla J. D. Supercoiling response of the lac ps promoter in vitro. J Mol Biol. 1985 Aug 20;184(4):587–598. doi: 10.1016/0022-2836(85)90305-5. [DOI] [PubMed] [Google Scholar]
- Bottomley W., Smith H. J., Bogorad L. RNA polymerases of maize: partial purification and properties of the chloroplast enzyme. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2412–2416. doi: 10.1073/pnas.68.10.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossland L. D., Rodermel S. R., Bogorad L. Single gene for the large subunit of ribulosebisphosphate carboxylase in maize yields two differentially regulated mRNAs. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4060–4064. doi: 10.1073/pnas.81.13.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebanier A. E., Coen D. M., Rich A., Bogorad L. Membrane proteins synthesized but not processed by isolated maize chloroplasts. J Cell Biol. 1978 Sep;78(3):734–746. doi: 10.1083/jcb.78.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. O., Bogorad L. Preferential transcription of cloned maize chloroplast DNA sequences by maize chloroplast RNA polymerase. Proc Natl Acad Sci U S A. 1980 Feb;77(2):822–826. doi: 10.1073/pnas.77.2.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. O., McIntosh L., Link G., Bogorad L. Differential transcription in vivo and in vitro of two adjacent maize chloroplast genes: The large subunit of ribulosebisphosphate carboxylase and the 2.2-kilobase gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6821–6825. doi: 10.1073/pnas.78.11.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd G. H., Bogorad L. A facile procedure for purifying maize chloroplast RNA polymerase from whole cell homogenates. Biochim Biophys Acta. 1980 Aug 26;609(1):14–30. doi: 10.1016/0005-2787(80)90197-5. [DOI] [PubMed] [Google Scholar]
- Lam E., Hanley-Bowdoin L., Chua N. H. Characterization of a chloroplast sequence-specific DNA binding factor. J Biol Chem. 1988 Jun 15;263(17):8288–8293. [PubMed] [Google Scholar]
- Link G., Coen D. M., Bogorad L. Differential expression of the gene for the large subunit of ribulose bisphosphate carboxylase in maize leaf cell types. Cell. 1978 Nov;15(3):725–731. doi: 10.1016/0092-8674(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Link G. DNA sequence requirements for the accurate transcription of a protein-coding plastid gene in a plastid in vitro system from mustard (Sinapis alba L.). EMBO J. 1984 Aug;3(8):1697–1704. doi: 10.1002/j.1460-2075.1984.tb02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Narita J. O., Rushlow K. E., Hallick R. B. Characterization of a Euglena gracilis chloroplast RNA polymerase specific for ribosomal RNA genes. J Biol Chem. 1985 Sep 15;260(20):11194–11199. [PubMed] [Google Scholar]
- Peterson M. L., Reznikoff W. S. Properties of lac P2 in vivo and in vitro. An overlapping RNA polymerase binding site within the lactose promoter. J Mol Biol. 1985 Oct 5;185(3):535–543. doi: 10.1016/0022-2836(85)90070-1. [DOI] [PubMed] [Google Scholar]
- Russell D., Bogorad L. Transcription analysis of the maize chloroplast gene for the ribosomal protein S4. Nucleic Acids Res. 1987 Feb 25;15(4):1853–1867. doi: 10.1093/nar/15.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Y., Bogorad L. Differential Expression in Bundle Sheath and Mesophyll Cells of Maize of Genes for Photosystem II Components Encoded by the Plastid Genome. Plant Physiol. 1988 Apr;86(4):1020–1026. doi: 10.1104/pp.86.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Y., Bogorad L. Expression of the ribulose-1,5-bisphosphate carboxylase large subunit gene and three small subunit genes in two cell types of maize leaves. EMBO J. 1986 Dec 20;5(13):3417–3422. doi: 10.1002/j.1460-2075.1986.tb04663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Smith H. J., Bogorad L. The polypeptide subunit structure of the DNA-dependent RNA polymerase of Zea mays chloroplasts. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4839–4842. doi: 10.1073/pnas.71.12.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., McIntosh L., Bogorad L., Arntzen C. J. Identification of the triazine receptor protein as a chloroplast gene product. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7463–7467. doi: 10.1073/pnas.78.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirdivant S. M., Crossland L. D., Bogorad L. DNA supercoiling affects in vitro transcription of two maize chloroplast genes differently. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4886–4890. doi: 10.1073/pnas.82.15.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger J., Baltimore D., Sharp P. A. Distinct factors bind to apparently homologous sequences in the immunoglobulin heavy-chain enhancer. 1986 Aug 28-Sep 3Nature. 322(6082):846–848. doi: 10.1038/322846a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]