Abstract
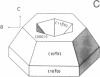
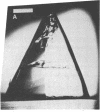
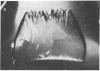
Peptide and glycopeptide antifreezes from a variety of cold-water fishes cause ice single crystals grown from the melt to assume unusual and strikingly similar habits. The antifreezes inhibit growth on the prism faces but allow limited growth on the basal plane. As new layers are deposited on the basal plane, pyramidal surfaces develop on the outside of the crystal, and large hexagonal pits form within the basal plane. The pits are rotated 30 degrees with respect to the normal orientation of hexagonal ice crystals. Growth inhibition on the prism, pyramidal, and pit faces indicates that these faces contain sites of adsorption of the antifreeze molecules. Several properties of the antifreeze pits are consistent with (but do not prove) an origin of the pits at dislocations. The similarity of crystal habit imposed on ice by antifreezes with wide differences in composition and structure indicates a common mechanism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addadi L., Weiner S. Interactions between acidic proteins and crystals: stereochemical requirements in biomineralization. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4110–4114. doi: 10.1073/pnas.82.12.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoba T., Fukae M., Tanabe T., Shimizu M., Moreno E. C. Selective adsorption of porcine-amelogenins onto hydroxyapatite and their inhibitory activity on hydroxyapatite growth in supersaturated solutions. Calcif Tissue Int. 1987 Nov;41(5):281–289. doi: 10.1007/BF02555230. [DOI] [PubMed] [Google Scholar]
- Blumenthal N. C., Betts F., Posner A. S. Effect of carbonate and biological macromolecules on formation and properties of hydroxyapatite. Calcif Tissue Res. 1975 Jul 25;18(2):81–90. doi: 10.1007/BF02546228. [DOI] [PubMed] [Google Scholar]
- DeVries A. L. Antifreeze peptides and glycopeptides in cold-water fishes. Annu Rev Physiol. 1983;45:245–260. doi: 10.1146/annurev.ph.45.030183.001333. [DOI] [PubMed] [Google Scholar]
- DeVries A. L., Komatsu S. K., Feeney R. E. Chemical and physical properties of freezing point-depressing glycoproteins from Antarctic fishes. J Biol Chem. 1970 Jun 10;245(11):2901–2908. [PubMed] [Google Scholar]
- Feeney R. E., Burcham T. S., Yeh Y. Antifreeze glycoproteins from polar fish blood. Annu Rev Biophys Biophys Chem. 1986;15:59–78. doi: 10.1146/annurev.bb.15.060186.000423. [DOI] [PubMed] [Google Scholar]
- Harrison K., Hallett J., Burcham T. S., Feeney R. E., Kerr W. L., Yeh Y. Ice growth in supercooled solutions of antifreeze glycoprotein. Nature. 1987 Jul 16;328(6127):241–243. doi: 10.1038/328241a0. [DOI] [PubMed] [Google Scholar]
- Hew C. L., Joshi S., Wang N. C., Kao M. H., Ananthanarayanan V. S. Structures of shorthorn sculpin antifreeze polypeptides. Eur J Biochem. 1985 Aug 15;151(1):167–172. doi: 10.1111/j.1432-1033.1985.tb09081.x. [DOI] [PubMed] [Google Scholar]
- Kibe A., Holzbach R. T., LaRusso N. F., Mao S. J. Inhibition of cholesterol crystal formation by apolipoproteins in supersaturated model bile. Science. 1984 Aug 3;225(4661):514–516. doi: 10.1126/science.6429856. [DOI] [PubMed] [Google Scholar]
- Li X. M., Trinh K. Y., Hew C. L., Buettner B., Baenziger J., Davies P. L. Structure of an antifreeze polypeptide and its precursor from the ocean pout, Macrozoarces americanus. J Biol Chem. 1985 Oct 25;260(24):12904–12909. [PubMed] [Google Scholar]
- Nakagawa Y., Abram V., Kézdy F. J., Kaiser E. T., Coe F. L. Purification and characterization of the principal inhibitor of calcium oxalate monohydrate crystal growth in human urine. J Biol Chem. 1983 Oct 25;258(20):12594–12600. [PubMed] [Google Scholar]
- Ng N. F., Trinh K. Y., Hew C. L. Structure of an antifreeze polypeptide precursor from the sea raven, Hemitripterus americanus. J Biol Chem. 1986 Nov 25;261(33):15690–15695. [PubMed] [Google Scholar]
- Price P. A., Otsuka A. A., Poser J. W., Kristaponis J., Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A. 1976 May;73(5):1447–1451. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J. A., DeVries A. L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2589–2593. doi: 10.1073/pnas.74.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag J. D., Cheng C. H., Panico M., Morris H. R., DeVries A. L. Primary and secondary structure of antifreeze peptides from arctic and antarctic zoarcid fishes. Biochim Biophys Acta. 1987 Oct 15;915(3):357–370. doi: 10.1016/0167-4838(87)90021-5. [DOI] [PubMed] [Google Scholar]
- Yang D. S., Sax M., Chakrabartty A., Hew C. L. Crystal structure of an antifreeze polypeptide and its mechanistic implications. Nature. 1988 May 19;333(6170):232–237. doi: 10.1038/333232a0. [DOI] [PubMed] [Google Scholar]