Abstract
The analyses of gene duplications by retroposition have revealed an excess of male-biased duplicates generated from X chromosome to autosomes in flies and mammals. Investigating these genes is of primary importance in understanding sexual dimorphism and genome evolution. In a particular instance in Drosophila, X-linked nuclear transport genes (Ntf-2 and ran) have given rise to autosomal retroposed copies three independent times (along the lineages leading to Drosophila melanogaster, D. ananassae, and D. grimshawi). Here we explore in further detail the expression and the mode of evolution of these Drosophila Ntf-2- and ran-derived retrogenes. Five of the six retrogenes show male-biased expression. The ran-like gene of D. melanogaster and D. simulans has undergone recurrent positive selection. Similarly, in D. ananassae and D. atripex, the Ntf-2 and ran retrogenes show evidence of past positive selection. The data suggest that strong selection is acting on the origin and evolution of these retrogenes. Avoiding male meiotic X inactivation, increasing level of expression of X-linked genes in male testes, and/or sexual antagonism might explain the recurrent duplication of retrogenes from X to autosomes. Interestingly, the ran-like in D. yakuba has mostly pseudogenized alleles. Disablement of the ran-like gene in D. yakuba indicates turnover of these duplicates. We discuss the possibility that Dntf-2r and ran-like might be involved in genomic conflicts during spermatogenesis.
CONVERGENT duplications of two genes involved in nuclear transport, Ntf-2 and ran in different lineages of Drosophila, have recently been described (Bai et al. 2007). The Drosophila Ntf-2 (Dntf-2) appears to have generated retroposed copies—retrogenes (Brosius 1991)—three independent times. The retrogenes are observed in different genomic locations in different Drosophila species with respect to the six evolutionarily conserved chromosomal arms of Drosophila (i.e., Muller elements A to F; Powell 1997): (1) Dntf-2r located in the 2L chromosomal arm (Muller element B) of the four species of the Drosophila melanogaster complex (D. melanogaster, D. simulans, D. mauritiana, and D sechellia; Betrán and Long 2003), (2) an unnamed retrogene in the D. ananassae lineage (GF23973) located in the chromosomal arm corresponding to Muller element D, and (3) a retrogene in the lineage leading to D. grimshawi (GH16820) located in the arm that corresponds to Muller element D. We call the Dntf-2-derived retrogenes of the D. ananassae and D. grimshawi lineages Da_Ntf-2r and Dg_Ntf-2r, respectively. The ran gene has also given rise to retrogenes three independent times—along some of the same lineages as the Dntf-2-derived retrogenes. It gave rise to ran-like, which is present in all the species of the D. melanogaster subgroup and is located in the 3L arm (Muller element D). D. ananassae and D. grimshawi also have independently derived ran retrogenes, GF24088 and GH16204, located in Muller elements B and D, respectively. We call the ran-derived retrogenes of the D. ananassae and D. grimshawi lineages Da_ran-like and Dg_ran-like, respectively.
The parental ran and Ntf-2 proteins physically interact with each other and play a central role in the transport of proteins into and out of the nucleus (Ribbeck et al. 1998). Both proteins are highly conserved and are required in all eukaryotes (Quimby et al. 2000). The presence of duplicates of both genes in several lineages may have an adaptive explanation, particularly if the expression of each duplicate overlaps the other. While the function of the duplicates is not known, all six independent retrogene events occurred from X-to-autosome locations—a direction of retroduplication known to be overrepresented in Drosophila, human, and mouse genomes (Betrán et al. 2002; Emerson et al. 2004). The X-to-autosome duplications may be the result of positive selection due to pressures relating to male meiotic X inactivation, sexual antagonism, and/or X chromosome dosage compensation (see below).
In the case of male meiotic X inactivation (Lifschytz and Lindsley 1972; Hense et al. 2007), it is thought to be beneficial to have gene copies off the X and onto autosomes where transcription can occur during X chromosome inactivation (Betrán et al. 2002). It has previously been shown that the D. melanogaster retrogene Dntf-2r exhibits a strong testis-biased expression (Betrán and Long 2003), and the same is true for ran-like (Chintapalli et al. 2007). In addition, both Dntf-2r and ran-like represent X-to-autosome movements (Bai et al. 2007) . Thus, it is possible that the multiple parallel retropositions to autosomes of Dntf-2 and ran have been driven by the selective advantage of providing a way of retaining nuclear transport function in males during meiotic X inactivation. Male meiotic X inactivation might be a consequence of the inactivation that unsynapsed meiotic chromosomes suffer (Turner et al. 2005; Turner 2007).
On the other hand, under the standard sexual antagonistic hypothesis, males and females need the same gene, but alleles that are good for males might be bad for females and vice versa (i.e., some homologous traits are selected in different directions in the different sexes; Chippindale et al. 2001). In this case, if mutations are partially dominant, it might be beneficial to have the male-biased genes located on the autosomes, as opposed to the X chromosome. The X chromosome spends two-thirds of its time in females; thus, the female would be able to outcompete the male for alleles on the X chromosome (Rice 1984; Charlesworth et al. 1987; Ranz et al. 2003).
In the case of X chromosome dosage compensation, it is known that compensation in Drosophila occurs through hypertranscription of the X chromosome in males. The need for additional increased X-linked gene expression in males could be a pressure to duplicate X-linked genes onto the autosomes (Vicoso and Charlesworth 2009).
Additionally, the duplicates attaining novel male testes functions could also explain the multiple duplication events of the nuclear transport genes Dntf-2 and ran. For example, it has been proposed that nuclear transport genes (including Dntf-2r) might play a role in segregation distortion and/or germline genomic conflicts involving transposable elements and viruses (Presgraves 2007; Presgraves and Stephan 2007). Interestingly, there is direct evidence that genes involved in nuclear transport play a role in the young SD segregation distortion system in spermatogenesis of D. melanogaster (see review, Kusano et al. 2003). The main distorter, Sd, is a truncated duplicate form of the nuclear transport gene RanGAP that (mis)localizes in the nucleus (Kusano et al. 2003). Nuclear transport genes (e.g., RanGAP and six different nucleoporins) and their duplicates (e.g., Dntf-2r) have been hypothesized to be involved in segregation distortion in Drosophila (Presgraves 2007). The arms race between distorters and suppressors of distortion could lead to the fixation of duplicate genes with testes expression and to fast gene evolution of these genes (Kusano et al. 2002; Burt and Trivers 2006; Presgraves 2007). Indeed, RanGAP, six nucleoporins, and Dntf-2r all have been shown to have evolved under positive selection (Betrán and Long 2003; Presgraves 2007; Presgraves and Stephan 2007). If this is the case, the selective advantage for the fixation, longevity, and fast evolution of duplicates like Dntf-2r will persist as long as segregation distortion conflicts involving nuclear transport keep emerging.
Transposable elements and viruses may also impose genomic conflicts involving nuclear transport since these elements often need to enter germline nuclei to replicate (Presgraves and Stephan 2007).
Previous work demonstrated that the D. melanogaster Dntf-2r has testis-biased expression and is under positive selection. D. melanogaster ran-like is similarly testes biased in expression. Here we explore the mode of evolution and the pattern of expression of other independently retroposed copies of Ntf-2 and ran. In both D. ananassae and D. grimshawi, we show that Dntf-2 and ran retrogenes are, for the most part, strongly male biased in their transcription. In addition, we reveal that all the retrogenes are evolving faster than the parental genes and are largely under positive selection. The ran-like gene is evolving under recurrent positive selection in several branches and for particular residues. In the lineages where ran-like is undergoing positive selection, some of the inferred selected amino acid changes appear to disturb specific protein–protein interaction surfaces. Polymorphism data for ran-like in the D. yakuba lineage indicate that D. yakuba's ran-like is a pseudogene in most alleles (9 of 10) and is wrongly annotated as functional in FlyBase. The retrogenes in the D. ananassae lineage, Da_Ntf-2r and Da_ran-like, are also under positive selection. These data reveal strong selective pressures on the origin and evolution of Dntf-2 and ran retrogenes.
MATERIALS AND METHODS
Strains used and DNA sequencing:
Genomic DNA was extracted from single flies using a Puregene kit (Qiagen, Valencia, CA). The ran-like gene was PCR amplified from genomic DNA from 12 D. melanogaster flies from different Zimbabwe strains (ZH13, ZH18, ZH19, ZH20, ZH21, ZH23, ZH26, ZH27, ZH28, ZH29, ZH32, and ZH40; Hollocher et al. 1997), nine D. simulans flies from different Madagascar strains (M1, M4, M5, M24, M37, M50, M242, M252, and M258), and 10 D. yakuba strains (Taï6, Taï15a, Taï15b, Taï18, Taï21, Taï26, Taï27, Taï30, Taï37, and Taï59) from the Taï forest in Ivory Coast. These strains were kindly provided by the Wu, Aquadro, Long, and Llopart laboratories. Oligonucleotide primers used for these reactions are given in the supporting information, Table S1.
Da_Ntf-2r and Da_Ran-like were PCR amplified from genomic DNA from 10 D. ananassae flies from different strains (14024-0371.16, 14024-0371.17, 14024-0371.18, 14024-0371.25, 14024-0371.30, 14024-0371.31, 14024-0371.32, 14024-0371.33, 14024-0371.34, and 14024-0371.35), and four D. atripex flies from different strains (14024-0361.00, 14024-0361.01, 14024-0361.02, and 14024-0361.03) obtained from the San Diego Stock Center. Oligonucleotide primers used in these reactions are given in Table S1. Da_Ntf-2r sequences of D. atripex are partial gene sequences (357 bp long).
PCR products were sequenced from both strands using an automated DNA sequencer and fluorescent BigDye terminators (Applied Biosystems, Foster City, CA). Internal primers were used in addition to the primers above to complete some of the sequencing. Heterozygotes were cloned using a TOPO cloning kit (Invitrogen, Carlsbad, CA), and one insert was sequenced to resolve haplotypes. Accession numbers for the sequences obtained are GU338155–GU338215.
Strains that were used for the whole genome sequence project of D. ananassae and D. grimshawi (Clark et al. 2007) were used to study pattern of expression of Dntf-2, ran, and their retrogenes in these species (see below). These strains were obtained from the former Tucson Drosophila Stock Center (currently the San Diego Stock Center).
Sequence analyses:
The nucleotide sequences of the Dntf-2 gene in the 12 sequenced Drosophila species (Clark et al. 2007) were retrieved from FlyBase along with the sequences of the paralog Dntf-2r in D. melanogaster, D. simulans, and D. sechellia. These 15 sequences were aligned with each other and with Dntf-2 retroposed sequences from D. ananassae and D. grimshawi with Clustal W software (Thompson et al. 1994). Sequences were analyzed initially using the CODEML software package implemented in PAML 4 (Yang 2007). The tree provided is shown in Figure S1. Branch models were employed to determine selective pressures on the parental and retroposed sequences by calculation of KA/KS ratios for each branch (Yang 1998). The one-ratio model sets all branches to evolve at the same rate. Additional models allowed for the genes to evolve at different rates, for the branches to evolve at different rates after duplication, or for the parental gene to evolve at a different rate after duplication. Models where KA/KS is set at one in particular lineages were also compared to detect purifying selection. These were all a priori hypotheses that we wanted to test. These models were compared by calculating two times the log likelihood values and comparing this value to a χ2 distribution with degrees of freedom equaling the difference in number of parameters estimated by each model.
Next, site models (NSsites) of CODEML software implemented in PAML were used to uncover the possibility of positive selection acting on a few sites. Site-specific likelihood models M7 and M8 were applied to the sequences with the appropriate tree topology (Nielsen and Yang 1998; Yang et al. 2000). Model M7, which does not allow for sites under positive selection, was compared to model M8, which does allow for sites under positive selection. M7 assumes a beta distribution for ω between 0 and 1 over all sites, while M8 adds an additional site class (ω ≥ 1), with ω estimated from the data. A likelihood ratio test was performed by calculating two times the log likelihood values and comparing this value to a χ2 distribution with two degrees of freedom. Posterior probabilities of codons under positive selection were computed in model M8 using Bayes empirical Bayes when the LRT was significant. The tree provided for the site analyses was ((Dntf-2r_D. simulans, Dntf-2r_D. sechellia), Dntf-2r_D. melanogaster).
The same PAML methods and comparisons were employed for ran and its retroposed sequences. We used parental and retrogene sequences that are present in the same species, with the addition of a ran-like ortholog in D. erecta. The ran-like ortholog in D. yakuba was not added to the analyses because a careful look at the annotation and polymorphism data revealed that it is likely a pseudogene. The tree provided for the KA/KS ratio analyses in different branches is shown in Figure S2. The fact that the ran-like ortholog in D. yakuba is evolving differently, similar to a pseudogene, prompted us to investigate if the other ran-like lineages evolve at different rates. The tree provided for the site-specific analyses was (((ran-like_D.simulans, ran-like_D.sechellia), ran-like_D.melanogaster), ran-like_D.erecta).
Sequences of Dntf-2r and ran-like were also analyzed using the HyPhy package. Dntf-2r sequences and tree topology were ((Dntf-2r_D. simulans, Dntf-2r_D. sechellia), Dntf-2r_D. melanogaster). The ran-like sequences and tree topology were (((ran-like_D. simulans, ran-like_D. sechellia), ran-like_D. melanogaster), ran-like_D. erecta). These sequences were uploaded to the HyPhy package available at http://www.datamonkey.org (Pond and Frost 2005). Random effects likelihood (REL) and fixed effects likelihood (FEL) analyses were performed in an attempt to detect positively selected codons in the Dntf-2r and ran-like phylogenies. A Bayes factor threshold of 50, which corresponds to very low probablity (P∼1/Bayes factor), was used for the REL analysis, and P < 0.10 for the FEL analysis (Kosakovsky Pond and Frost 2005). These codon analyses are presumed to be more realistic than the PAML site models because they allow for synonymous rate variation across sites (Kosakovsky Pond and Frost 2005).
A McDonald–Kreitman test (McDonald and Kreitman 1991) was performed for the polymorphism data obtained for ran-like in D. melanogaster and D. simulans. Sequenced products were aligned using Clustal W (Thompson et al. 1994) and imported into DnaSP 5.0 (Librado and Rozas 2009) to perform the test. Lineage-specific McDonald–Kreitman tests were also performed for ran-like in D. melanogaster and D. simulans (Akashi 1995; Presgraves 2007). A modified McDonald–Kreitman test was also used. The latter test removes synonymous unpreferred changes from the calculations because they might contribute more to polymorphisms than to divergence (Akashi 1995; Schlenke and Begun 2003).
D. yakuba ran-like sequences obtained in this work were aligned with two genes of ran-like of D. yakuba annotated in FlyBase (GE22850; 3L random and GE19852; 3L) to reveal the disablements that we detected in 9 of the 10 alleles and all the genome sequences (see results and Figure S3). We infer that the two annotated genes of ran-like of D. yakuba in FlyBase are likely alleles of the same gene. Both genes are flanked by the same region but have different disablements that likely prevented initial assembly.
Standard McDonald–Kreitman tests (McDonald and Kreitman 1991) were also performed for the polymorphism data obtained for Da_ran-like and Da_Ntf-2r in D. ananassae and D. atripex. Sequenced products were aligned using Clustal W (Thompson et al. 1994) and imported into DnaSP 5.0 (Librado and Rozas 2009) to perform the tests. The lineage-specific and the modified McDonald–Kreitman tests were not performed in this case because of the absence of a close enough outgroup sequence and because of a lack of information on codon preferences in these species.
The action of recent positive selection can be addressed using proposed population genetics statistics that test for skew in the frequency spectrum of alleles when compared to the neutral model. Tajima's D (Tajima 1989) compares θπ (the average number of nucleotide differences per site between two random sequences) and θW (Watterson's estimate of θ from the number of segregating sites; Watterson 1975). Differences between θπ and θW (Tajima's D) reveal nonequilibrium conditions in the history of the gene. Negative significant values are consistent with recent events of positive selection. The H statistic, the difference between θπ and θH estimates (Fay and Wu 2000), measures the excess of derived variants at high frequency. Again, negative significant values of this statistic are consistent with recent events of positive selection. Tajima's D and Fay and Wu's H values were tested using neutral coalescence simulations. They were computed and tested by 10,000 simulations using DNAsp 5.1 (Librado and Rozas 2009). Recombination rates for all those simulations were considered zero, to be conservative. When these tests were applied to the ran-like polymorphism data in D. yakuba, deletions were manually removed and coded as nucleotide changes.
Expression analyses:
Tissues were homogenized, and total RNA was prepared as described by the Qiagen protocol (RNeasy kit, Qiagen). Total RNA was obtained from 30 virgin gonadectomized females, 30 gonadectomized males, 30 ovaries, and 100 testes plus accessory glands of D. ananassae. Gonadectomized males and females are flies from which we removed ovaries/testes and accessory glands by dissecting mature males and females in saline solution. After dissection, tissues were preserved in RNAlater solution (Applied Biosystems/Ambion, Austin, TX) at −20° after soaking them at 4° overnight until they were processed. Total RNA was also obtained from two virgin females and three males from D. grimshawi.
RT–PCR was conducted on total RNA for Dntf-2, ran, and the retrogenes derived from these genes in D. ananassae and D. grimshawi. Gapdh2 and Gapdh were used as internal standards for the quantitative real-time RT–PCR (qRT–PCR) performed for the retrogenes in D. ananassae and D. grimshawi, respectively. Analysis of expression of intronless genes (such as Dntf-2 and ran retrogenes) is challenging because genomic contamination can produce a band of the same size as that expected from the cDNA. Therefore, we digested possible contaminating DNA from the total RNA (DNase I amplification grade, Invitrogen) and ran controls including DNA digested total RNA without reverse transcriptase (RT−). Single strand complementary DNA (cDNA) was synthesized using Superscript and oligo-dT (Promega, Madison, WI). RT–PCRs were carried out using specific oligonucleotide primers given in Table S1. qRT–PCRs were performed using the ABI 7300 Real Time PCR system and the SYBR Green PCR Core reagents from Applied Biosystems. Two or three replicates of RT+ and RT− were performed for every retrogene, normalizing gene, and RNA extraction. The primers described above were used and produced similar amplification plots in the logarithmic scale that corresponds to similar efficiency (Schmittgen and Livak 2008). RT–PCR products were run in gels to control for any spurious amplification. Threshold cycle numbers (CT values) were obtained with the default ABI software parameters. CT values obtained for the retrogenes were normalized by subtracting the CT value of the normalizing gene (ΔCT). The mean difference in normalized threshold cycle number (ΔCT) in different tissues was tested using ANOVA analyses. Post hoc Tukey tests were also performed. Fold changes in the expression were calculated using the expression 2−ΔΔCT (Schmittgen and Livak 2008).
RESULTS AND DISCUSSION
Dntf-2 and ran retrogenes are mostly male biased in expression:
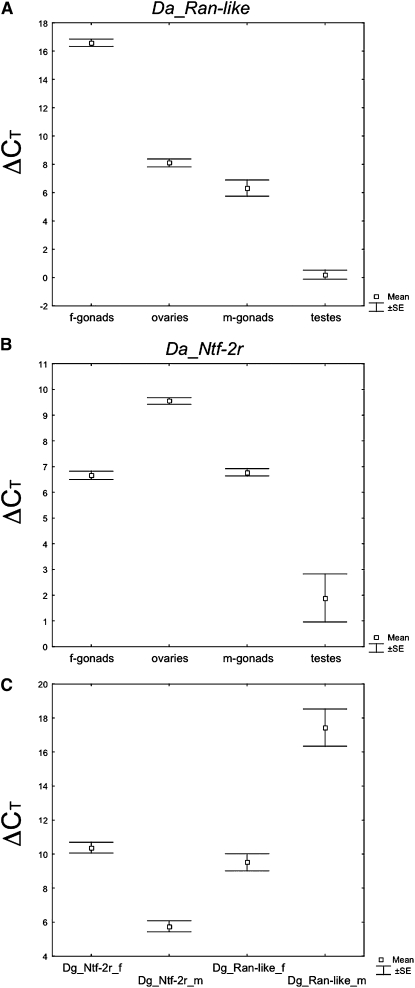
In D. ananassae, RT–PCR results reveal that both parental genes, Dntf-2 and ran, are transcribed equally in both males and females across all tested tissue types (data not shown). The qRT–PCR results for the Da_Nntf-2r and Da_ran-like retrogenes are shown in Figure 1, A and B. The average of the retrogene CT difference to Gapdh2 (i.e., ΔCT) and standard error are shown for each tissue type. Small values of ΔCT correspond to high values of transcription. An ANOVA comparing values for Da_ ran-like expression in different tissues (Figure 1A) reveals that these values are significantly different (F(3,8) = 317.5; P < 10−5). Post hoc Tukey tests show that all values are statistically significantly different from each other (P < 0.05 in all comparisons). Da_ran-like is highly expressed in testes and expressed at low or very low levels in gonadectomized male bodies, gonadectomized female bodies, and ovaries. Testes expression is 60-fold higher than in other tissues.
Figure 1.—
qRT–PCR results for the D. ananassae and D. grimshawi retrogenes (Da_ran-like, Da_Ntf-2r, Dg_ran-like, and Dg_ Ntf-2r) are shown. The average of the retrogene CT difference to the normalizing gene (i.e., ΔCT) and standard errors are shown for every tissue type. f, females; m, males.
An ANOVA comparing values for Da_ Ntf-2r expression in different tissues (Figure 1B) shows that these values are significantly different (F(3,7) = 77.472; P < 10−4). Post hoc Tukey tests reveal that all values are statistically significantly different from each other (P < 0.05 in all comparisons), except the comparison between male and female gonadectomized body (P = 0.9939). Da_ Ntf-2r is highly expressed in testes and expressed at much lower levels in gonadectomized male bodies, gonadectomized female bodies, and ovaries. Expression in testes is 27-fold higher than in other tissues.
In D. grimshawi RT–PCR results reveal again that the parental genes Dntf-2 and ran are transcribed equally in males and in females (data not shown). The retrogene Dg_Nntf-2r is highly expressed in males compared to females (F(1,4) = 103.96; P = 0.0005; Figure 1C). We do not know whether this bias is due to testes expression as we were not able to obtain separate tissues for D. grimshawi. Unlike Dg_Nntf-2r, Dg_ran-like is expressed higher in females than males (F(1,4) = 43.40; P = 0.0028; Figure 1C).
We conclude that Dntf-2 and ran retrogenes are typically male biased in expression. The retrogenes are strongly testes biased in the D. melanogaster lineage (Betrán and Long 2003; Chintapalli et al. 2007) and the D. ananassae lineage (Figure 1). Dg_Nntf-2r is male biased in the D. grimshawi lineage (Figure 1), although it remains unknown whether Dg_Nntf-2r is testes biased.
Sequence evolution of Dntf-2 and its retrogenes:
To understand the mode of evolution of Dntf-2 and its retrogenes, we performed sequence analyses using PAML software as described in materials and methods. Results of the branch PAML analyses for Dntf-2 genes and Dntf-2 retrogenes appear in Table S2. The free-ratio model (data not shown, l = −2507.6789, P = 65) was found to be significantly better (P = 1.787 × 10−13) than the one-ratio model in revealing rate differences. The two-ratio model, which estimates one KA/KS ratio for Dntf-2 and one ratio for the Dntf-2 retrogene branches, was found to fit the data better than the one-ratio model (P < 1.110 × 10−16). A four-ratio model was implemented to allow differing rates of evolution for the three retrogenes and Dntf-2. This model is significantly better than the two-ratio model (P = 8.390 × 10−5), indicating different rates of evolution among the recurrently recruited retrogenes. The retrogene in the D. melanogaster complex is evolving the fastest (KA/KS = 0.5311). That said, all of the retrogene evolution rates are significantly higher than the parental rate—3- to 21-fold higher.
The retrogene rates can be explained either by positive selection acting on the retrogenes or by relaxation of constraint in the retrogene lineages. We have evidence of positive selection acting on Dntf-2r provided by the McDonald–Kreitman test (Betrán and Long 2003) and on Da_Ntf-2r (see below). The results of the Da_Ntf-2r McDonald–Kreitman test is consistent with a high KA/KS ratio (0.3309) estimated in the D. ananassae lineage. However, no lines are available from D. grimshawi or close relatives to gather polymorphism data, and we have no evidence supporting selection in the D. grimshawi lineage at this point.
A five-ratio model is an extension of the four-ratio model. It allows a branch to evolve under a different KA/KS ratio postduplication. When the five-ratio model was applied to the retrogene in the D. melanogaster complex, it did not fit significantly better than the simpler four-ratio model. Models that considered a different rate for the parental genes postduplication were significantly worse at fitting the data than the four-ratio model (data not shown). Four additional models were constructed to determine whether the estimated KA/KS ratio for every gene was significantly less than one to ascertain whether purifying selection is acting. All four models were significantly worse at fitting the data than the four-ratio model, indicating that all of the KA/KS values in the phylogeny are significantly less than one and that purifying selection is acting in each of the lineages.
Although it has been pointed out that KS will saturate at large evolutionary distances (e.g., at distances larger than the D. melanogaster group that includes D. melanogaster, D. simulans, D. sechellia, D. yakuba, D. erecta, and D. ananassae; Larracuente et al. 2008), all of our Dntf-2 retrogenes are younger than the distance represented by the D. melanogaster group. Dntf-2r is present only in D. melanogaster, D. simulans, and D. sechellia. Da_Ntf-2r is present only in D. ananassae. Dg_Ntf-2r is present only in D. grimshawi, whose lineage is estimated to be shorter than the D. ananassae lineage (Clark et al. 2007). However, because the estimates of Dntf-2 KA/KS listed in Table S2 suffer from saturation, we reestimated the KA/KS ratio for Dntf-2 using only the D. melanogaster group of species. The PAML KA/KS ratio obtained was 0.0188. This KA/KS value confirms that the parental genes have evolved much slower than the retrogenes.
Site models (M7 and M8 and FEL) were also fitted to the data to test for positive selection acting on particular sites of Dntf-2r (see materials and methods). From these results we did not infer positive selection acting on any sites of the retroposed Dntf-2 sequences. However, REL analysis detected two codons [47 (Bayes factor = 52.9636), 87 (Bayes factor = 52.6307)] that are likely under positive selection. Nucleoporins are known to interact with amino acid number 47 of the parental NTF-2 (File S1).
Polymorphism data for part of Da_Ntf-2r in the D. ananassae and D. atripex lineages (Table S3) were used to perform the McDonald–Kreitman and Tajima's D and Wu's H tests. The results of the McDonald–Kreitman test reveal a statistically significant excess of replacement substitutions (Table 1) that is usually interpreted as recurrent positive selection acting on the protein. Tajima's D and Fay and Wu's H tests were not significant for either sample set (data not shown). The above result, along with published results for Dntf-2r (Betrán and Long 2003), suggest that most retroposed Dntf-2 sequences have been under strong selective pressures. We discuss the possible functional consequences of these protein changes in File S2.
TABLE 1.
McDonald–Kreitman tests for Da_Ntf-2r and Da_ran-like in the D. ananassae and D. atripex lineages
|
Da_Ntf-2r |
Da_ran-like |
|||
|---|---|---|---|---|
| Fixed | Polymorphic | Fixed | Polymorphic | |
| Replacement | 36 | 2 | 15 | 0 |
| Synonymous | 27 | 16 | 33 | 21 |
| GWilliams correction = 12.991 P = 0.0003 | Fisher's exact test P(two tailed)= 0.0031 | |||
Sequence evolution of ran and its retrogenes:
To understand the mode of evolution of ran and its retrogenes, we performed sequence analyses similar to the ones performed for Dntf-2 and its retrogenes. PAML-derived log likelihood values and maximum likelihood estimates of KA/KS ratios for the branches in ran and ran-like, as well as the other ran retrogenes, are given in Table S4. A free-ratio model (data not shown) was fitted (l = −4410.17775, P = 69) and compared to the one-ratio model. The free-ratio model gave a much better fit (P = 0) to the data due to differing KA/KS ratios along the branches of the tree. The one-ratio model was then compared to a two-ratio model (P = 0), showing a 43-fold increase in the rate of evolution in the ran retrogene lineages when compared to the ran branches (e.g., 0.1793 for retrogene vs. 0.0042 for parental gene). Next, a four-ratio model was fitted to the data to allow differing rates of evolution for the branches that correspond to the three recurrent recruitments of ran retrogenes. This model shows accelerated evolution of ran-like in the D. melanogaster subgroup relative to all other branches in the tree when compared to the two-ratio model (P = 0). Other retrogene lineages are evolving much faster than the parental genes as well (9–10 times faster).
To determine the mode of evolution in the D. melanogaster subgroup immediately after duplication of ran, a five-ratio model was generated. Models that consider a different rate for the parental genes after duplication were significantly worse at fitting the data (data not shown), while models that consider a different rate for the retrogenes after duplication are significantly better than the previous four-ratio model (P = 1.8873 × 10−15). Our five-ratio model shows a marked increase in the KA/KS (0.7023) in ran-like of the D. melanogaster subgroup. Immediately after duplication of ran in the D. melanogaster subgroup, purifying selection (KA/KS = 0.0249) was acting on the newly retroposed gene, even though it was still evolving roughly six times faster than the parental gene. In fact, all ratios in the five-ratio model, including this last one, are significantly smaller than one (Table S4). Our conclusions (i.e., that retrogenes evolve much faster than parental genes) remained unchanged when we reestimated the ran KA/KS ratio using only the species of the D. melanogaster group, where estimates should have higher accuracy (i.e., KS should not be saturated). The ran KA/KS ratio had a value of 0.0065 in this calculation, confirming that retrogenes evolve one to two orders of magnitude faster than the parental gene (Table S4). Again, either relaxation of constraint or positive selection could explain the large increase in evolutionary rate among the ran-like lineages, although additional analyses (see below) reveal that selection is more likely.
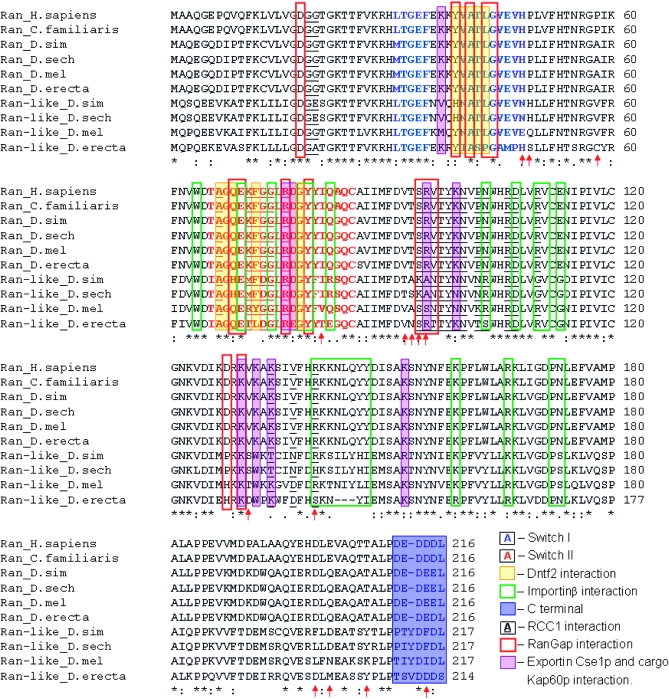
Site models (M7 vs. M8, REL, and FEL) were applied to test for positive selection acting on ran-like. The M8 site model allows for positively selected sites, while M7 does not. The likelihood ratio test between site models M7 and M8 was statistically significant (2Δl = 8.885, d.f. = 2; P = 0.0118), which is indicative of positive selection acting on ran-like because it reveals that model M8 fits the data significantly better than model M7. Site model M8 estimated that 36.4% of sites in the ran-like alignment experienced positive selection (KA/KS = 2.52). Codons that are most likely under positive selection as revealed by Bayes empirical Bayes analysis (posterior probabilities ≥0.95%) are shown in Table 2. REL analyses using the Hyphy package detected 14 sites (Table 2 and Figure 2) with a Bayes factor >50 (P < 0.02) that have likely been under positive selection. The more stringent FEL analyses detected only one codon (131) as being likely under positive selection (P = 0.0973).
TABLE 2.
Comparison of likely positively selected codons identified by site-specific model M8 BEB and REL analyses in ran-like
| Positively selected codons |
||
|---|---|---|
| Codon | M8 BEB probability | REL Bayes factor |
| 48 N | 0.911 | 572.1 |
| 49 H | 0.955 | 558.1 |
| 58 V | 0.810 | 52.2 |
| 81 I | 0.748 | 53.1 |
| 92 T | 0.785 | 54.2 |
| 93 A | 0.913 | 598.3 |
| 94 K | 0.825 | 53.1 |
| 95 A | 0.958 | 469.5 |
| 131 S | 0.939 | 583.9 |
| 140 R | 0.960 | 467.6 |
| 200 F | 0.985 | 3574.9 |
| 202 D | 0.888 | 443.9 |
| 207 Y | 0.842 | 55.3 |
| 215 F | 0.921 | 389.1 |
Codons that had Bayes empirical Bayes (BEB) posterior probabilities ≥0.95 or a Bayes factor ≥50 were included in the results. Boldface values represent sites identified with statistical significance by either method. Codon number and amino acids are relative to D. simulans full-length protein.
Figure 2.—
Alignment of ran and Drosophila ran-like proteins highlighting amino acid residues of known function. Arrows point to sites that have likely been under positive selection in the retrocopies (Table 2).
Polymorphism data in D. melanogaster, D. simulans, and D. yakuba for ran-like were obtained. Table S5 shows the polymorphism data for D. melanogaster and D. simulans. McDonald–Kreitman and modified McDonald–Kreitman tests were performed for ran-like using polymorphism data for D. simulans and D. melanogaster (Table 3). Both McDonald–Kreitman tests showed a significantly higher ratio of replacements per substitution, as opposed to replacements per silent polymorphism. These data support recurrent positive selection acting on ran-like in D. melanogaster and D. simulans. Lineage-specific McDonald–Kreitman tests performed for ran-like in D. melanogaster and ran-like in D. simulans were not significant (data not shown). Likewise, Tajima's D and Fay and Wu's H tests were not significant, indicating that the positive selection revealed above was not recent.
TABLE 3.
McDonald–Kreitman and modified McDonald–Kreitman tests for ran-like in the D. melanogaster and D. simulans lineages
| Fixed | Polymorphic | |
|---|---|---|
| Replacement | 47 | 21 |
| Synonymous | 12 (2) | 14 (5) |
G (with Williams correction) = 4.062, P = 0.0438. Values in parentheses correspond to the changes to preferred codons used for the modified McDonald–Kreitman test: G (with Williams correction) = 4.028, P = 0.0447.
D. yakuba polymorphism data were left out of the McDonald–Kreitman analysis because most ran-like alleles (9 of 10) were found to have deletions or insertions in the coding region (Figure S3). All of the deletions and insertions result in frameshifts and/or premature stop codons. The ran-like gene in D. yakuba is likely incorrectly annotated in FlyBase as two genes (one with an intron), but they are likely two disabled alleles (Figure S3). There are five kinds of disabling deletions and one kind of disabling insertion in our Taï ran-like data set. Some of the disablements overlap with the ones observed in the FlyBase sequences. These disablements are at frequencies that vary from 10 to 30% in our sample. This observation means that no single disablement has swept through the population under directional selection in agreement with the nonsignificant Tajima's D value (data not shown). We estimate the age of the alleles (Slatkin and Rannala 2000) to be less than ∼1.03 × 106 generations, assuming an effective population size of 106. If, in addition, we assume two generations per year in D. yakuba, this estimate leads to an age of 0.515 MY for the alleles present at 0.30 frequency. Since D. santomea and D. yakuba are estimated to have diverged ∼400,000 years ago (Llopart et al. 2002), we conclude that some of the disablements may perhaps be shared between these species.
Polymorphism data were also obtained in D. ananassae and D. atripex for Da_ran-like (Table S6). The McDonald–Kreitman test was performed and revealed a significantly higher ratio of replacements per silent substitution when compared to the ratio of replacements per silent polymorphism. Fisher's exact test was applied in this case because some cells have no observations (Table 1). This result is compatible with recurrent positive selection occurring in these lineages. However, Tajima's D and Fay and Wu's H tests were not significant for either sample set (data not shown). Taken together, the site-specific analyses and McDonald–Kreitman tests for ran-like in D. melanogaster and D. simulans and Da_ran-like in D. ananassae and D. atripex strongly support the action of positive selection on ran retrogenes. We discuss the possible functional consequences of these protein changes in File S2.
Are X-to-autosome retrogenes convergently duplicated because of their function in genomic conflicts in Drosophila?
A strong selective force, when acting across separate lineages, can often lead to convergent evolution. Utp14 in mammals is an example of convergent evolution that involves the recurrent emergence and recruitment of retrogenes (Bradley et al. 2004). In this case, male meiotic X inactivation was suggested as the selective force driving the convergent recruitment of Utp14 retrogenes.
In the present case, two interacting Drosophila nuclear transport genes, Dntf-2 and ran, gave rise to three retrogenes each, in overlapping lineages. All these retrogenes represent X-to-autosome duplications. We further show that most of the retroposed genes have a male-biased transcription and have evolved under recurrent positive selection. We reveal that strong selection is associated with the origin and evolution of the duplicates of Dntf-2 and ran. That said, ran-like appears to be pseudogenizing in D. yakuba. In further support to the X-to-autosome bias, we have identified another independent X-to-autosome retroposed copy of Ntf-2 in Anopheles gambiae (data not shown).
In the Introduction, we outlined several selective hypotheses that could explain the recurrent duplication of Ntf-2 and ran: male meiotic X inactivation, increasing level of expression of X-linked genes in male testes, sexual antagonism, and new function (e.g., segregation distortion or other). In particular, meiotic genomic conflicts, be they meiotic drive driven or parasite driven, are a powerful force in shaping genes and genomes as these conflicts lead to an arms race and rapid evolution (Burt and Trivers 2006). Genes (or their duplicates) involved in conflicts evolve under recurrent positive selection (Kingan et al. 2009) and may become fixed in the populations fairly quickly (Burt and Trivers 2006). These genes, particularly the duplicates, might be rendered of no use when the conflicts disappear or are dealt with in a different way. Indeed, examples exist of genes responding to strong selective pressures only to disappear after the pressures on them dissipate. Seminal fluid proteins have been observed to evolve under positive selection and have high turnover (Begun and Lindfors 2005). In fact, this type of evolutionary dynamic characterizes many male-biased genes (Zhang et al. 2007), possibly because male-biased genes are more likely to be engaged in male competition, sexual antagonism, segregation distortion, and/or parasite-related conflicts more often than non-male-biased genes. Very complex situations might emerge in meiotic drive systems as observed in the case of the Winters sex ratio genes in D. simulans (Kingan et al. 2009) where three genes of a gene family—one of them not even encoding for a protein anymore—are part of a meiotic drive system. Each of the Winters sex ratio genes are under positive selection in one population or another and are polymorphic for presence–absence of alleles.
In our case, if we hypothesize that nuclear transport is routinely involved in male germline-based genomic conflicts—segregation distortion or other—then this could explain recurrent duplication of Ntf-2 and ran as well as the fast evolution of the their duplicates. We postulate that the Ntf-2 and ran parental genes are (or have been) involved in male germline genomic conflicts. Different alleles of Ntf-2 and ran in the population could act either as drivers or as suppressors of the conflict system. Alleles that increase in frequency in males, but are detrimental to females, would often end up being present at intermediate frequencies (Rice 1984; Patten and Haig 2009). Such an antagonism could promote the emergence of new genes under the model proposed by Proulx and Phillips (2006). Duplicates of Ntf-2 and ran would then help alleviate the antagonism. At the same time, factors such as the male meiotic X inactivation, increasing level of expression of X-linked genes in male testes, and/or sexual antagonism may determine the location of the duplicated genes (e.g., X to autosome), as described in the Introduction. The partial loss of function that we infer (File S2) might have occurred in some lineages (e.g., ran-like in D. melanogaster subgroup species) would be explained by a segregation distortion or genomic conflict function followed by selective pressure to differentiate from the parental gene. The complete loss of ran-like in some lineages would occur if the conflicts become resolved through an alternative mechanism in that lineage (postrecruitment of ran-like) or vanish altogether. The retrogene would then become quickly pseudogenized or deleted (Petrov et al. 1996).
Finally, a knockout of Dntf-2r (D. melanogaster P-element insertion line EY05573) shows no obvious male fertility effects (M. Motiwale and E. Betrán, unpublished results), supporting a degree of dispensability of the gene. Experiments are being carried out to reveal the potential role of Dntf-2r and ran-like in segregation distortion in D. melanogaster using the SD system. Functions related to conflicts caused by transposable elements or viruses should also be tested.
Acknowledgments
We thank Elena de la Casa-Esperón, Daven Presgraves, Dave Begun, and several reviewers for comments on this work. We also thank Mao-Lien Wu, Chung-I Wu, Tessa Bauer DuMont, Chip Aquadro, Hongzheng Dai, Manyuan Long, Ana Llopart, and the Tucson and San Diego Drosophila Stock Centers for providing stocks. Research was supported by the University of Texas at Arlington startup funds and grant GM071813 from National Institutes of Health (to E.B.).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.113522/DC1.
References
- Akashi, H., 1995. Inferring weak selection from patterns of polymorphism and divergence at “silent” sites in Drosophila DNA. Genetics 139 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Y., C. Casola, C. Feschotte and E. Betrán, 2007. Comparative genomics reveals a constant rate of origination and convergent acquisition of functional retrogenes in Drosophila. Genome Biol. 8 R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begun, D. J., and H. A. Lindfors, 2005. Rapid evolution of genomic Acp complement in the melanogaster subgroup of Drosophila. Mol. Biol. Evol. 22 2010–2021. [DOI] [PubMed] [Google Scholar]
- Betrán, E., and M. Long, 2003. Dntf-2r: a young Drosophila retroposed gene with specific male expression under positive Darwinian selection. Genetics 164 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betrán, E., K. Thornton and M. Long, 2002. Retroposed new genes out of the X in Drosophila. Genome Res. 12 1854–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, J., A. Baltus, H. Skaletsky, M. Royce-Tolland, K. Dewar et al., 2004. An X-to-autosome retrogene is required for spermatogenesis in mice. Nat. Genet. 36 872–876. [DOI] [PubMed] [Google Scholar]
- Brosius, J., 1991. Retroposons–seeds of evolution. Science 251 753. [DOI] [PubMed] [Google Scholar]
- Burt, A., and R. Trivers, 2006. Genes in Conflict. The Biology of Selfish Genetic Elements. Harvard University Press, Cambridge, MA.
- Charlesworth, B., J. A. Coyne and N. H. Barton, 1987. The relative rates of evolution of sex chromosomes and autosomes. Am. Nat. 130 113–146. [Google Scholar]
- Chintapalli, V. R., J. Wang and J. A. Dow, 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39 715–720. [DOI] [PubMed] [Google Scholar]
- Chippindale, A. K., J. R. Gibson and W. R. Rice, 2001. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl. Acad. Sci. USA 98 1671–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, A. G., M. B. Eisen, D. R. Smith, C. M. Bergman, B. Oliver et al., 2007. Evolution of genes and genomes on the Drosophila phylogeny. Nature 450 203–218. [DOI] [PubMed] [Google Scholar]
- Emerson, J. J., H. Kaessmann, E. Betrán and M. Long, 2004. Extensive gene traffic on the mammalian X Chromosome. Science 303 537–540. [DOI] [PubMed] [Google Scholar]
- Fay, J. C., and C. I. Wu, 2000. Hitchhiking under positive Darwinian selection. Genetics 155 1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hense, W., J. F. Baines and J. Parsch, 2007. X chromosome inactivation during Drosophila spermatogenesis. PLoS Biol. 5 e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocher, H., C. T. Ting, M. L. Wu and C. I. Wu, 1997. Incipient speciation by sexual isolation in Drosophila melanogaster: variation in mating preference and correlation between sexes. Evolution 51 1175–1181. [DOI] [PubMed] [Google Scholar]
- Kingan, S. B., D. Garrigan and D. L. Hartl, 2009. Recurrent selection on the Winters sex-ratio genes in Drosophila simulans. Genetics 184 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosakovsky Pond, S. L., and S. D. Frost, 2005. Not so different after all: a comparison of methods for detecting amino acid sites under selection. Mol. Biol. Evol. 22 1208–1222. [DOI] [PubMed] [Google Scholar]
- Kusano, A., C. Staber and B. Ganetzky, 2002. Segregation distortion induced by wild-type RanGAP in Drosophila. Proc. Natl. Acad. Sci. USA 99 6866–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano, A., C. Staber, H. Y. Chan and B. Ganetzky, 2003. Closing the (Ran)GAP on segregation distortion in Drosophila. Bioessays 25 108–115. [DOI] [PubMed] [Google Scholar]
- Larracuente, A. M., T. B. Sackton, A. J. Greenberg, A. Wong, N. D. Singh et al., 2008. Evolution of protein-coding genes in Drosophila. Trends Genet. 24 114–123. [DOI] [PubMed] [Google Scholar]
- Librado, P., and J. Rozas, 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25 1451–1452. [DOI] [PubMed] [Google Scholar]
- Lifschytz, E., and D. L. Lindsley, 1972. The role of X-chromosome inactivation during spermatogenesis. Proc. Natl. Acad. Sci. USA 69 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopart, A., S. Elwyn, D. Lachaise and J. A. Coyne, 2002. Genetics of a difference in pigmentation between Drosophila yakuba and Drosophila santomea. Evolution 56 2262–2277. [DOI] [PubMed] [Google Scholar]
- McDonald, J. H., and M. Kreitman, 1991. Adaptive protein evolution at the Adh locus in Drosophila. Nature 351 652–654. [DOI] [PubMed] [Google Scholar]
- Nielsen, R., and Z. Yang, 1998. Likelihood models for detecting positively selected amino acid sites and applications to the HIV-1 envelope gene. Genetics 148 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten, M. M., and D. Haig, 2009. Maintenance or loss of genetic variation under sexual and parental antagonism at a sex-linked locus. Evolution 63 2888–2895. [DOI] [PubMed] [Google Scholar]
- Petrov, D. A., E. R. Lozovskaya and D. L. Hartl, 1996. High intrinsic rate of DNA loss in Drosophila. Nature 384 346–349. [DOI] [PubMed] [Google Scholar]
- Pond, S. L., and S. D. Frost, 2005. Datamonkey: rapid detection of selective pressure on individual sites of codon alignments. Bioinformatics 21 2531–2533. [DOI] [PubMed] [Google Scholar]
- Powell, J. R., 1997. Progress and Prospects in Evolutionary Biology: The Drosophila Model. Oxford University Press, New York.
- Presgraves, D. C., 2007. Does genetic conflict drive rapid molecular evolution of nuclear transport genes in Drosophila? Bioessays 29 386–391. [DOI] [PubMed] [Google Scholar]
- Presgraves, D. C., and W. Stephan, 2007. Pervasive adaptive evolution among interactors of the Drosophila hybrid inviability gene, Nup96. Mol. Biol. Evol. 24 306–314. [DOI] [PubMed] [Google Scholar]
- Proulx, S. R., and P. C. Phillips, 2006. Allelic divergence precedes and promotes gene duplication. Evolution 60 881–892. [PubMed] [Google Scholar]
- Quimby, B. B., T. Lamitina, S. W. L'Hernault and A. H. Corbett, 2000. The mechanism of ran import into the nucleus by nuclear transport factor 2. J. Biol. Chem. 275 28575–28582. [DOI] [PubMed] [Google Scholar]
- Ranz, J. M., C. I. Castillo-Davis, C. D. Meiklejohn and D. L. Hartl, 2003. Sex-dependent gene expression and evolution of the Drosophila transcriptome. Science 300 1742–1745. [DOI] [PubMed] [Google Scholar]
- Ribbeck, K., G. Lipowsky, H. M. Kent, M. Stewart and D. Gorlich, 1998. NTF2 mediates nuclear import of Ran. EMBO J. 17 6587–6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, W. R., 1984. Sex chromosomes and the evolution of sexual dimorphism. Evolution 38 735–742. [DOI] [PubMed] [Google Scholar]
- Schlenke, T. A., and D. J. Begun, 2003. Natural selection drives Drosophila immune system evolution. Genetics 164 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen, T. D., and K. J. Livak, 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3 1101–1108. [DOI] [PubMed] [Google Scholar]
- Slatkin, M., and B. Rannala, 2000. Estimating allele age. Annu. Rev. Genomics Hum. Genet. 1 225–249. [DOI] [PubMed] [Google Scholar]
- Tajima, F., 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J. D., D. G. Higgins and T. J. Gibson, 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, J. M., 2007. Meiotic sex chromosome inactivation. Development 134 1823–1831. [DOI] [PubMed] [Google Scholar]
- Turner, J. M., S. K. Mahadevaiah, O. Fernandez-Capetillo, A. Nussenzweig, X. Xu et al., 2005. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat. Genet. 37 41–47. [DOI] [PubMed] [Google Scholar]
- Vicoso, B., and B. Charlesworth, 2009. The deficit of male-biased genes on the D. melanogaster X chromosome is expression-dependent: A consequence of dosage compensation? J. Mol. Evol. 68 576–583. [DOI] [PubMed] [Google Scholar]
- Watterson, G. A., 1975. On the number of segragating sites in genetical models without recombination. Theor. Popul. Biol. 7 256–276. [DOI] [PubMed] [Google Scholar]
- Yang, Z., 1998. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol. Biol. Evol. 15 568–573. [DOI] [PubMed] [Google Scholar]
- Yang, Z., 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24 1586–1591. [DOI] [PubMed] [Google Scholar]
- Yang, Z., R. Nielsen, N. Goldman and A. M. Pedersen, 2000. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics 155 431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., D. Sturgill, M. Parisi, S. Kumar and B. Oliver, 2007. Constraint and turnover in sex-biased gene expression in the genus Drosophila. Nature 450 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]