Abstract
The polyvagal theory states that social behavior is linked to cardiac vagal control. This theory has been tested widely in infants and children, but less so in adults. Thus, we examined if resting or stress-related changes in high-frequency heart rate variability (HF-HRV; a presumed index of vagal control) varied with social functioning in 50 healthy women (mean age 68 years). After completing assessments of social functioning, women were exposed to laboratory stressors with concurrent psychophysiological monitoring. Although stressor-induced suppression of HF-HRV was common, women with less stressor-induced suppression of HF-HRV reported more positive social functioning. Resting HF-HRV was not related to social functioning. These findings are at apparent odds with the polyvagal theory; however, they complement prior work suggesting that emotional self-regulation could plausibly modulate cardiac vagal control in association with social functioning.
Descriptors: Individual differences, Social factors, Blood pressure, Heart rate variability
Respiratory-related cardiac vagal control has been linked to the regulation of attention, emotion, and communication within the conceptual framework of Porges’ polyvagal theory (Porges, 2003; Porges, Doussard-Roosevelt, & Maiti, 1994). According to this theory, respiratory sinus arrhythmia (RSA), also called high-frequency heart rate variability (HF-HRV), is primarily controlled by tonic and phasic vagal cardiac influences and is thought to be involved in modulating how individuals interact with their environment (Eckberg, 2000; Gautier et al., 2007; Porges, 2001; Stein & Kleiger, 1999; Zara & Lombardi, 2001). In particular, the polyvagal theory posits that early development of adaptive social behavior is linked to the control of tonic and phasic vagal activity (Porges, 2007b; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996).
Potential Mechanisms Linking HRV and Social Functioning
During rest, the vagus nerve exerts high levels of regulatory cardiac control. By contrast, during stress, the vagus reduces its cardiac control, facilitating increases in cardiac output necessary for metabolic mobilization (Porges et al., 1996). The homeostatic vagal control over resting cardiac activity has been termed the “vagal brake” (Porges, 2007b). Cortical and subcortical brain regions are thought to modulate autonomic control and cognitive and emotional processes such that changes in cardiac vagal control in particular are concomitant with central processing and motivated behavior (Gianaros, Van Der Veen, & Jennings, 2004; Jennings & van der Molen, 2002; Thayer & Brosschot, 2005). The polyvagal theory (Porges, 1995) posits that vagal control and social behavior are anatomically linked via the vagus nerve through a so-called Social Engagement System. This refers to a network of brain stem nuclei that regulate the myelinated vagus and presumably underlie adaptive control over facial expressions, auditory reception, and vocalization. The system is seen as active during resting states, concomitant with the vagal brake, and is thought to promote social behavior (Porges, 2001). During stress, when the vagal brake is typically withdrawn, there is a presumptive increase in cardiac output and concurrent downregulation of the Social Engagement System (Porges, 2001, 2003, 2007a). Thus, individuals who show decreased cardiac vagal control at times of stress and increased vagal control at times of rest should report better social functioning owing to the efficient disengagement and engagement of the vagal brake supporting emergent adaptive social behavior (Porges, 2003). However, the specific anatomical features of this pathway are currently debated (Grossman & Taylor, 2007).
Cardiac Vagal Control and Social Functioning in Children
The polyvagal theory has been tested chiefly in infants and children. Resting cardiac vagal control, measured by RSA, in infancy and childhood has been associated with better social skills and affective regulation, a possible determinant of social behavior (Doussard-Roosevelt, Porges, Scanlon, Alemi, & Scanlon, 1997; Fox & Field, 1989; Richards & Cameron, 1989; Stifter, Fox, & Porges, 1989). Some studies report that the level of suppression of RSA during stressful laboratory challenges may also be linked to positive social behavior. Specifically, infants and children who demonstrate greater RSA suppression during experimental challenges exhibit fewer socially mediated behavioral problems, higher peer status, higher levels of sociability and social skills, and better temperament (Calkins & Keane, 2004; Doussard-Roosevelt, Montgomery, & Porges, 2003; Graziano, Keane, & Calkins, 2007; Porges et al., 1996; Richards & Cameron, 1989). This literature is not definitive, however. Other reports show that less suppression of and even increases in RSA during laboratory challenges vary with lower rates of child and parent reports of family conflict (Salomon, 2005; Salomon, Matthews, & Allen, 2000). There are possible explanations for these discrepant results. First, the various experimental tasks used in research protocols may evoke different levels of social or attentive vigilance or differentially alter affective state in participants, potentially impacting cardiac vagal control. Second, studies reporting an association between cardiac vagal suppression and positive social functioning used measures that focused on the child’s behavior as reported by parents and teachers. By contrast, studies reporting an association between less cardiac vagal suppression and positive social function used measures that focused on perceived family conflict, not the child’s actual behavior. Thus, differences in both tasks and social measures may account for the heterogeneous results.
Cardiac Vagal Control and Social Functioning in Adults
Few studies have tested predictions from the polyvagal theory in adults. Regarding resting HF-HRV, some reports suggest an association between anxiety and depressive disorders and decreased resting HF-HRV (Beauchaine, Gatzke-Kopp, & Mead, 2007; Carney et al., 2000; Chambers & Allen, 2002; Friedman, 2007; Friedman & Thayer, 1998; Rottenberg, 2007). Also, higher levels of resting HF-HRV have been associated with better coping and less negative emotional reactivity in response to environmental stressors (Brosschot & Thayer, 1998; Fabes & Eisenberg, 1997). Moreover, Horsten and colleagues (1999) found that social isolation and low social support were associated with lower HF-HRV. Together, these studies modestly support an association between higher resting cardiac vagal control and more positive affective regulation and social engagement.
Regarding stressful laboratory task-related changes in HF-HRV, some studies suggest an association between decreased suppression of cardiac vagal control and psychopathology. Specifically, social anxiety and depression have been linked to decreased HF-HRV suppression (Friedman & Thayer, 1998; Movius & Allen, 2005; Rottenberg, Clift, Bolden, & Salomon, 2007). No studies to our knowledge have examined social factors in conjunction with stressful laboratory challenges.
The Current Study
There is limited research in older adults on social functioning and indicators of cardiac vagal control. This may be particularly significant because cardiac vagal control decreases with age (De Meersman & Stein, 2007; Jennings et al., 1997; Shahar et al., 2007). Therefore, we examined the relationships between social functioning and HF-HRV in a sample of healthy, postmenopausal women. Women completed measures of social support, negative interactions, and marital adjustment as well as a week-long daily diary prior to participating in a psychophysiological study involving two stressful laboratory challenges. We hypothesized that women with higher resting HF-HRV would report more positive social functioning. Because our participants were not depressed or anxious and our experimental tasks and outcome measures were most similar to those used in the portion of the child literature that focused on individual social behavior, we hypothesized that suppression of HF-HRV during cognitive challenges would be associated with positive social functioning. Depression, hostility, and expressed anger were controlled for to ensure that results were due to social functioning rather than negative affect or personality factors.
Method
Participants
Participants were 50 healthy postmenopausal women recruited as a subsample from the Pittsburgh Healthy Women Study (Matthews et al., 1989). Inclusion criteria stipulated that participants did not have a histories of (a) cardiovascular or cerebrovascular disease (hypertension, coronary artery disease, angina, transient ischemic attacks, blood clotting, myocardial infarction, and congestive heart failure), (b) stroke or cerebrovascular incident involving loss of consciousness, (c) Type I or II diabetes, (d) cancer, and (e) psychiatric or neurological disorders (including dementia or suspected Alzheimer’s disease) or had ever taken psychotropic medications. Women were excluded if they had been hospitalized or had major surgery in the past 3 years. Demographic characteristics of the sample can be found in Table 1. The University of Pittsburgh Institutional Review Board approved all procedures and all women provided informed consent. Prior to the psychological stress protocol, participants refrained from caffeine, tobacco products, and exercise for 3 h and from drinking alcohol for 12 h. They also underwent an ancillary neuroimaging protocol described elsewhere (Gianaros, Jennings, Sheu, Derbyshire, & Matthews, 2007; Gianaros, Jennings, Sheu, Greer, et al., 2007).
Table 1.
Participant Demographics
| Characteristic | Mean | SD |
|---|---|---|
| Age (years) | 67.98 | 1.38 |
| Number of years of school completed | 15.43 | 1.59 |
| Number of alcoholic beverages consumed/week |
2.37 | 3.69 |
| Number of days smoked at least one cigarette |
0.28 | 1.39 |
| Height (in.) | 63.38 | 3.02 |
| Weight (lbs.) | 154.34 | 22.09 |
| Body mass index (kg/m2) | 27.09 | 4.17 |
| Basal SDNN | 34.98 | 35.6 |
| Basal MSD | 26.99 | 50.7 |
| Basal total power (ms2) | 76,282.74 | 168,758.5 |
| Basal HF-HRV (ln ms2) | 4.42 | 1.3 |
Note: SDNN: standard deviation of normal-to-normal interbeat intervals; MSD: mean square successive differences; HF-HRV: high-frequency heart rate variability. The natural log of basal HF-HRV is reported; the original mean is 83.10.
Materials
Assessment of social functioning
Four measures assessed social functioning: the Interpersonal Support Evaluation List (ISEL), the Marital-Adjustment Test (MRS), the Social Rhythm Metric (SRM), and the Negative Interactions Scale. The ISEL assesses an individual’s perceived availability of four types of social support: appraisal (ISELAP), belonging (ISELBL), tangible (ISELTN), and self-esteem (Cohen, Mermelstein, Kamarck, & Hoberman, 1985). Appraisal refers to the perceived availability of someone to talk to about one’s problems, belonging refers to the perceived availability of people with whom one can interact, and tangible refers to the perceived availability of material aid (Cohen & Hoberman, 1983). We used an abbreviated, 12-item version of the ISEL that excluded the self-esteem subscale and included only the four highest-loading items for each of the other subscales. Participants rated the degree to which they agreed with self-descriptive statements on a 4-point scale. This scale is both reliable (α = .88–.90 in the general population) and valid, as it correlates positively with similar measures of social support including the Inventory of Socially Supportive Behaviors (Barrera, Sandler, & Ramsay, 1981).
Marital adjustment was assessed by the MRS (Locke & Wallace, 1959). Participants who were married or in a long-term committed relationship responded to 16 questions about their general level of happiness in the relationship, their perceived level of agreement with their significant other on a number of issues, their methods of handling disagreement, and regrets they may have about their current relationship. The reliability (α = .90) and validity of this measure have been established (Locke & Wallace, 1959). The MRS distribution in our sample was bimodal. Accordingly, we reclassified the continuous scale into septiles. The original and transformed variables remained highly correlated (r = .97, p<.05).
The SRM is a measure that quantifies an individual’s daily activities, indicating the degree of regularity in their cognitive and social structuring of the day and biological circadian rhythms (Monk, Flaherty, Frank, Hoskinson, & Kupfer, 1990; Monk, Kupfer, Frank, & Ritenour, 1990). Establishing a schedule of activities, particularly those of a potentially social nature, is important in the treatment of psychopathology; it serves as an indicator of how lifestyle regularity facilitates social engagement. Thus, the SRM may be a marker of psychological well-being and capacity for effective social interaction. Test–retest administrations show the SRM to be reliable (rho = .44, p<.001). The current study used the 5-item version of the SRM, an abbreviated measure that is highly correlated with the full-length 17-item version (r = .80, p<.05) (Monk, Frank, Potts, & Kupfer, 2002). Participants’ SRMs were calculated using information provided in their week-long daily diaries. The time at which an individual began each of five activities, starting housework or work at her place of employment, exercising, eating dinner, and the time at which she last drank an alcoholic or caffeinated beverage every day was recorded. These start times were used to calculate a habitual time for beginning that activity. The SRM calculates the number of times that an individual’s activity occurs within 45 min of their habitual time and scores this relative to the total number of social activities in that week.
Negative interactions were assessed using a five-item questionnaire asking participants to indicate how often others have been critical of or have made demands upon them in the past month. This measure combines Krause’s four-item negative interaction scale for older adults with one item from a similar measure developed by Schuster and colleagues (Krause, 1995; Schuster, Kessler, & Aseltine, 1990). Participants responded to items using a 4-point scale ranging from never to very often. This measure is reliable (α = .87 in a sample of older adults). One woman was excluded from all analyses due to reporting a score 4 standard deviations above the mean on the Negative Interactions Scale.
Pairwise correlations between the social variables showed that the ISEL total score (ISEL-TOT) was significantly correlated with the MRS (.67, p<.01), SRM (.39, p<.01), and Negative Interactions Scale (−.43, p<.01). The Negative Interactions Scale was also correlated with the SRM (−.32, p<.05) and the MRS (−.48, p<.01). A principal components factor analysis revealed a single factor, referred to here as the social functioning factor. When examining the factor loadings, the SRM (.49) contributed slightly less variance to the factor than the MRS (.81), ISEL-TOT (.83), or Negative Interactions Scale (−.60). These four measures share approximately 49% variance (Eigen-value = 1.95).
Given the positive and negative loadings, the factor can be described as bipolar, where the capacity for adaptive social functioning, as captured by the SRM, MRS, and ISEL-TOT, extends to the absence of such facility, that is, maladaptive social interaction. These measures are based on an individual’s perception of how she interacts with others and, to some extent, how satisfying those relations are. As such, it is likely that the factor represents the degree to which an individual adaptively maximizes her social interactions in order to obtain some level of pleasure. Given that the ISEL, Negative Interactions Scale, and MRS all assess an individual’s perceptions of the quality of her relations with others and the SRM examines an individual’s psychological well-being and capacity for interaction as a product of lifestyle regularity, it is possible that the common core element tapped by this composite can be construed as “ease of social engagement.”
Following the factor analysis, we created unit-weighted factor scores by converting all of the component variables’ scores to z scores and averaging them (McDonald, 1985). Fifteen women who were not married or in a monogamous relationship at the time of data collection were unable to complete the MRS. Thus, the sample size for the factor including the MRS was truncated. To verify that the factor was still coherent given the larger sample including these women, a principal components factor analysis using only the SRM, ISEL-TOT, and Negative Interactions Scale was conducted. All analyses were conducted using both factors, yielding similar results. Thus, this report focuses on the factor that incorporated the MRS.
Laboratory challenges
The Stroop color-word interference task and a mental arithmetic task were administered. Participants rested quietly for an 8-min baseline period prior to completing two repetitions each of the Stroop color-word interference task and the mental arithmetic task in a counterbalanced order. The Stroop task included conditions with congruent and incongruent trials, each lasting 2 min. The higher-demand incongruent condition was performance titrated such that the interword interval decreased when participants achieved scores over 65% correct (Kamarck et al., 1992; Kamarck, Jennings, Pogue-Geile, & Manuck, 1994). For each trial, participants used a four-button response device to select the color of the target word to its identifier word. During incongruent trials, the target word was presented in a color that did not match the color of the word named. All identifier words were also presented in colors that were incongruent to the colors they named. The target word and identifier words were presented in a congruent manner during congruent trials.
The mental arithmetic task contained a lower (single digit) and higher-demand condition (two or three digits; Kamarck et al., 1992, 1994). Participants solved addition and subtraction problems by selecting one of two solutions. There were an equivalent number of addition and subtraction problems. The more difficult condition was performance titrated such that more difficult problems were presented when participants achieved scores over 65% correct.
For both challenges, task accuracy was calculated as the percentage of total trials that were correctly completed. Following completion of the tasks, participants were asked to rest quietly for 8 min until the experimenter returned to the room; this defined a recovery period. Participants also rated their level of stress and how demanding they perceived the tasks on scales ranging from 0 (not at all) to 4 (extremely) upon completion.
Measurement of blood pressure
Blood pressure was measured during the baseline, task, and recovery periods from the brachial artery of the left arm (not the arm used for task response). Participants were seated upright with their feet flat on the floor. Blood pressure was measured with an automated device (auscultatory-Korotkoff method: IBS Model SD-700A; IBS Corp., Waltham, MA). Blood pressure was measured once every 2 min during the 8-min baseline and recovery periods and once during each of the two repetitions of the Stroop and mental arithmetic tasks. To compute blood pressure during baseline and recovery, the four measurements from the baseline period were averaged. To calculate task-induced blood pressure, the two measurements from the incongruent Stroop condition were averaged and the two measurements from the more challenging mental arithmetic task were averaged.
Measurement of HF-HRV
Cardiac vagal activity was estimated by HF-HRV obtained via electrocardiogram (ECG). ECG measurements were collected during the resting baseline, Stroop and mental arithmetic tasks, and recovery. A modified lead II electrode placement using three silver-silver chloride electrodes (Conmed; Andover Medical, Haverhill, MA) was used. Whereas ECG monitoring was continuous during the tasks, ECG signals were recorded only during the last 6 min of the initial 10-min baseline period, allowing for adaptation. The ECG signal was digitized (12 bit), sampled (at 1000 Hz), and stored for off-line processing using LabView acquisition software (National Instruments Corp., Austin, TX).
Prior to HF-HRV calculation, R wave markers in the ECG signal were assessed for artifacts by visual inspection and by an automatic artifact detection algorithm available in a customized software package (Mindware Heart Rate Variability Scoring Module, version 2.16; Mindware Technologies Ltd., Columbus, OH). Following manual corrections of suspected artifacts, minute-by-minute estimates of heart rate and HF-HRV were established as directed by current guidelines and detailed elsewhere (Gianaros et al., 2005). First, for each minute of the baseline, Stroop task, mental arithmetic task, and recovery a 60-s time series of interbeat intervals (IBIs; the time in milliseconds between sequential ECG R spikes) was created using an interpolation algorithm with a 250-ms sampling time. The time series was then linearly detrended, mean centered, and tapered using a Hamming window. Spectral power values were determined (in ms2/Hz) with fast Fourier transformations, and the power values in the 0.15- to 0.40-Hz spectral bandwidth were integrated (ms2). Prior to statistical analyses, a natural log (ln) transformation was applied to the spectral power values, correcting for distributional violations. Resulting minute-by-minute estimates of heart rate and HF-HRV were normalized and averaged for the baseline period, for each of the tasks, and for the recovery period. Baseline measures of the standard deviation of normal-to-normal interbeat intervals (SDNN), mean square successive differences (MSD), total spectral power, and HF-HRV are reported in Table 1. The mean resting HF-HRV measure obtained for this sample (83.10 ms2) is comparable to that reported for a similar sample of older individuals (Zhang, 2007). For each woman, the average baseline value of HF-HRV was subtracted from its corresponding Stroop and mental arithmetic task values (more demanding task epochs), yielding a task-minus-baseline change score for each. These change scores were later averaged to create a single change score from baseline to task.
Assessment of covariates
In addition to the variables of interest, we included demographic and health-related variables in the analyses as covariates. These variables were educational attainment (highest level of education completed) and body mass index (BMI; weight in kg/height in meters2). We also examined age, smoking status (current, former, never), and the number of alcoholic beverages consumed per week as potential confounds. However, as seen in Table 1, the ranges of these variables were limited and none were significantly correlated with any of the variables of interest. Thus, these variables were not included in the final analyses.
Statistical Analyses
Following study hypotheses, we first tested whether baseline HF-HRV and the average change in HF-HRV from baseline to the two tasks was associated with the social functioning factor. A three-step hierarchical regression analysis was conducted for this purpose. Regression models were subjected to functional form and residual analyses, ensuring that function form was linear, heteroscedasticity was not present, residuals were normally distributed, and outliers were not present. At Step 1, BMI and educational attainment were entered as covariates. At Step 2, baseline HF-HRVwas entered both to assess its own relationship with the dependent variable after the effects of BMI and education were parsed out and to control for this variable in the following step. At Step 3, the average change in HF-HRV from baseline to task was entered. The unique percentage of variance in the social functioning factor explained by baseline HF-HRV and the average change in HF-HRVwas evaluated by the Δ R2 at Steps 2 and 3.
Results
Efficacy of Cognitive Challenge Manipulations
Both the Stroop and mental arithmetic challenges elicited cardiovascular and behavioral responses indicating effective stress induction. On self-report measures, participants indicated that the incongruent condition of the Stroop task was more stressful, t = 15.52, df = 47, p<.01, and demanding, t = 14.72, df = 47, p<.01, than the congruent condition. Similar results were found between the more difficult and less challenging conditions of the mental arithmetic challenge for stress, t = 8.77, df = 47, p<.01, and demand, t = 9.71, df = 47, p<.01, ratings. Also, participants’ systolic blood pressure significantly increased from baseline during the incongruent condition of the Stroop task and the more difficult condition of the mental arithmetic task and their systolic blood pressure measured during the recovery period was significantly decreased from baseline, F(1, 42) = 80.55, p<.01 (see Table 2). Measures of heart rate showed a similar pattern, F(1,43) = 36.15, p<.01 (see Table 2)
Table 2.
Systolic Blood Pressure and Heart Rate Changes throughout the Experiment
| Systolic blood pressure |
Heart rate |
|||
|---|---|---|---|---|
| Variable | Mean | Standard error | Mean | Standard error |
| Baseline | 125.67 | 1.85 | 69.08 | 1.21 |
| Stroop | 140.24 | 2.44 | 72.89 | 1.22 |
| Mental arithmetic | 135.72 | 1.99 | 71.86 | 1.15 |
| Recovery | 118.01 | 2.25 | 67.94 | 1.13 |
Note: Comparisons between baseline and Stroop, mental arithmetic, and recovery systolic blood pressures were significant (p<.01); comparisons between baseline, Stroop, mental arithmetic, and recovery heart rates were significant (p<.01).
As expected, participants’ HF-HRV decreased while they were performing the tasks, indicating cardiac vagal suppression (M = −.021, SD = 0.47, SE = 0.07, t = −2.99, p<.01). Moreover, baseline and task-related changes in HF-HRV were significantly correlated (r = −.45, p<.01) as were baseline HF-HRV and actual levels of HF-HRV recorded during the tasks (averaged across the tasks), (r = .93, p< .01). This suggests that women with higher baseline HF-HRV showed less change in HF-HRV during the tasks. However, baseline HF-HRV and recovery HF-HRV were also significantly correlated with task-related changes in systolic blood pressure during the incongruent condition of the Stroop task and more demanding condition of the mental arithmetic task; all correlations indicated comparable associations (r = ~ .40, p<.01).
HF-HRV and Social Functioning
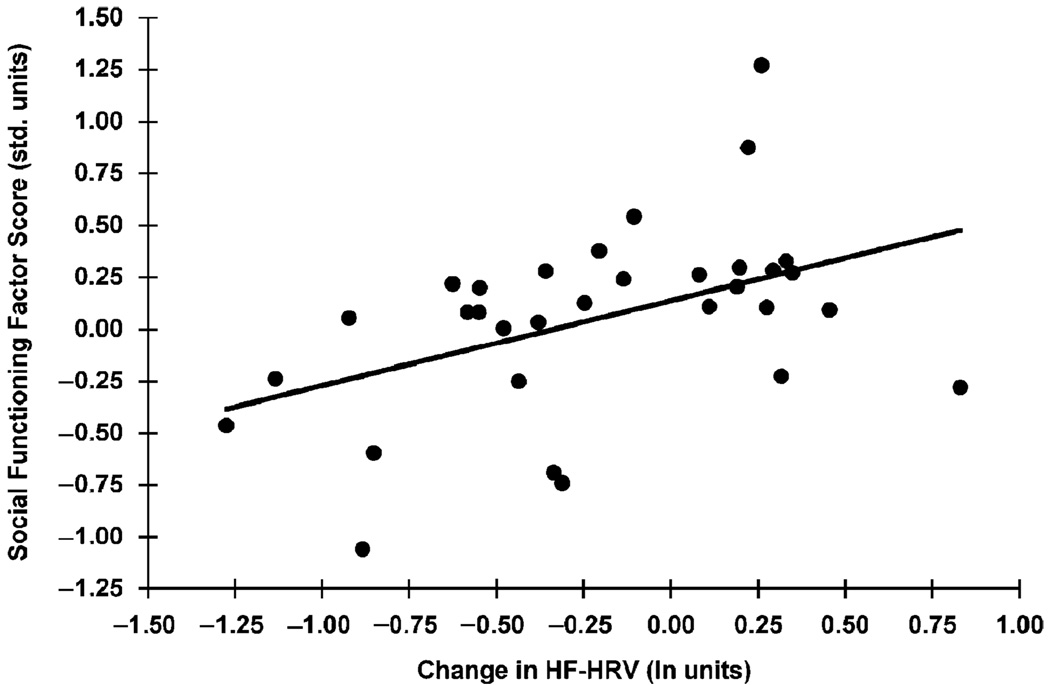
Correlational analyses showed that baseline HF-HRV was not significantly correlated with the social functioning factor; however, the average change in HF-HRV from baseline to the Stroop and mental arithmetic tasks was (r = .47, p<.05; see Figure 1). A regression analysis was used to examine this relationship. As seen in Table 3, in a three-step hierarchical regression, baseline HF-HRV did not predict the social functioning factor when BMI and educational attainment were entered in Step 1. However, the smaller average decline in HF-HRV from baseline to task did predict higher social functioning factor scores above and beyond BMI, educational attainment, and baseline HF-HRV. The average change in HF-HRV from baseline to task accounted for 25% of the variance in the social functioning factor. Upon examination of the component measures of the social functioning factor (the SRM, MRS, ISEL-TOT, and Negative Interactions Scale), we found that the average change in HF-HRV systematically accounted for more of the variance in the composite social functioning factor than any of the individual component measures (data available from the authors upon request).
Figure 1.
Correlation between the task-related change in high-frequency heart rate variability and the social functioning factor; r = .47, p<.05.
Table 3.
Summary of Hierarchical Regression Analysis for HF-HRVand the Social Functioning Factor
| Variable | Beta | t | SE | ΔR2 |
|---|---|---|---|---|
| Step 1 | ||||
| BMI | −0.01 | −0.52 | 0.02 | |
| Education | 0.08 | 1.69 | 0.05 | |
| Step 2 | ||||
| Baseline HF-HRV | −0.01 | −0.21 | 0.06 | 0.03 |
| Step 3 | ||||
| Average Δ in HF-HRV | 0.50 | 3.16 | 0.16 | 0.25* |
Note: HF-HRV:High-frequency heart rate variability; BMI: body mass index.
p<.05.
To ensure that our results reflected social functioning and not dispositional traits, we also conducted analyses controlling for measures of affect and personality that are often independently related to social functioning and cardiac autonomic control, including the Center for Epidemiologic Studies Depression Scale, the Cook–Medley Hostility Scale, and Spielberger’s Anger Expression Scale (Cook & Medley, 1954; Houston, Smith, & Cates, 1989; Krause, 2005; Krause & Rook, 2003; Radloff, 1977; Smith & Houston, 1987; Smith, Ruiz, & Uchino, 2004; Spielberger et al., 1985; Suls & Bunde, 2005). None of these analyses revealed significant findings.
Exploratory analyses of the association between levels of HF-HRV during recovery and social functioning were also completed. In a two-step hierarchical regression analysis controlling for BMI, educational attainment, and the average change in HF-HRV from baseline to task, recovery levels of HF-HRV were not associated with the composite social functioning factor. Analyses using the individual component measures provided similar results. Baseline HF-HRV was not controlled for in these analyses, as it was highly correlated with the recovery measure (r = .93, p<.01).
To determine whether the cognitive challenge manipulations were comparably effective for participants whose HF-HRV increased from baseline to the tasks and those who showed decreases, correlations between the average change in participants’ HF-HRV from baseline to task conditions and ratings of the stressfulness and demand of the harder and easier versions of the Stroop and mental arithmetic tasks were examined. None of these correlations, nor correlations between blood pressure change and HF-HRVchange, were significant.
Discussion
A smaller decrease in HF-HRV from a resting baseline to conditions of stressful psychological challenge predicted better social functioning, as measured by a composite social functioning factor. This finding would suggest that maintaining greater cardiac vagal control during psychological stress is related to more positive social functioning. For the most part, participants’ HF-HRV decreased from baseline while they were performing the Stroop and mental arithmetic stressor tasks, presumptively indicating cardiac vagal suppression. No statistically significant relationships between social functioning and baseline HF-HRV or recovery HF-HRV were found.
In aggregate, the present findings are consistent with prior studies demonstrating that cardiac vagal suppression in response to laboratory challenges or stressors is typical for most individuals, as hypothesized, but that some individuals exhibit a stress-related increase in cardiac vagal control (Berntson et al., 1994; Gianaros, Quigley, Mordkoff, & Stern, 2001; Salomon, 2005; Salomon et al., 2000). This finding, although at apparent odds with predictions derived from the polyvagal theory, may be understood from prior work on emotion regulation and social support.
Studies have shown associations between increases in cardiac vagal control during laboratory challenges and positive mood, social engagement, and active emotion regulation (Bazhenova, Plonskaia, & Porges, 2001; Butler, Wilhelm, & Gross, 2006; Hansen, Johnsen, & Thayer, 2003; Ingjaldsson, Laberg, & Thayer, 2003; Thayer, Friedman, & Borkovec, 1996). Thus, changes in cardiac vagal control during laboratory challenges may correspond to individual differences in emotion regulation processes. Although we make this interpretation cautiously because some studies have not replicated the association between cardiac vagal control and emotion regulation (Demaree, Schmeichel, & Robinson, 2004; Demaree et al., 2006), it is plausible that women in our study who demonstrated relatively greater task-related HF-HRVthat was predictive of better social functioning approached the laboratory challenges in a more emotionally positive manner.
Moreover, reports suggest that immediate and ongoing social support buffers cardiovascular (e.g., blood pressure and heart rate) reactivity in response to laboratory challenges (Lepore, Allen, & Evans, 1993; Nausheen, Gidron, Gregg, Tissarchodou, & Peveler, 2007; Uchino & Garvey, 1997). Similarly, Salomon and colleagues (Salomon, 2005; Salomon et al., 2000) reported that smaller decreases and even increases in HF-HRV during laboratory challenges vary with lower rates of family conflict. Bridging the emotion regulation and social support findings, perceived social connectedness has been shown to predict extended positive emotion, decreased blood pressure reactivity, and more rapid recovery from negative mood states among older adults (Ong & Allaire, 2005). These reports emphasize not only that emotion regulatory processes may influence cardiovascular function, but also the potentially protective power of positive social functioning for older adults. As the first study to our knowledge to examine the relationship between cardiac vagal control and social functioning in older adults, our findings suggest that greater cardiac vagal control may also be involved in promoting better social function in this population.
Our findings, however, do not agree with predictions derived from the polyvagal theory. We found that less task-related cardiac vagal suppression varied with more positive social functioning across women. Also, we failed to find a relationship between resting HF-HRV and social functioning. Although the literature suggests that indicators of baseline cardiac vagal control are associated with social functioning, our findings may be affected by the fact that resting cardiac vagal control decreases with age (Doussard-Roosevelt, McClenny, & Porges, 2001; Horsten et al., 1999; Jennings et al., 1997; Richards & Cameron, 1989; Stifter et al., 1989). Additionally, many of the studies reporting a positive relationship between resting vagal control social functioning are conducted in children and use outcome measures such as parent and teacher reports of the child’s behavior, whereas we used self-report measures that required participants to rate their social functioning in more developmentally advanced contexts (e.g., within marriage). Thus, it is unclear whether our results are masked by measurement differences, by declines in cardiac parameters common in this age group, or by unique developmentally or contextually dependent relationships between cardiac vagal control and social functioning.
Furthermore, this sample of older women showed minimal changes in HF-HRV from baseline to task conditions combined with substantial changes in blood pressure. This pattern, although perhaps anomalous in a younger sample, is consistent with biological changes related to aging, namely decreases in muscarinic receptor sensitivity and increased blood pressure responsivity (Jennings & Yovetich, 1991). This finding underlines the possibility that cardiac vagal changes and social functioning may relate differently in younger versus older individuals. Our results may also have physical health-related implications for older individuals, particularly postmenopausal women. We have reported findings from a larger cohort of the Healthy Women Study showing that women who exhibit less of a stress-induced suppression in HF-HRV to a speech preparation stressor also show reduced coronary atherosclerosis (Gianaros et al., 2005).
There are limitations of the current study. We did not account for the potential influence of respiration rate and tidal volume on HF-HRV. Although both have been identified as potential confounds in the measurement of HF-HRV by some authors, others report that the relationship between inter- and intra-individual changes in respiratory parameters and vagal control are not improved by controlling for respiration (Gianaros & Quigley, 2001; Gianaros et al., 2001; Grossman, Karemaker, & Wieling, 1991; Grossman & Kollai, 1993; Grossman & Taylor, 2007; Houtveen, Rietveld, & De Geus, 2002; Ritz & Dahme, 2006). The latter is particularly found when experimental tasks only elicit modest changes in respiration, as it is likely that our paradigm did. Also, our results must be interpreted with the caveat that HF-HRV is only an estimate of cardiac vagal control and not an absolute measure of tonic or phasic vagal outflow to the heart (Grossman & Taylor, 2007). Moreover, although prior studies, like our own, have examined associations between cardiovascular reactivity to psychological challenges and psychosocial functioning, cardiovascular reactivity to social stressors may also be relevant. It is also important to note that our interpretations are somewhat limited by the fact that we did not assess individual differences in emotion regulation per se. Although correlations between the average change in participants’ HF-HRV from baseline to task conditions and their ratings of stress and demand levels were not significant, these were only single-item assessments of participants’ appraisals of the experiment. It is possible that more explicit measures of affect and emotion regulation may have provided different results. Also, it is likely that the lack of association between depression, hostility, anger expression, and HF-HRV was due to the highly selective nature of this sample of emotionally and physically healthy women. Given our relatively small sample size and the minimal varianceinparticipants’ scores on these psychosocial measures, we were unlikely to detect significant relationships.
To build on the present findings and speculations, future work should incorporate individual difference measures of dispositional implicit and explicit emotion regulation into similar experimental paradigms in order to assess the role emotion regulation may have in physiological regulation. Moreover, although relevant measures of affect and personality were not associated with our measures of HF-HRV in this study, similar measures may be promising in future research. Also, although we did not find significant associations between task-related blood pressure reactivity and the social functioning factor, we did find associations between baseline and recovery HF-HRVand blood pressure reactivity. Given these findings and other positive reports linking blood pressure reactivity and social factors (Uchino, Cacioppo, Malarkey, Glaser, & Kiecolt-Glaser, 1995; Uchino, Holt-Lunstad, Uno, Betancourt, & Garvey, 1999), research regarding the relationship between social behavior and cardiac vagal control studied in conjunction with other forms of cardiovascular reactivity is warranted, particularly in samples of older adults.
Overall, this study contributes new findings to the growing number of studies investigating the relationships between social functioning and resting and task-related cardiac vagal control. We have shown that, among older women, modulation of cardiac vagal control in response to a stressful laboratory challenge paradigm is related to social functioning in a nuanced manner. As it relates to the influential polyvagal theory, the present study underscores the need for future research into the mechanisms by which vagal regulation and emotional and behavioral reactivity relate to social functioning, particularly among older adults.
Acknowledgments
Research support was provided by the Pittsburgh Mind-Body Cente (National Institutes of Health grant HL 076852/076858), by Nationa Institutes of Health grants MH K01 070616-03, HL 28266, and T32HL007560. We also thank Dr. Lewis Kuller for his support as prin cipal investigator of the Healthy Women Study.
REFERENCES
- Barrera M, Sandler IN, Ramsay TB. Preliminary development of a scale of social support: Studies on college students. Journal of Community Psychology. 1981;9:435–447. [Google Scholar]
- Bazhenova OV, Plonskaia O, Porges SW. Vagal reactivity and affective adjustment in infants during interaction challenges. Child Development. 2001;72:1314–1326. doi: 10.1111/1467-8624.00350. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, Fieldstone A. Autononic cardiac control III: Psychological stress and cardiac response in autonomic space as revealed by pharmocological blockade. Psychophysiology. 1994;31:599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Thayer JF. Anger inhibition, cardiovascular recovery, and vagal function: A model of the link between hostility and cardiovascular disease. Annals of Behavioral Medicine. 1998;20:326–332. doi: 10.1007/BF02886382. [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45:101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosomatic Medicine. 2000;62:639–647. doi: 10.1097/00006842-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Chambers AS, Allen JJB. Vagal tone as an indicator of treatment response in major depression. Psychophysiology. 2002;39:861–864. doi: 10.1111/1469-8986.3960861. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hoberman H. Positive events and social supports as buffers of life change stress. Journal of Applied Social Psychology. 1983;13:99–125. [Google Scholar]
- Cohen S, Mermelstein R, Kamarck T, Hoberman H. Measuring the functional components of social support. In: Sarason IG, Sarason B, editors. Social support: Theory, research, and applications. The Hague: Martinus Nijhoff; 1985. pp. 74–93. [Google Scholar]
- Cook WW, Medley DM. Proposed hostility and pharisaicvirtue scales for the MMPI. Journal of Applied Psychology. 1954;38:414–418. [Google Scholar]
- Demaree HA, Schmeichel BJ, Robinson JL. Behavioural, affective, and physiological effects of negative and positive emotional exaggeration. Cognition and Emotion. 2004;18:1079–1097. [Google Scholar]
- Demaree HA, Schmeichel BJ, Robinson JL, Pu J, Everhart DE, Berntson GG. Up- and down-regulating facial disgust: Affective, vagal, sympathetic, and respiratory consequences. Biological Psychology. 2006;71:90–99. doi: 10.1016/j.biopsycho.2005.02.006. [DOI] [PubMed] [Google Scholar]
- De Meersman RE, Stein PK. Vagal modulation and aging. Biological Psychology. 2007;74:165–173. doi: 10.1016/j.biopsycho.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, McClenny BD, Porges SW. Neonatal cardiac vagal tone and school-age developmental outcome in very low birth weight infants. Developmental Psychobiology. 2001;38:56–66. [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Montgomery LA, Porges SW. Short-term stability of physiological measures in kindergarten children: Respiratory sinus arrhythmia, heart period, and cortisol. Developmental Psychobiology. 2003;43:230–242. doi: 10.1002/dev.10136. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Porges SW, Scanlon JW, Alemi B, Scanlon KB. Vagal regulation of heart rate in the rediction of developmental outcome for very low birth weight preterm infants. Child Development. 1997;68:173–186. [PubMed] [Google Scholar]
- Eckberg DL. Physiological basis for human autonomic rhythms. Annals of Medicine. 2000;32:341–349. doi: 10.3109/07853890008995937. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N. Regulatory control and adult’s stress-related responses to daily life events. Journal of Personality and Social Psychology. 1997;73:1107–1117. doi: 10.1037//0022-3514.73.5.1107. [DOI] [PubMed] [Google Scholar]
- Fox NA, Field TM. Individual differences in preschool entry behavior. Journal of Applied Developmental Psychology. 1989;10:527–540. [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Friedman BH, Thayer JF. Autonomic balance revisited: Panic anxiety and heart rate variability. Journal of Psychosomatic Research. 1998;44:133–151. doi: 10.1016/s0022-3999(97)00202-x. [DOI] [PubMed] [Google Scholar]
- Gautier C, Stine L, Jennings JR, Sutton-Tyrrell K, Muldoon MB, Kamarck T, et al. Reduced low-frequency heart rate variability relates to greater intimal-medial thickness of the carotid wall in two samples. Coronary Artery Disease. 2007;18:97–104. doi: 10.1097/MCA.0b013e328011ac01. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SWG, Matthews KA. Heightened frontal neural reactivity to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension. 2007;49:134–140. doi: 10.1161/01.HYP.0000250984.14992.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage. 2007;35:795–802. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Quigley KS. Autonomic origins of a nonsignal stimulus-elicited bradycardia and its habituation in humans. Psychophysiology. 2001;38:540–547. doi: 10.1017/s004857720100004x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Quigley KS, Mordkoff JT, Stern RM. Gastric myoelectrical and autonomic cardiac reactivity to laboratory stressors. Psychophysiology. 2001;38:642–652. [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Salomon K, Zhou F, Owens JF, Edmundowicz D, Kuller LH, et al. A greater reduction in high-frequency heart rate variability to a psychological stressor is associated with subclinical coronary and aortic calcification in postmenopausal women. Psychosomatic Medicine. 2005;67:553–560. doi: 10.1097/01.psy.0000170335.92770.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano PA, Keane SP, Calkins SD. Cardiac vagal regulation and early peer status. Child Development. 2007;78:264–278. doi: 10.1111/j.1467-8624.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: The need for respiratory control. Psychophysiology. 1991;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Kollai M. Respiratory sinus arrhythmia, cardiac vagal tone, and respiration: Within- and between-individual relations. Psychophysiology. 1993;30:486–495. doi: 10.1111/j.1469-8986.1993.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. International Journal of Psychophysiology. 2003;48:263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Horsten M, Ericson M, Perski A, Wamala SP, Schenck-Gustafsson K, Orth-Gomer K. Psychosocial factors and heart rate variability in healthy women. Psychosomatic Medicine. 1999;61:49–57. doi: 10.1097/00006842-199901000-00009. [DOI] [PubMed] [Google Scholar]
- Houston BK, Smith MA, Cates DS. Hostility patterns and cardiovascular reactivity to stress. Psychophysiology. 1989;26:337–342. doi: 10.1111/j.1469-8986.1989.tb01930.x. [DOI] [PubMed] [Google Scholar]
- Houtveen JH, Rietveld S, De Geus EJ. Contribution of tonic vagal modulation of heart rate, central respiratory drive, respiratory depth, and respiratory frequency to respiratory sinus arrhythmia during mental stress and physical exercise. Psychophysiology. 2002;39:427–436. doi: 10.1017.S0048577202394022. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: Relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Manuck S, Everson SA, Kaplan G, Salonen JT. Aging or disease? Cardiovascular reactivity in Finnish men over the middle years. Psychology and Aging. 1997;12:225–238. doi: 10.1037//0882-7974.12.2.225. [DOI] [PubMed] [Google Scholar]
- Jennings JR, van der Molen MW. Cardiac timing and the central regulation of action. Psychological Research. 2002;66:337–349. doi: 10.1007/s00426-002-0106-5. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Yovetich N. Autonomic nervous system indices. In: Jennings JR, Coles MGH, editors. Handbook of cognitive psychophysiology: Central and autonomic nervous system approaches Chichester. England: John Wiley and Sons, Ltd; 1991. [Google Scholar]
- Kamarck T, Jennings JR, Debski TT, Glickman-Weiss E, Johnson PS, Eddy MJ, et al. Reliable measures of behaviorally evoked cardiovascular reactivity from a PC-based test battery: Results from student and community samples. Psychophysiology. 1992;29:17–28. doi: 10.1111/j.1469-8986.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Kamarck T, Jennings JR, Pogue-Geile M, Manuck SB. A multidimensional measurement model for cardiovascular reactivity: Stability and cross-validation in two adult samples. Health Psychology. 1994;13:471–478. doi: 10.1037//0278-6133.13.6.471. [DOI] [PubMed] [Google Scholar]
- Krause N. Negative interaction and satisfaction with social support among older adults. The Journals of Gerontoloy. Series B, Psychological Sciences and Social Sciences. 1995;50B:59–73. doi: 10.1093/geronb/50b.2.p59. [DOI] [PubMed] [Google Scholar]
- Krause N. Negative interaction and heart disease in late life. Journal of Aging and Health. 2005;17:28–55. doi: 10.1177/0898264304272782. [DOI] [PubMed] [Google Scholar]
- Krause N, Rook KS. Negative interaction in late life: Issues in the stability and generalizability of conflict across relationships. Journal of Gerontology. 2003;58B:P88–P99. doi: 10.1093/geronb/58.2.p88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore SJ, Allen KA, Evans GW. Social support lowers cardiovascular reactivity to an acute stressor. Psychosomatic Medicine. 1993;55:518–524. doi: 10.1097/00006842-199311000-00007. [DOI] [PubMed] [Google Scholar]
- Locke H, Wallace K. Short marital-adjustment and prediction tests: Their reliability and validity. Marriage and Family Living. 1959;21:251–255. [Google Scholar]
- Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. New England Journal of Medicine. 1989;321:641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- McDonald RP. Factor analysis and related methods. Hillsdale, NJ: Lawrence Erlbaum Associates; 1985. [Google Scholar]
- Monk TH, Flaherty JF, Frank E, Hoskinson K, Kupfer DJ. The social rhythm metric: An instrument to quantify the daily rhythyms of life. The Journal of Nervous and Mental Disease. 1990;178:120–126. doi: 10.1097/00005053-199002000-00007. [DOI] [PubMed] [Google Scholar]
- Monk TH, Frank E, Potts JM, Kupfer DJ. A simple way to measure daily lifestyle regularity. Journal of Sleep Research. 2002;11:183–190. doi: 10.1046/j.1365-2869.2002.00300.x. [DOI] [PubMed] [Google Scholar]
- Monk TH, Kupfer DJ, Frank E, Ritenour AM. The social rhythm metric (SRM): Measuring daily social rhythms over 12 weeks. Psychiatry Research. 1990;36:195–207. doi: 10.1016/0165-1781(91)90131-8. [DOI] [PubMed] [Google Scholar]
- Movius HL, Allen JJB. Cardiac vagal tone, defensiveness, and motivational style. Biological Psychology. 2005;68:147–162. doi: 10.1016/j.biopsycho.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Nausheen B, Gidron Y, Gregg A, Tissarchodou HS, Peveler R. Loneliness, social support, and cardiovascular reactivity to laboratory stress. Stress. 2007;10:37–44. doi: 10.1080/10253890601135434. [DOI] [PubMed] [Google Scholar]
- Ong AD, Allaire JC. Cardiovascular intraindividual variability in later life: The influence of social connectedness and positive emotions. Psychology and Aging. 2005;20:476–485. doi: 10.1037/0882-7974.20.3.476. [DOI] [PubMed] [Google Scholar]
- Porges SW. Cardiac vagal tone: A physiological index of stress. Neuroscience and Biobehavioral Reviews. 1995;19:225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Phylogenetic contributions to social behavior. Physiology and Behavior. 2003;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. A phylogenetic journey through the vague and ambiguous Xth cranial nerve: A commentary on contemporary heart rate variability research. Biological Psychology. 2007a;74:301–307. doi: 10.1016/j.biopsycho.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007b;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development. 1994;59:167–186. [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Richards JE, Cameron D. Infant heart-rate variability and behavioral developmental status. Infant Behavior and Development. 1989;12:45–58. [Google Scholar]
- Ritz T, Dahme B. Implementation and interpretation of respiratory sinus arrhythmia measures in psychosomatic medicine: Practice against better evidence? Psychosomatic Medicine. 2006;68:617–627. doi: 10.1097/01.psy.0000228010.96408.ed. [DOI] [PubMed] [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: A critical analysis. Biological Psychology. 2007;74:200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44:450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Salomon K. Respiratory sinus arrhythmia during stress predicts resting respiratory sinus arrhythmia 3 years later in a pediatric sample. Health Psychology. 2005;24:68–76. doi: 10.1037/0278-6133.24.1.68. [DOI] [PubMed] [Google Scholar]
- Salomon K, Matthews KA, Allen MT. Patterns of sympathetic and parasympatheitc reactivity in a sample of children and adolescents. Psychophysiology. 2000;37:842–849. [PubMed] [Google Scholar]
- Schuster TL, Kessler RC, Aseltine RH. Suppportive interactions, negative interactions, and depressed mood. American Journal of Community Psychology. 1990;18:423–438. doi: 10.1007/BF00938116. [DOI] [PubMed] [Google Scholar]
- Shahar L, Nevo O, Thaler I, Rosenfeld R, Dayan L, Hirshoren N, et al. Effect of aging on the cardiovascular regulatory systems in healthy women. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2007;292:788–793. doi: 10.1152/ajpregu.00352.2006. [DOI] [PubMed] [Google Scholar]
- Smith MA, Houston BK. Hostility, anger expression, cardiovascular responsivity, and social support. Biological Psychology. 1987;24:39–48. doi: 10.1016/0301-0511(87)90098-6. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ruiz JM, Uchino BN. Mental activation of supportive ties, hostility, and cardiovascular reactivity to laboratory stress in young men and women. Health Psychology. 2004;23:476–485. doi: 10.1037/0278-6133.23.5.476. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Johnson EH, Russell SF, Crane RJ, Jacobs GA, Worden TJ. The experience and expression of anger: Construction and validation of an anger expression scale. In: Chesney MA, Rosenman RH, editors. Anger and hostility in cardiovascular and behavioral disorders. New York: Hemisphere; 1985. pp. 5–30. [Google Scholar]
- Stein PK, Kleiger RE. Insights from the study of heart rate variability. Annual Review of Medicine. 1999;50:249–261. doi: 10.1146/annurev.med.50.1.249. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Fox NA, Porges SW. Facial expressivity and vagal tone in 5- and 10-month-old infants. Infant Behavior and Development. 1989;12:127–137. [Google Scholar]
- Suls J, Bunde J. Anger, anxiety, and depression, as risk factors for cardiovascular disease: The problems and implications of overlapping affective dispositions. Psychological Bulletin. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Brosschot JF. Psychosomatics and psychpathology: Looking up and down from the brain. Psychoneuroendocrinology. 2005;30:1050–1058. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Malarkey W, Glaser R, Kiecolt-Glaser JK. Appraisal support predicts age-related differences in cardiovascular function in women. Health Psychology. 1995;14:556–562. doi: 10.1037//0278-6133.14.6.556. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Garvey TS. The availability of social support reduces cardiovascular reactivity to acute psychological stress. Journal of Behavioral Medicine. 1997;20:15–27. doi: 10.1023/a:1025583012283. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Holt-Lunstad J, Uno D, Betancourt D, Garvey TS. Social support and age-related differences in cardiovascular function: An examination of potential mediators. Annals of Behavioral Medicine. 1999;21:135–142. doi: 10.1007/BF02908294. [DOI] [PubMed] [Google Scholar]
- Zara A, Lombardi F. Autnomic indexes based on the analysis of heart rate variability: A view from the sinus node. Cardiovascular Research. 2001;50:434–442. doi: 10.1016/s0008-6363(01)00240-1. [DOI] [PubMed] [Google Scholar]
- Zhang J. Effect of age and sex on heart rate variability in healthy subjects. Journal of Manipulative and Physiological Therapeutics. 2007;30:374–379. doi: 10.1016/j.jmpt.2007.04.001. [DOI] [PubMed] [Google Scholar]