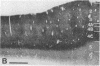
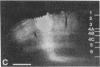
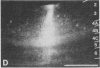
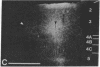
Abstract
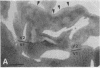
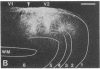
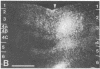
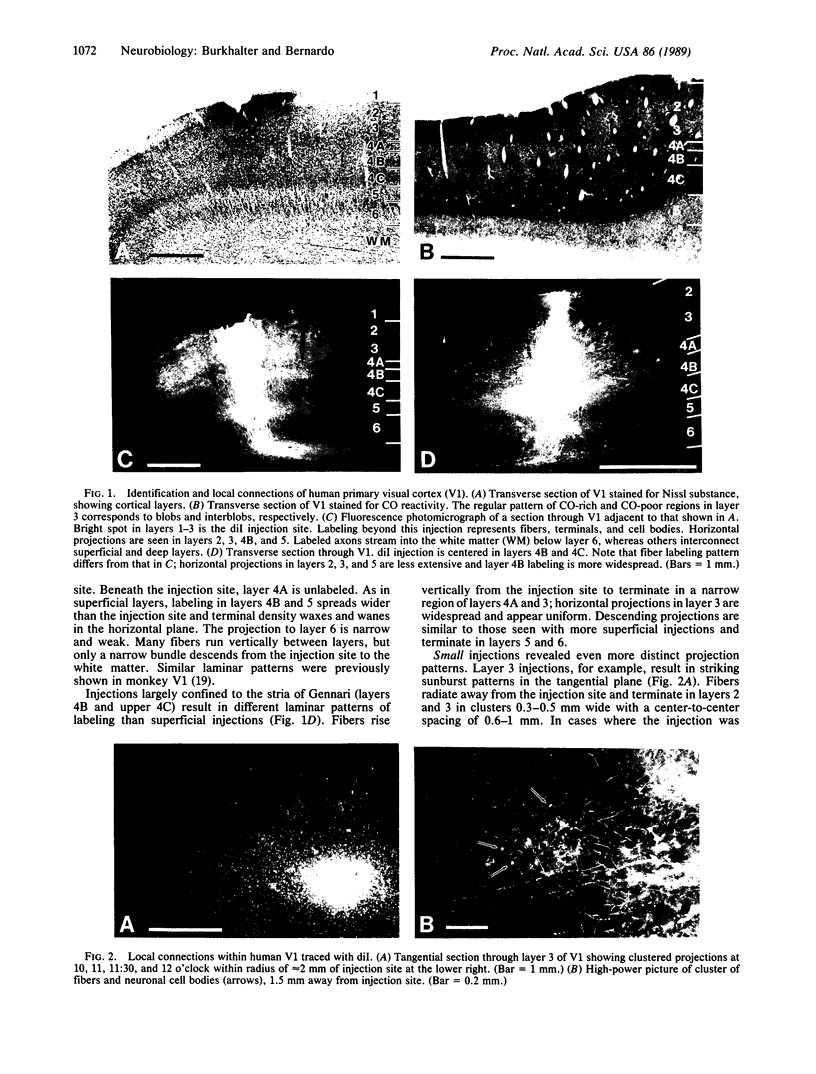
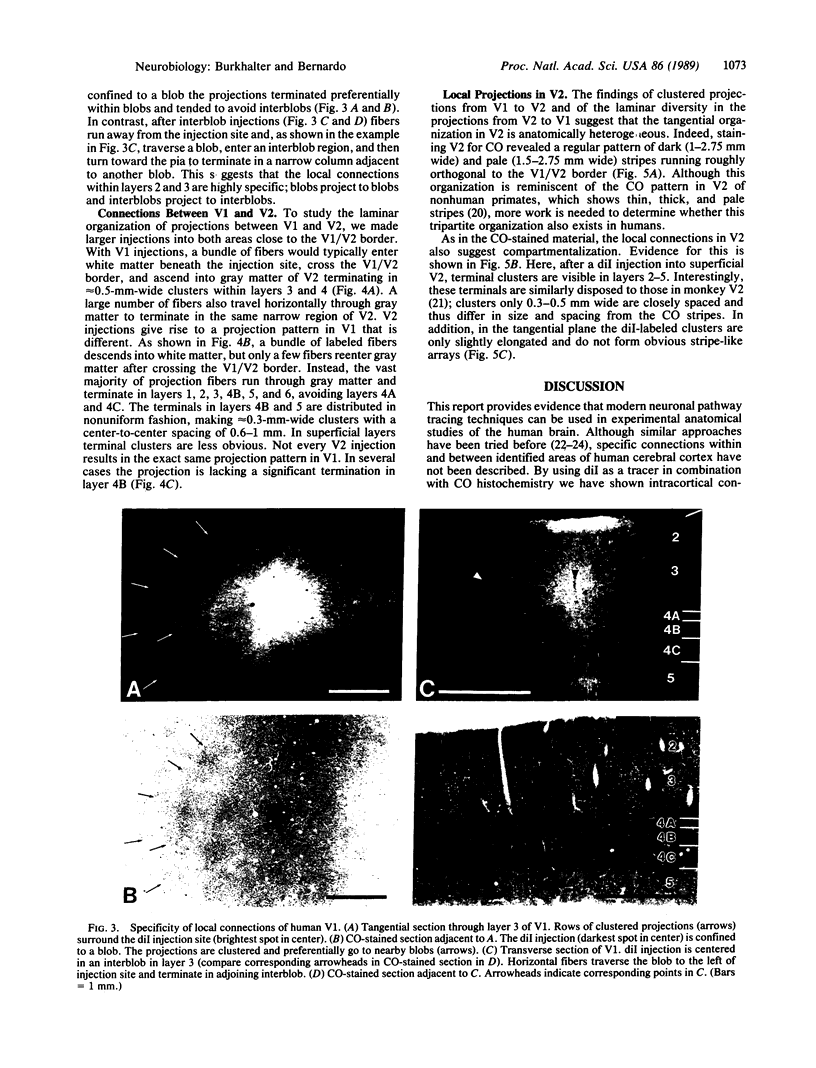
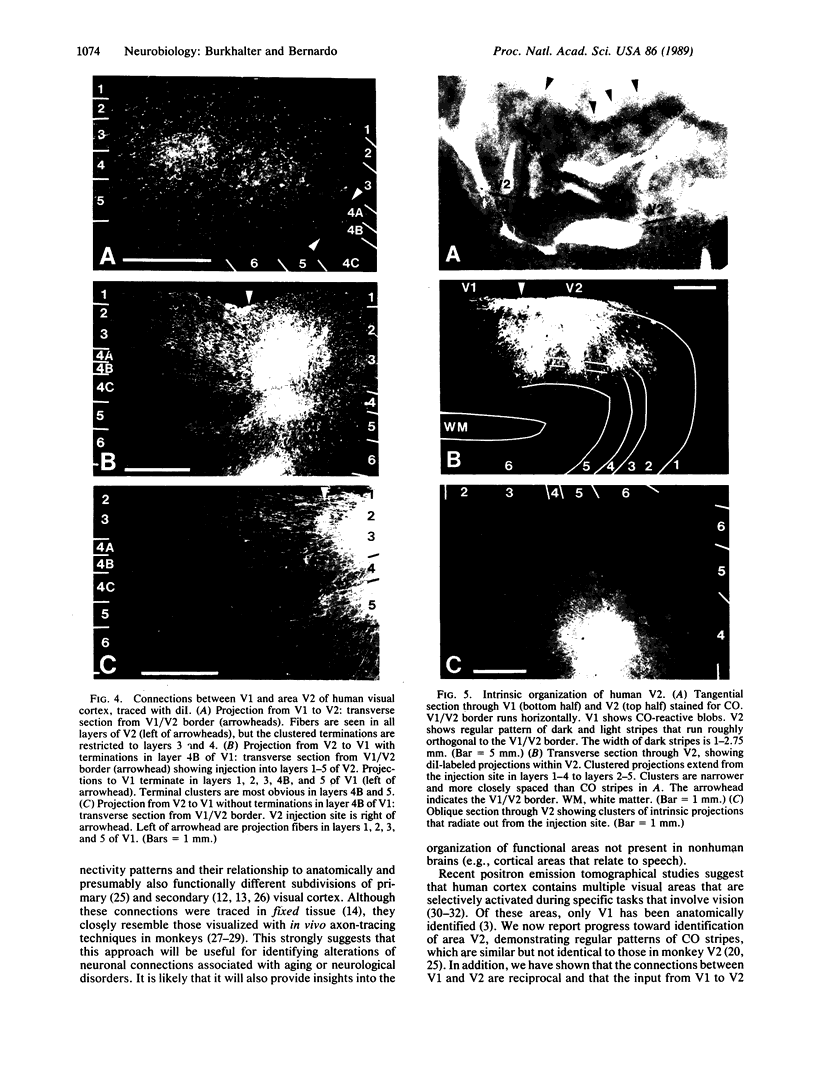
Clinical and psychophysical observations indicate that the visual cortex is critical for the perception of color, form, depth, and movement. Little, however, is known about the cortical circuitry that underlies these functions in humans. In an attempt to learn more about these connections, we have traced projections of primary (V1) and secondary (V2) visual cortex in the postmortem, fixed human brain, using the fluorescent dye 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate as an axonal marker. The results show that V1 makes a forward projection to layers 3 and 4 of V2, and V2 projects back to layers 1, 2, 3, 5, and 6 of V1. Some V2 injections also show an input to layer 4B of V1. Projections to 4B probably originate from cytochrome oxidase (CO)-reactive stripes that we have identified in V2. Differential connections between CO-rich (blobs) and CO-poor regions (interblobs) also exist within V1; blobs are connected to blobs and interblobs are connected to interblobs. The results show that the connections in human visual cortex are similar to those of nonhuman primates and that their organization is consistent with the concept of multiple processing streams in the visual system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach T. G., McGeer E. G. Retrograde filling of pyramidal neurons in postmortem human cerebral cortex using horseradish peroxidase. J Neurosci Methods. 1988 Apr;23(3):187–193. doi: 10.1016/0165-0270(88)90002-7. [DOI] [PubMed] [Google Scholar]
- Beach T. G., McGeer E. G. Tract-tracing with horseradish peroxidase in the postmortem human brain. Neurosci Lett. 1987 Apr 23;76(1):37–41. doi: 10.1016/0304-3940(87)90188-1. [DOI] [PubMed] [Google Scholar]
- Blasdel G. G., Lund J. S., Fitzpatrick D. Intrinsic connections of macaque striate cortex: axonal projections of cells outside lamina 4C. J Neurosci. 1985 Dec;5(12):3350–3369. doi: 10.1523/JNEUROSCI.05-12-03350.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H. The pigment architecture of the human occipital lobe. Anat Embryol (Berl) 1977 Mar 30;150(2):229–250. doi: 10.1007/BF00316652. [DOI] [PubMed] [Google Scholar]
- Damasio A., Yamada T., Damasio H., Corbett J., McKee J. Central achromatopsia: behavioral, anatomic, and physiologic aspects. Neurology. 1980 Oct;30(10):1064–1071. doi: 10.1212/wnl.30.10.1064. [DOI] [PubMed] [Google Scholar]
- DeYoe E. A., Van Essen D. C. Concurrent processing streams in monkey visual cortex. Trends Neurosci. 1988 May;11(5):219–226. doi: 10.1016/0166-2236(88)90130-0. [DOI] [PubMed] [Google Scholar]
- DeYoe E. A., Van Essen D. C. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature. 1985 Sep 5;317(6032):58–61. doi: 10.1038/317058a0. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Miezin F. M., Allman J. M., Van Essen D. C., Raichle M. E. Retinotopic organization of human visual cortex mapped with positron-emission tomography. J Neurosci. 1987 Mar;7(3):913–922. doi: 10.1523/JNEUROSCI.07-03-00913.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein M. The discovery of the visual cortex. Sci Am. 1988 Sep;259(3):118–127. doi: 10.1038/scientificamerican0988-118. [DOI] [PubMed] [Google Scholar]
- Godement P., Vanselow J., Thanos S., Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987 Dec;101(4):697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- Haber S. Tracing intrinsic fiber connections in postmortem human brain with WGA-HRP. J Neurosci Methods. 1988 Feb;23(1):15–22. doi: 10.1016/0165-0270(88)90017-9. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. F., Hickey T. L. Ocular dominance columns: evidence for their presence in humans. Brain Res. 1980 Jan 20;182(1):176–179. doi: 10.1016/0006-8993(80)90841-0. [DOI] [PubMed] [Google Scholar]
- Holmes G. DISTURBANCES OF VISION BY CEREBRAL LESIONS. Br J Ophthalmol. 1918 Jul;2(7):353–384. doi: 10.1136/bjo.2.7.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. C., Hedley-Whyte E. T. Mapping of cytochrome oxidase patches and ocular dominance columns in human visual cortex. Philos Trans R Soc Lond B Biol Sci. 1984 Jan 17;304(1119):255–272. doi: 10.1098/rstb.1984.0022. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Livingstone M. S. Segregation of form, color, and stereopsis in primate area 18. J Neurosci. 1987 Nov;7(11):3378–3415. doi: 10.1523/JNEUROSCI.07-11-03378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci. 1984 Jan;4(1):309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Connections between layer 4B of area 17 and the thick cytochrome oxidase stripes of area 18 in the squirrel monkey. J Neurosci. 1987 Nov;7(11):3371–3377. doi: 10.1523/JNEUROSCI.07-11-03371.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci. 1987 Nov;7(11):3416–3468. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Specificity of intrinsic connections in primate primary visual cortex. J Neurosci. 1984 Nov;4(11):2830–2835. doi: 10.1523/JNEUROSCI.04-11-02830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell J. H., van Essen D. C. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983 Dec;3(12):2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Newsome W. T., Wurtz R. H., Dürsteler M. R., Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci. 1985 Mar;5(3):825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S. E., Fox P. T., Posner M. I., Mintun M., Raichle M. E. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988 Feb 18;331(6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Posner M. I., Petersen S. E., Fox P. T., Raichle M. E. Localization of cognitive operations in the human brain. Science. 1988 Jun 17;240(4859):1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Rockland K. S. A reticular pattern of intrinsic connections in primate area V2 (area 18). J Comp Neurol. 1985 May 22;235(4):467–478. doi: 10.1002/cne.902350405. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Pandya D. N. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979 Dec 21;179(1):3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- Shipp S., Zeki S. Segregation of pathways leading from area V2 to areas V4 and V5 of macaque monkey visual cortex. Nature. 1985 May 23;315(6017):322–325. doi: 10.1038/315322a0. [DOI] [PubMed] [Google Scholar]
- Tootell R. B., Silverman M. S., De Valois R. L., Jacobs G. H. Functional organization of the second cortical visual area in primates. Science. 1983 May 13;220(4598):737–739. doi: 10.1126/science.6301017. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Columnar cortico-cortical interconnections within the visual system of the squirrel and macaque monkeys. Brain Res. 1979 Feb 23;162(2):201–217. doi: 10.1016/0006-8993(79)90284-1. [DOI] [PubMed] [Google Scholar]
- Zihl J., von Cramon D., Mai N. Selective disturbance of movement vision after bilateral brain damage. Brain. 1983 Jun;106(Pt 2):313–340. doi: 10.1093/brain/106.2.313. [DOI] [PubMed] [Google Scholar]