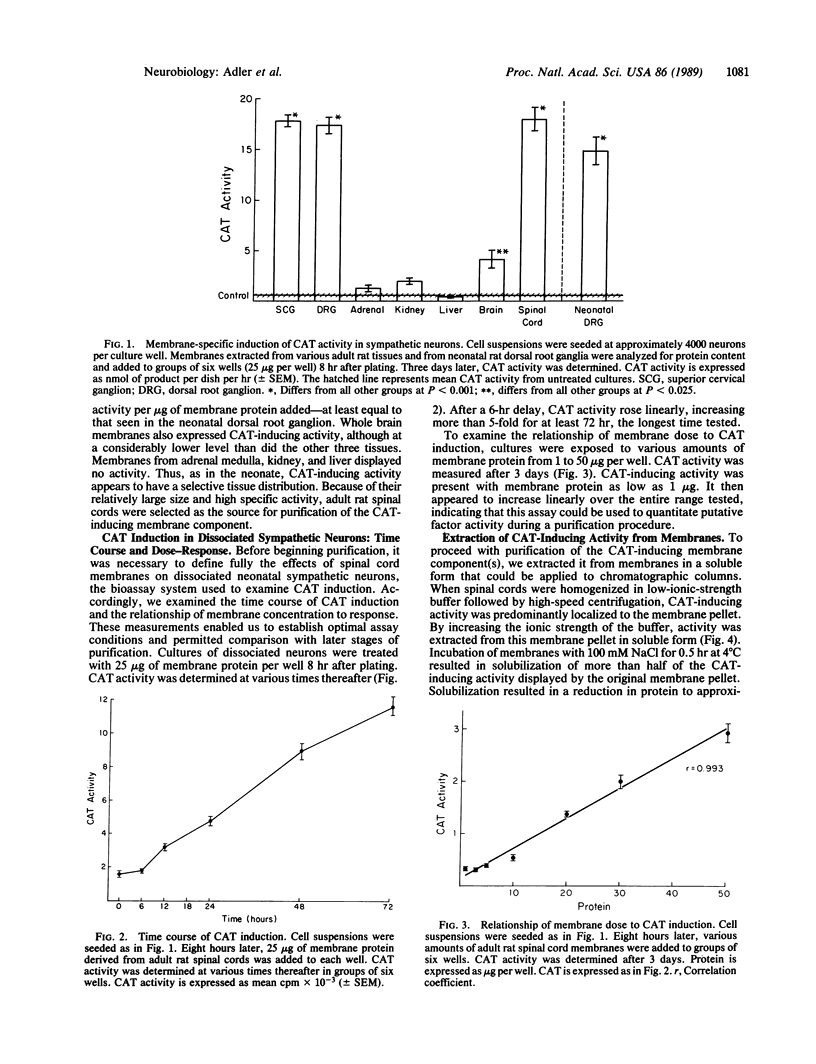
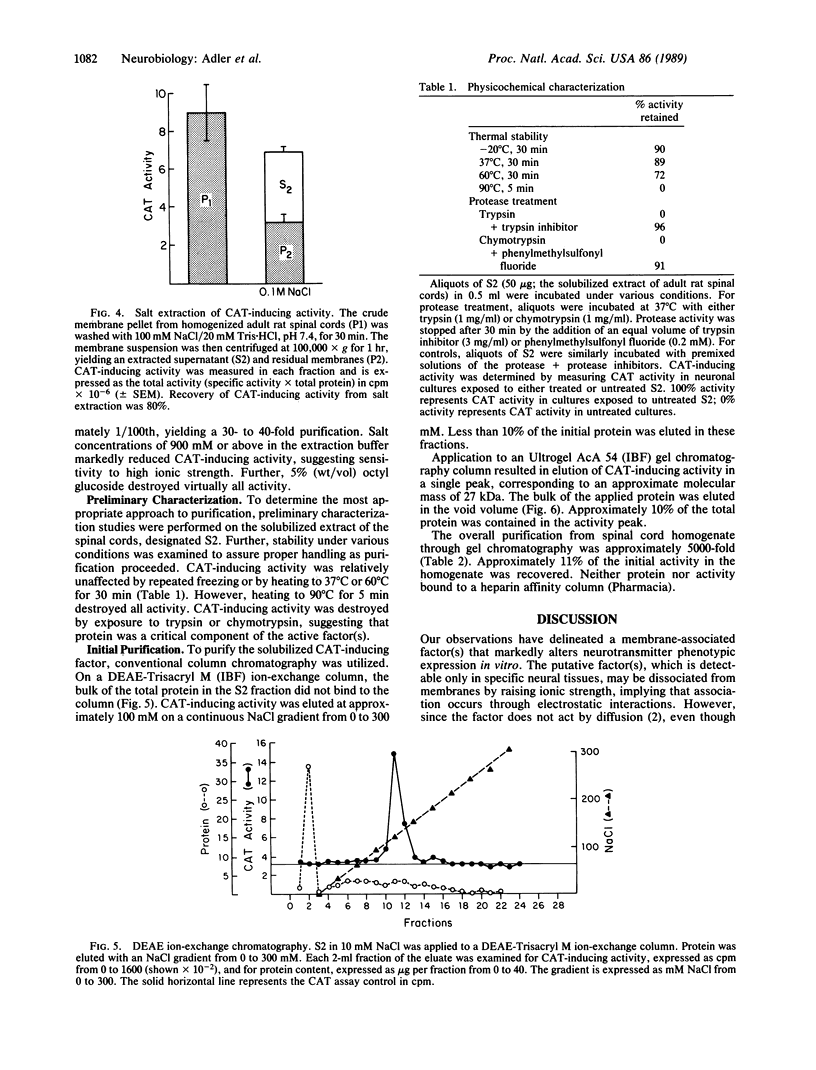
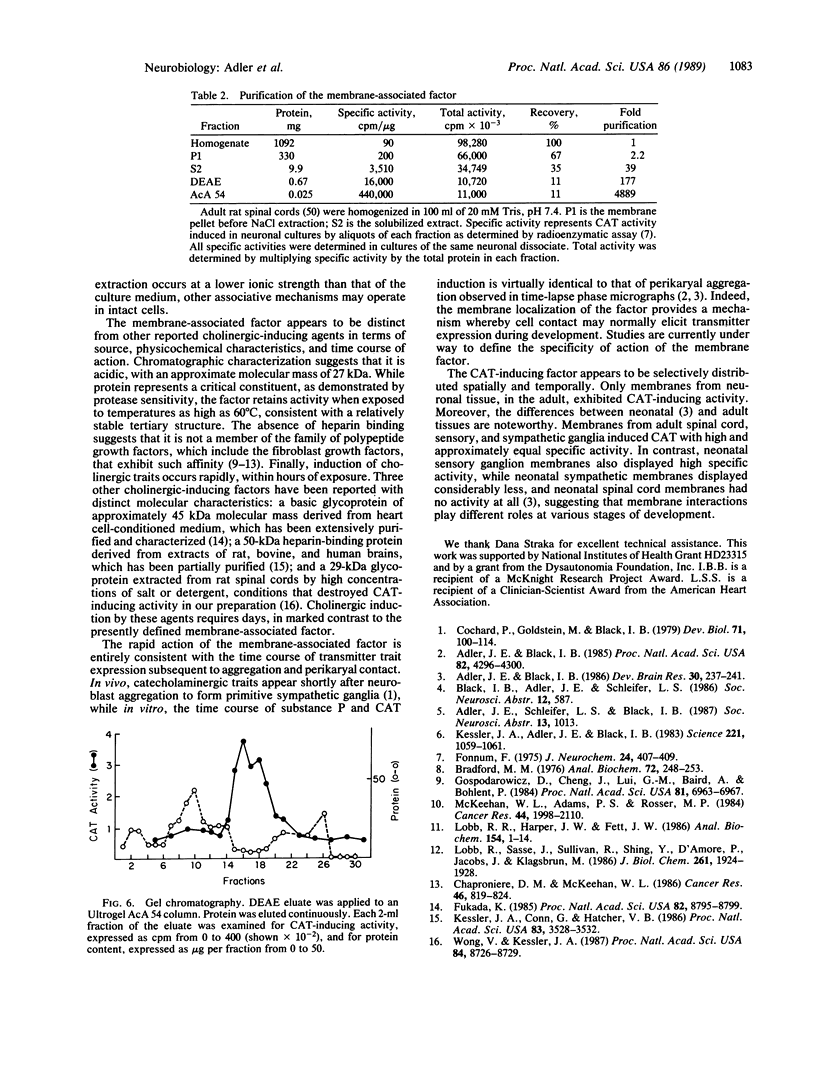
Abstract
Cell membrane contact induces the de novo expression of choline O-acetyltransferase (CAT; acetyl-CoA: choline O-acetyltransferase, EC 2.3.1.6) activity in cultures of virtually pure neonatal rat dissociated sympathetic neurons. To identify molecular mechanisms underlying membrane-associated CAT induction, the responsible membrane component was characterized and partially purified. Substantial CAT-inducing activity was found in membranes from adult rat spinal cord and sensory and sympathetic ganglia. Whole brain membranes demonstrated significantly less activity. CAT induction in sympathetic neurons in response to spinal cord membranes was linear with respect to time, after an initial 6-hr lag. It was also linear with respect to concentrations of spinal cord protein from 2 to 100 micrograms per ml. CAT-inducing activity was extracted from spinal cord membranes by incubation with 100 mM NaCl and was purified approximately 5000-fold by DEAE ion-exchange and gel filtration chromatography. The active factor appears to be an extrinsic protein with an apparent molecular mass of 27 kDa. It is inactivated by trypsin and chymotrypsin but is moderately thermostable, retaining activity at 60 degrees C but not at 90 degrees C.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. E., Black I. B. Membrane contact regulates transmitter phenotypic expression. Brain Res. 1986 Dec;395(2):237–241. doi: 10.1016/s0006-8993(86)80202-5. [DOI] [PubMed] [Google Scholar]
- Adler J. E., Black I. B. Sympathetic neuron density differentially regulates transmitter phenotypic expression in culture. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4296–4300. doi: 10.1073/pnas.82.12.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chaproniere D. M., McKeehan W. L. Serial culture of single adult human prostatic epithelial cells in serum-free medium containing low calcium and a new growth factor from bovine brain. Cancer Res. 1986 Feb;46(2):819–824. [PubMed] [Google Scholar]
- Cochard P., Goldstein M., Black I. B. Initial development of the noradrenergic phenotype in autonomic neuroblasts of the rat embryo in vivo. Dev Biol. 1979 Jul;71(1):100–114. doi: 10.1016/0012-1606(79)90085-x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Fukada K. Purification and partial characterization of a cholinergic neuronal differentiation factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8795–8799. doi: 10.1073/pnas.82.24.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J. A., Adler J. E., Black I. B. Substance P and somatostatin regulate sympathetic noradrenergic function. Science. 1983 Sep 9;221(4615):1059–1061. doi: 10.1126/science.6192502. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Conn G., Hatcher V. B. Isolated plasma membranes regulate neurotransmitter expression and facilitate effects of a soluble brain cholinergic factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3528–3532. doi: 10.1073/pnas.83.10.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R. R., Harper J. W., Fett J. W. Purification of heparin-binding growth factors. Anal Biochem. 1986 Apr;154(1):1–14. doi: 10.1016/0003-2697(86)90487-2. [DOI] [PubMed] [Google Scholar]
- Lobb R., Sasse J., Sullivan R., Shing Y., D'Amore P., Jacobs J., Klagsbrun M. Purification and characterization of heparin-binding endothelial cell growth factors. J Biol Chem. 1986 Feb 5;261(4):1924–1928. [PubMed] [Google Scholar]
- McKeehan W. L., Adams P. S., Rosser M. P. Direct mitogenic effects of insulin, epidermal growth factor, glucocorticoid, cholera toxin, unknown pituitary factors and possibly prolactin, but not androgen, on normal rat prostate epithelial cells in serum-free, primary cell culture. Cancer Res. 1984 May;44(5):1998–2010. [PubMed] [Google Scholar]
- Wong V., Kessler J. A. Solubilization of a membrane factor that stimulates levels of substance P and choline acetyltransferase in sympathetic neurons. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8726–8729. doi: 10.1073/pnas.84.23.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]


