Abstract
Bcl-2 proteins are over-expressed in many tumors, and are critically important for cell survival. Their anti-apoptotic activities are determined by intracellular localization and post-translational modifications (such as phosphorylation). Here we showed that WAVE1, a member of the Wiskott-Aldrich syndrome protein family, was over-expressed in blood cancer cell lines, and functioned as a negative regulator of apoptosis. Further enhanced expression of WAVE1 by gene transfection rendered leukemia cells more resistant to anti-cancer drug-induced apoptosis; whereas suppression of WAVE1 expression by RNA interference restored leukemia cells' sensitivity to anti-drug-induced apoptosis. WAVE1 was found to be associated with mitochondrial Bcl-2, and its depletion led to mitochondrial release of Bcl-2, and phosphorylation of ASK1/JNK and Bcl-2. Furthermore, depletion of WAVE1 expression increased anti-cancer drug-induced production of reactive oxygen species in leukemia cells. Taken together, these results suggest WAVE1 as a novel regulator of apoptosis, and potential drug target for therapeutic intervention of leukemia.
Keywords: WAVE1, Bcl-2, Apoptosis, ROS, Calcium, Leukemia
INTRODUCTION
Apoptosis has now been widely accepted as a prominent tumor-suppression mechanism 1, 2. It is mediated by two central pathways: an extrinsic pathway triggered by cell death receptors (such as Fas), and an intrinsic pathway triggered by the mitochondrial release of cytochrome c and its subsequent complex formation with Apaf-1, ATP, and caspase-9 1, 3. A pivotal event in the mitochondrial pathway mitochondrial outer membrane permeabilization (MOMP), which is mainly regulated by Bcl-2 family members [anti-apoptotic (e.g., Bcl-2 and Bcl-XL) or pro-apoptotic (e.g., Bax, Bid, Bad and Bak)] 4.
Bcl-2 is an anti-apoptotic member of the Bcl-2 family, and plays an important role in the regulation of apoptosis, tumorigenesis and cellular responses to anti-cancer therapy 4. Overexpression of Bcl-2 inhibits stress-induced cell death and promotes haemopoietic cell survival 4, 5. Bcl-2 is localized to the mitochondrial membrane as well as endoplasmic reticulum (ER) and nuclear membranes. The anti-apoptotic activity of Bcl-2 is regulated by its intracellular localization and post-translational modification such as phosphorylation 6-9. However, the intricate molecular mechanisms underlying Bcl-2 subcellular localization and intermolecular interactions are not fully understood.
The family of Wiskott Aldrich syndrome proteins including WAVE1, WAVE2, WAVE3, WASP, N-WASP, are involved in transduction of signals from receptors on the cell surface to the actin cytoskeleton 10. As an essential process for cell morphologic changes and motility, cytoskeleton reorganization is also an important regulator of apoptotic cell death 11. WAVE1 is expressed most abundantly in murine brain tissue, and at extremely low levels in other tissues including heart, liver, lung, kidney, pancreas, and peripheral blood 12. Although essential for the normal central nervous system development and mammalian fertilization 13, 14, WAVE1 can also interact with a number of mitochondrial proteins (e.g., glucokinase and PKA), thereby coordinating death and glycolysis 15. In addition, WAVE1 also interacts with pancortin-2 in a mitochondria-associated protein complex, and mediates ischemic neuronal death 16, 17. These findings support a general role for WAVE1 in the regulation of mitochondria function.
Leukemia is a malignant disease of the bone marrow and blood, and is the most common form of cancers in children. We have recently found that WAVE1 was involved in multi-drug-resistance and oxdative stress of human leukemia cells 18-20, but its pathogenic role in leukemia were not fully understood. Here we showed that WAVE1 was over-expressed in both human blood cancer cells lines and primary bone marrow mononuclear cells of patients with childhood leukemia. Elevation of WAVE1 expression by gene transfection rendered leukemia cells resistant to apoptosis; whereas suppression of WAVE1 expression by RNA interference increased the sensitivity of leukemia cells to anti-cancer drugs. Furthermore, our experimental data suggested a role for WAVE1 in the regulation of apoptosis partly through controlling Bcl-2 localization and phosphorylation, supporting a potential pathogenic role for the WAVE1-Bcl-2 axis in leukemia.
RESULTS
WAVE1 is over-expressed in human leukemia cell lines and renders cells resistant to chemotherapeutics- induced apoptosis
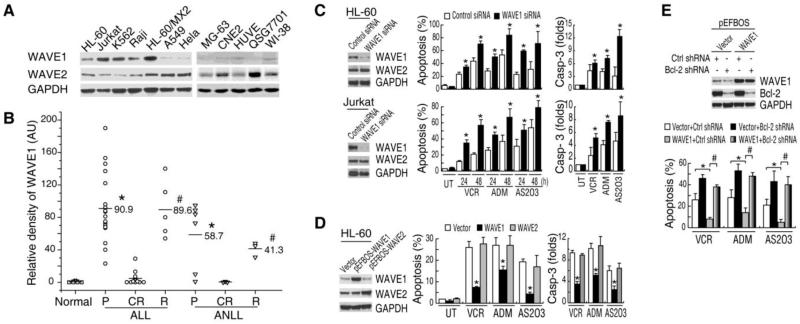
We firstly determined levels of WAVE1 expression in five leukemia cell lines (HL60, K562, HL-60/MX2, Jurkat, and Raji) by Western blotting analysis. Levels of WAVE1 expression were high in all five human leukemia cancer cell lines (Figure 1A). In contrast, the constitutive expression levels of WAVE1, but not WAVE2, were noticeably lower in non-blood cancer cell-lines, including human lung A549 cancer cells, Hela cervical cancer cells, MG-63 osteosarcoma cells, CNE2 nasopharyngeal carcinoma cells, human umbilical vein endothelial cell (HUVE), QSG7701 hepatocarcinoma cells, and WI-38 lung fibroblastoma cells (Figure 1A), suggesting that a different role of WAVE1 in leukemia tumorigenesis.
Figure 1. WAVE1 is over-expressed in human leukemia cell lines and renders cells resistant to chemotherapeutics- induced apoptosis.
(A) WAVE1 was over-expressed in blood cancer cell lines. Western blotting analysis of WAVE1 and WAVE2, and GAPDH in various human cancer cell lines as indicated. (B) Relative expression levels of WAVE1 in childhood leukemia. Total protein was extracted from normal or patients' BMMCs, and equal amounts of proteins (30 μg) were loaded, and WAVE1 level was determined by the relative optical intensity (in arbitrary units, AU) of the immunoreactive bands on Western blots. Each dot represents relative WAVE1 level in each individual sample. ALL, acute lymphoblastic leukemia; ANLL, acute nonlymphocytic leukemia; P, primary; CR, complete remission; R, relapse. *P < 0.05 versus normal. #P < 0.05 versus CR. (C) WAVE1 knockdown increased the sensitivity of leukemia cells to anti-cancer drug induced apoptosis. Knockdown WAVE1 by siRNA in leukemia cells as indicated and then treated with VCR (1 μg/ml), ADM (1 μg/ml), and AS2O3 (5 μM). Cell apoptosis was examined at 24 and 48 h, and caspase-3 activity was examined at 24 h (n=3). UT, Untreated group. *P < 0.05 versus Control siRNA group. (D) WAVE1 overexpression rendered HL-60 leukemia cells resistant to anti-cancer drug -induced apoptosis. HL-60 cells transfected with WAVE1, WAVE2 and pEFBOS vector plasmid and then treat with VCR (1 μg/ml), ADM (1 μg/ml), and AS2O3 (5 μM). Cell apoptosis and caspase 3 activity was examined at 24 h (n=3). UT, Untreated group. *P < 0.05 versus empty vector group. (E) Knockdown of Bcl-2 by shRNA in WAVE1 over-expressing cells restored sensitivity to anti-cancer drug-induced apoptosis. HL-60 cells transfected with various plasmid were stimulated with VCR (1 μg/ml), ADM (1 μg/ml), and AS2O3 (5 μM). At 24 h post treatment, cell apoptosis was assayed (n=3, *, # P < 0.05).
To evaluate the clinical relevance, we determined the relative WAVE1 protein expression levels in BMMCs obtained from 37 patients with acute lymphocytic leukemia (18 patients in primary phase, 5 in relapse phase, and 14 in complete remission phase) and 12 patients with acute nonlymphocytic leukemia (6 patients in primary phase, 3 in relapse phase, and 3 in complete remission phase). Higher levels of WAVE1 expression were found in BMMCs derived from patients with primary and relapse leukemia (Figure 1B). In contrast, consistent with the absence of WAVE1 expression in normal PBMCs, WAVE1 was not detectable in BMMCs derived from normal healthy subjects, or patients with complete remission (Figure 1B), suggesting that WAVE1 maybe reflects stages of haemocyte differentiation and maturation.
To explore the potential role for WAVE1 in the regulation of apoptotic cell death of leukemia cells, a target-specific siRNA duplex against WAVE1 was transferred into HL-60 and Jurkat leukemia cells. Transfection of WAVE1-siRNA led to a significant decrease in WAVE1 protein in these cells (Figure 1C). Depletion of WAVE1 expression in these cells rendered them significantly more sensitive to vincristine (VCR), arsenic trioxide (AS2O3) and adriamycin (ADM)-induced apoptotic cell death, which were associated with high levels of caspase-3 activities (Figure 1C).
To further characterize the role of WAVE1 in leukemia after chemotherapy, we transfected HL-60 leukemia cells with full-length human WAVE1 and WAVE2 cDNA. These WAVE1-overexpressing cells became resistant to apoptosis induced by VCR, ADM, and AS2O3 (Figure 1D), suggesting a potential anti-apoptotic role for WAVE1 in leukemia cells. In contrast, overexpression of WAVE2 did not increase the resistance to anti-cancer drugs by apoptosis analysis or caspase-3 activity assay (Figure 1D), supporting a specific role for WAVE1 in the pathogenesis of leukemia. In contrast, knockdown of Bcl-2 in WAVE1 over-expressing cells partially restored their sensitivity to anti-cancer drug-induced apoptosis, suggesting that WAVE1-mediated anti-apoptotic effects are partly Bcl-2 dependent (Figure 1E).
WAVE1 regulates ROS production and Ca2+ homeostasis in leukemia cell apoptosis
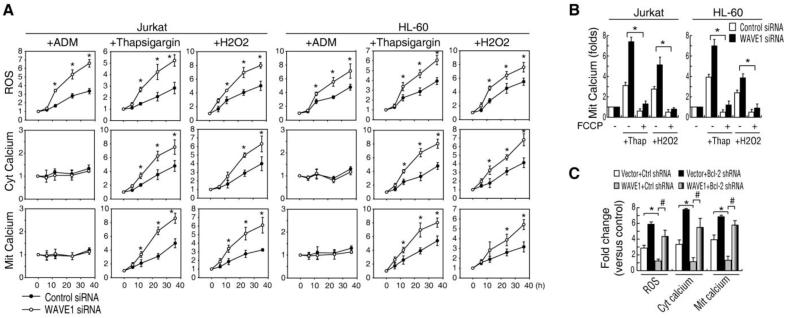
Many cellular processes including apoptosis are regulated by the ROS and calcium signal pathway 4, 21. To gain insight into the intricate molecular mechanism by which WAVE1 regulates apoptosis in leukemia cells, we firstly investigated the effect of WAVE1 on intracellular ROS and Ca2+ homeostasis in apoptosis. Consistent with previous study 22, ADM increased ROS production, but not calcium. In contrast, thapsigargin (an inhibitor of sarco/endoplasmic reticulum Ca2+ ATPase involved in calcium sequestration by the ER) and H2O2 (an agonist that induces the opening of the InsP3 receptors) increased ROS production, as well as cytosolic-free/mitochondrial Ca2+. Notably, suppression of WAVE1 expression by siRNA increased these drugs-induced ROS production and cytosolic-free/mitochondria Ca2+ (Figure 2A), suggesting a potential role for WAVE1 in regulation of ROS production and calcium homeostasis in leukemia. Moreover, addition of p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (FCCP), a protonophore that leads to collapse of the mitochondrial membrane potential 23, decreased the load of mitochondria Ca2+ in WAVE1 siRNA cells after thapsigargin and H2O2 treatment (Figure 2B). Furthermore, overexpression of WAVE1 rendered cells resistant to thapsigargin-induced ROS production, and elevation of cytosolic or mitochondrial Ca2+ levels (Figrue 2C). In contrast, knockdown of Bcl-2 in WAVE1 over-expressing cells partially restored thapsigargin-induced production of ROS production, and elevation of cytosolic free or mitochondrial Ca2+ levels (Figure 2C), suggesting that WAVE1-mediated effects are partly Bcl-2 dependent.
Figure 2. WAVE1 regulates ROS production and Ca2+ homeostasis in apoptosis.
(A) Knockdown WAVE1 by siRNA in Jurkat or HL-60 cells and treated with ADM (1 μg/ml), thapsigargin (100 nM),or H2O2 (250 μmol) for 24 h, and ROS production, ER Ca2+ release (cytosolic free calcium) and mitochondria Ca2+ load was analyzed by flow cytometry (n=3, * P < 0.05 versus control siRNA group). UT, Untreated group, set as 1. (B) Knockdown WAVE1 cells were treated with thapsigargin (100 nM) or H2O2 (250 μmol) with or without FCCP (2.5 μmol) for 24 h, and mitochondria Ca2+ load was analyzed by flow cytometry (n=3, * P < 0.05). Untreated group, set as 1. (C) Knockdown of Bcl-2 by shRNA in WAVE1 overexpressin cells restored ROS and Ca2+ production. HL-60 cells transfected with various plasmids were stimulated with thapsigargin (100 nM) for 24 h, and cellular levels of ROS, cytosolic free calcium, and mitochondria Ca2+ load was analyzed by flow cytometry (n = 3, * P < 0.05).
WAVE1 regulates intracellular localization of Bcl-2 in leukemia cell
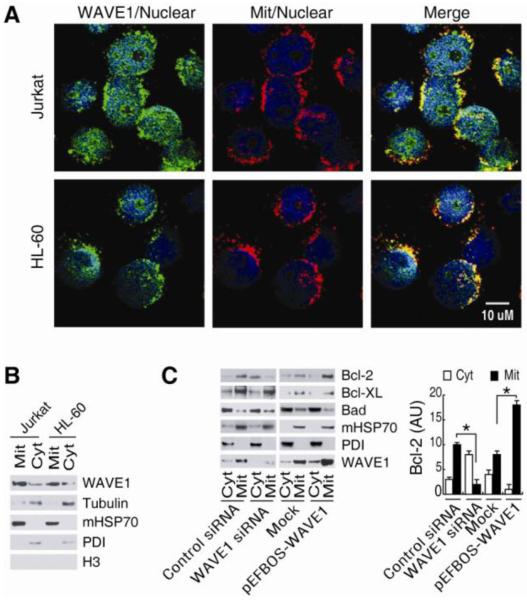
Similar to the mitochondrial localization of WAVE1 in neuronal cells 17, WAVE1 was localized to the mitochondrial as well as cytosol and nuclear in leukemia cells (e.g. Jurkat and HL-60) by image and /or Western blotting analysis (Figure 3A and B). Its mitochondrial localization provided a basis for its molecular interaction with divergent mitochondrial proteins, such as Bcl-2 and Bcl-XL 6, 24-26. To further investigate the role of WAVE1 in leukemia, we determined the effects of WAVE1 depletion on mitochondrial localization of anti-apoptotic proteins Bcl-2. We found that WAVE1 knockdown decreased mitochondrial levels of (Mit)-Bcl-2, but increased cytosol levels of Bcl-2 in Jurkat cells (Figure 3C). In contrast, WAVE1 overexpression significantly increased mitochondrial levels of Mit-Bcl-2 in Jurkat cells. However, WAVE1 did not influence Bcl-XL and Bad localization (Figure 3C), indicating a specific role for WAVE1 in the regulation of Bcl-2 intracellular localization in leukemia cells.
Figure 3. WAVE1 regulates intracellular localization of Bcl-2 in leukemia cell.
(A) Localization of WAVE1 in leukemia cell. Jurkat and HL-60 cells were stained with WAVE1-specific antibodies (Green) and Mitotracker (Red) and nuclear Hoechst 33258 (Blue). (B) Localization of WAVE1 in mitochondria. Western blotting analysis of WAVE1 in cytoplasmic and mitochondrial fractions of Jurkat and HL-60 cells. The successful separation of cytoplasmic (“Cyt”) and mitochondria (“Mit”) fraction was confirmed by western blotting analysis of each fraction for known cytoplasmic (tubulin), mitochondria (mHSP70), nuclear (H3) and ER (PDI). (C) WAVE1 regulated Bcl-2 intracellular localization. Western blotting analysis of Bcl-2, Bcl-xL and Bad levels in cytoplasmic (“Cyt”) and mitochondria (“Mit”) fractions of Jurkat cells with or without WAVE siRNA or gene transfection. PDI and mHSP70 were used as fraction isolation quality control. * P < 0.05
WAVE1 binds Bcl-2 in mitochondria in leukemia cell
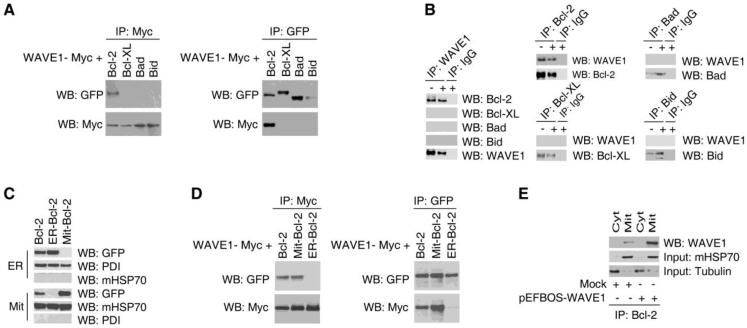
A recent study suggests that WAVE1 interacts with Bcl-XL in mitochondria in neuronal cells 16. In a sharp contrast, CoIP assay revealed an association between WAVE1 and Bcl-2 (but not Bcl-XL, Bad and Bid) in Jurkat cells co-transfected with WAVE1-myc and Bcl-2-GFP cDNA (Figure 4A). To confirm this interaction, we performed CoIP experiment using WAVE1 and Bcl-2 antibodies in Jurkat cells. Among many proteins tested, only Bcl-2 was found to be associated with WAVE1 in leukemia cells (Figure 4B). To determine the functional localization for WAVE1/Bcl-2 interaction, plasmids encoding a mitochondria-restricted Bcl-2 (Mit-Bcl-2 containing a mitochondrial insertion sequence of monoamine oxidase B), or ER-restricted Bcl-2 (ER-Bcl-2 containing a ER-specific sequence of cytochrome b5) 9 were transfected into Jurkat cells. Fractionation experiments revealed that Bcl-2 was detected only in the mitochondrial fraction of the Mit-Bcl-2-expressing Jurkat cells, but in the ER fraction of ER-Bcl-2-expressing cells (Figure 4 C). CoIP assay indicated an association between WAVE1 and Mit-Bcl-2, but not ER-Bcl-2 in Jurkat cells co-transfected with WAVE1-Myc, Mit-Bcl-2-GFP or ER-Bcl-2-GFP plasmids (Figure 4D). Consistently, CoIP assay of distinct subcellular fractions confirmed an interaction between WAVE1 and Bcl-2 in the mitochondrial fraction (Figure 4E), suggesting that WAVE1 functions as an important anchor protein for Bcl-2 in mitochondria in leukemia cells.
Figure 4. WAVE1 binds Bcl-2 in mitochondria in leukemia cell.
(A) CoIP assay Jurkat cells were transfected with plasmids encoding Myc-tagged WAVE1 and GFP-tagged Bcl-2, Bcl-xl, Bad and Bid. Cell lysates normalized for total protein content were analyzed directly or subjected to IP using anti-Myc or anti-GFP antibody and were analyzed by western blotting using anti-Myc or anti-GFP antibodies. (B) Endogenous WAVE1 bind Bcl-2. Jurkat cells were treat with or without ADM for 24 h, and lysates were prepared for IP with control IgG or anti-WAVE1, anti-Bcl-2, anti-Bcl-xl, anti-Bad, anti-Bid. The resulting immune complexes were analyzed by western blotting using antibodies recognizing WAVE1, Bcl-2, Bcl-xl, Bad, or Bid. (C) Jurkat cells were transfected with plasmids encoding GFP-tagged Bcl-2, mitochondria-restricted Bcl-2 (Mit-Bcl-2), ER-restricted Bcl-2 (ER-Bcl-2). Cells were fractionated into mitochondrial (Mit), and nuclear (ER), and were analyzed by western blotting. PDI and mHSP70 were used as fraction isolation quality control. (D) CoIP assay Jurkat cells were transfected with plasmids encoding Myc-tagged WAVE1 and GFP-tagged Bcl-2, mitochondria-restricted Bcl-2 (Mit-Bcl-2), and ER-restricted Bcl-2 (ER-Bcl-2). Cell lysates normalized for total protein content were analyzed directly or subjected to IP using anti-Myc or anti-GFP antibody and were analyzed by western blotting using anti-Myc or anti-GFP antibodies.
(E) Endogenous WAVE1 bind mitochondria Bcl-2. The cytosolic and mitochondria fractions were prepared and lysates were prepared for IP with anti-Bcl-2. The resulting immune complexes were analyzed by western blotting using antibodies recognizing WAVE1. The inputs were analyzed by western blotting using anti-mHSP70 and tubulin.
WAVE1 regulates Bcl-2-mediated ROS production in leukemia cell
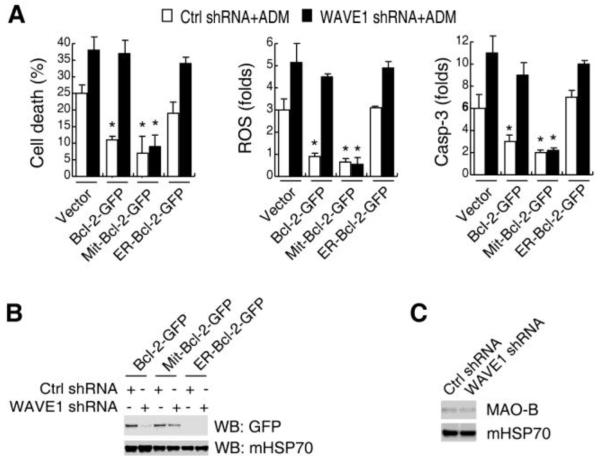
Bcl-2 is required to balance mitochondria ROS production and subsequent release of pro-death molecules 6-9. To determine the influence of WAVE1 on Bcl-2 function, we measured the ROS production and caspase-3 activity in cells transfected with various Bcl-2-expression or WAVE1- inhibition plasmid constructs. Transfection with wild type Bcl-2 and Mit-Bcl-2, but not ER Bcl-2-GFP decreased ADM-induced ROS production, caspase-3 activity, and cell death (Figure 5A), suggesting that Mit-Bcl-2 was important for mitochondria ROS production. Consistently, transfection of WAVE1-depleted (shRNA) cells with Mit-Bcl-2-GFP, but not the wild type Bcl-2, also blocked ADM-induced ROS production and apoptosis (Figure 5A). Furthermore, WAVE1 depletion impaired mitochondrial localization of wild type Bcl-2-GFP, but not Mit-Bcl-2-GFP (Figure 5B). The Mit-Bcl-2-GFP is constructed in human Bcl-2 gene by linking a mitochondrial targeting domain from monoamine oxidase B (MAO-B) protein. Similarly, the mitochondrial localization of MAO-B was not influenced by WAVE1 knockdown in Jurkat cells (Figure 5C), suggesting that WAVE1 regulated native Bcl-2 mitochondrial localization, rather than the restricted-mitochondrial targeting domain of MAO-B protein.
Figure 5. WAVE1 regulates Bcl-2-mediated ROS production in leukemia cell.
(A) Effects of WT Bcl-2, mitochondria-Bcl-2, and ER-Bcl-2 on ADM-induced cell death, ROS production, and caspase-3 activity with or without WAVE1 shRNA. Jukat cells were co-transfected with expression plasmids (Bcl-2-GFP, Mit-Bcl-2-GFP, and ER-Bcl-2-GFP) and/or shRNA plasmids (WAVE1 shRNA and control shRNA), then stimulated with ADM (1 μg/ml) for 24 h, and assayed ROS production, cell death and caspase-3 activity as indicated (n=3, * P < 0.05 versus vector group). (B) WAVE1 knockdown impaired mitochondria localization of nature Bcl-2, but not mitochondria-restricted Bcl-2. Jukat cells were co-transfected with expression plasmids (Bcl-2-GFP, Mit-Bcl-2-GFP, and ER-Bcl-2-GFP) and/or shRNA plasmids (WAVE1 shRNA and control shRNA), then isolated mitochondria, and western blotting assay GFP and mHSP70 levels. (C) No influence of WAVE1 on mitochondria localization of monoamine oxidase B (MAO-B) protein. Knockdown WAVE1 by shRNA in Jurkat cells, then isolated mitochondria, and western blotting assay MAO-B and mHSP70 levels.
WAVE1 regulates Bcl-2-mediated Ca2+ homeostasis in leukemia cell
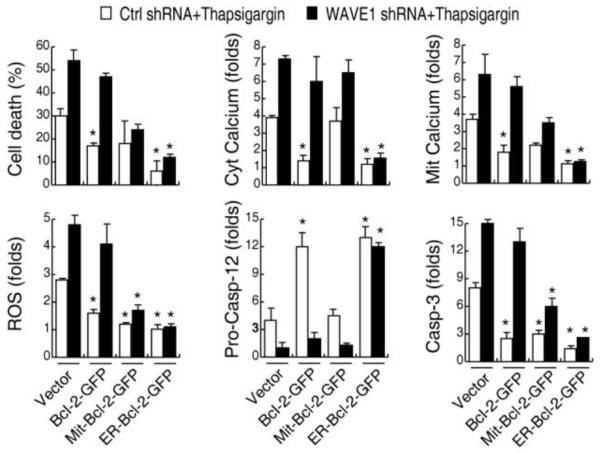
ER-targeted Bcl-2 protects cell following most stress such as staurosporine, brefeldin A/cycloheximide, tunicamycin, thapsigargin and ceramide 4. Over-expression of ER-Bcl-2 decreased thapsigargin-induced cytolic free Ca2+ and mitochondria Ca2+ load, ROS generation, and caspase-12 and -3 activity in control shRNA Jurkat cell (Figure 6). In contrast, over-expression of Mit-Bcl-2 did not inhibit ER-stress induced ER Ca2+ release and mitochondria Ca2+ load in control shRNA Jurkat cell, indicated that the function of Bcl-2 was dependent on its subcellular localizations. Interestingly, WAVE1 deletion led to Bcl-2 translocation from mitochondria to ER in leukemia cells (Figure 7A). The observations that WAVE1 knockdown increased thapsigargin-induced cytosolic-free/mitochondria Ca2+ level and cell death (Figure 6), and that transfection with wild-type Bcl-2 in WAVE1 shRNA cells did not block thapsigargin-induced cytolic free/mitochondria Ca2+ production and apoptosis (Figure 6), suggest that WAVE1 may not only regulates Bcl-2 subcellular localization, but also its intracellular functions.
Figure 6. WAVE1 regulates Bcl-2-mediated Ca2+ homeostasis in leukemia cell.
Jurkat cells were co-transfected with expression plasmids (Bcl-2-GFP, Mit-Bcl-2-GFP, and ER-Bcl-2-GFP) and/or shRNA plasmids (WAVE1 shRNA and control shRNA), then stimulated with thapsigargin (100 nM) for 24 h, and assayed cell death, ER Ca2+ release (cytosolic free calcium) and mitochondria Ca2+ load, ROS production, pro-caspase-12 level, and caspase3 activity as indicated (n=3, * P < 0.05 versus vector group).
Figure 7. WAVE1 regulates Bcl-2 phosphorylation in an ASK1/JNK-dependent pathway.
(A) WAVE1 knockdown increased Bcl-2 phosphorylation in ER. Knockdown WAVE1 by shRNA in Jurkat cells, then isolated mitochondria (Mit) and ER, and western blotting assay phosphorylation-Bcl-2 (p-Bcl-2) levels. The successful separation of cytoplasmic (“Cyt”) and mitochondria (“Mit”) fraction was confirmed by Western blotting analysis of each fraction for known mitochondrial (mHSP70, COXIV), nuclear (H3) and ER (PDI, Calnexin) markers. (B) Effects of Bcl-2 phosphorylation on ER stress-induced cell death. Jurkat cells expressing neo vector, Bcl-2 WT, or Bcl-2 AAA (non-phosphorylation Bcl-2) were treated thapsigargin (100 nM) for 24 h, and assayed cell death. * P < 0.05 versus Bcl-2 WT group. (C) Effects of Bcl-2 WT, Bcl-2 AAA with or without WAVE1 on ER stress-induced cell death. Jurkat cells were co-transfected with expression plasmids (Bcl-2-WT, and Bcl-2 AAA) and/or shRNA plasmids (WAVE1 shRNA and control shRNA), then stimulated with thapsigargin (100 nM) for 24 h, and assayed cell death. * P < 0.05 versus Bcl-2 WT group. (D) Effects of WAVE1 on ER stress-induced activation of JNK pathway. Jurkat cells expressing either control shRNA or WAVE1 shRNA vector were stimulated with thapsigargin at indicated dose for 30 min, and analyzed phosphorylation of JNK (p-JNK1/2), ASK1 (p-ASK1), p38 (p38), and RAC1 (p-RAC1) by western blotting. Data showed as the relative optical intensity (in arbitrary units, AU) of the immunoreactive bands on Western blots. UT, untreated group, set as 1. * P < 0.05 versus ctrl shRNA group. (E) Effects of JNK/ASK1 kinase and JNK inhibitor (SP600125) on ER stress-induced cell death with or without WAVE1. Jurkat cells expressing either control shRNA or WAVE1 shRNA vector were pretreated with kinase and inhibitor as indicated for 1 h and then treated with thapsigargin (100 nM) for 24 h, and analyzed cell death. (F) ASK1/JNK, but not RAC/CDC42/JNK mediated Bcl-2 phosphorylation. Jurkat cells were pretreated with kinase and inhibitor as indicated for 1 h and then treated with thapsigargin (100 nM) for 24 h, and analyzed p-Bcl-2 by western blotting. Tubulin was used as loading control. (G) A proposed model for WAVE1 regulated apoptosis by control of Bcl-2 intracellular localization and phosphorylation in leukemia cell. See text for details.
WAVE1 regulates Bcl-2 phosphorylation in an ASK1/JNK-dependent pathway
Since phosphorylation of Bcl-2 is implicated in regulating Bcl-2 function 27, 28, we further studied the role of WAVE1 in regulation of Bcl-2 phosphorylation. Consistent with previous studies 27, 28, phosphorylated Bcl-2 was located in the ER, but not mitochondria (Figure 7A). However, WAVE1 knockdown increased the phosphorylation of ER-bcl-2 (Figure 7A). Consistently, Jurkat cells expressing a nonphosphorylatable Bcl-2AAA (T69A, S70A, S87A) mutant were found to be more resistant to thapsigargin-induced cell death (Figure 7B) 27. Transfection with Bcl-2 AAA but not Bcl-2 WT in WAVE1 knockdown Jurkat cells decreased calcium production and cell death (Figure 7C and data not shown), suggesting that WAVE1 regulates Bcl-2 phosphorylation and function involving in ER Ca 2+ homeostasis.
The JNK pathway is responsible for phosphorylation of Bcl-2 in response to microtubule-damaging agents and calcium-dependent death stimuli 27-29. Depletion of WAVE1 expression led to an increase in thapsigargin-induced phosphorylation of JNK1/2, but not p38 (Figure 7D). Furthermore, WAVE1 depletion led to an increase in phosphorylation of ASK1 (apoptosis signal-regulating kinase 1), but not small GTPases RAC1 (Ras-related C3 botulinum toxin substrate 1) (Figure 7D). Pretreatment with JNK and ASK1 kinase, but not p38 and CDC42 kinase, promoted thapsigargin-induced Bcl-2 phosphorylation (Figure 7F) and cell death (Figure 7E), especially in control shRNA transfected Jurkat cells (Figure 7E). In contrast, JNK inhibitor SP600125, but not p38 inhibitor SB203580 decreased thapsigargin-induced Bcl-2 phosphorylation (Figure 7F) and cell death (Figure 7E). Collectively, these data indicate that WAVE1 regulates Bcl-2 phosphorylation in an ASK1/JNK-dependent pathway.
DISCUSSION
Although WAVE1-mediated actin-dependent morphological processes in mammals have been well known since the 1996 10, 30-32, its expression and function under pathological conditions is poorly understood. In the present study, we demonstrated that WAVE1 was expressed abundantly in human blood cancer cell lines, and barely in non-blood tumor cell-lines. To establish its clinical relevance, we determined WAVE1 expression levels in BMMCs derived from patients with childhood leukemia. WAVE1 expression was not detectable in PBMCs of normal healthy subjects, but detected at high levels in BMMCs of patients with ALL or ANLL, Furthermore, its expression levels correlated with the clinical symptoms: higher in active (e.g., primary and relapse) phase, and returning to normal in complete remission, implicating a potential contributory role of WAVE1 in the pathogenesis of leukemia. Furthermore, elevation of WAVE1 expression by gene transfection rendered leukemia cells resistant to apoptosis induced by VCR, ADM, AS2O3, which are widely used anti-cancer drugs in clinic leukemia. In contrast, suppression of WAVE1 expression by RNA interference increased the sensitivity of leukemia cells to anti-cancer drugs or ER stress (e.g. thapsigargin), suggesting an important anti-apoptotic role for WAVE1 in leukemia cells.
Our experimental data suggest that WAVE1 may be involved in the counter-regulation of ROS production and Ca2+ homeostasis in leukemia cells. ROS can be induced by many chemotherapeutic agents (such as ADM, AS2O3) and radiation therapy during cancer therapy 21, 33, and has been proposed as common mediators for apoptosis in leukemia cells 34. Recent studies have demonstrated that the mode of cell death depends on the severity of the oxidative damage 33. We found that suppression of WAVE1 expression by RNAi increased ADM- and thapsigargin-induced ROS production, and Ca2+ released into the cytoplasm from the ER. Alterations in Ca2+ homeostasis and accumulation of misfolded proteins in the ER cause ER stress that ultimately leads to apoptosis 2, 35. ER Ca2+ release leads to rapid calcium uptake and accumulation in mitochondria (ER/mitochondria coupling)36, leading to mitochondrial permeability transition pore opening and cytochrome c release prior onset of apoptosis 36.
Furthermore, WAVE1 confers anti-apoptotic activities potentially via controlling Bcl-2 intracellular localization and post-translational modification, which in turn regulates intracellular ROS levels and Ca2+ homeostasis. Bcl-2, an integral membrane protein located on the ER and mitochondria, is an important inhibitor of apoptosis, although its mechanism of localization is not fully understood 37, 38. Mitochondria bcl-2 prevents the increase in MOMP and protects cells from various death insults partly by regulated ROS 6, 24-26. Our experimental data suggest that WAVE1 may function as an anchoring protein for mitochondrial Bcl-2, because: 1) WAVE1 is co-immunoprecipitated with Bcl-2 from mitochondrial fraction of leukemia cells; 2) deletion of WAVE1 expression led to a parallel decrease in mit-Bcl-2 and increase in ER-Bcl-2, thus the mitochondrial localization of mature full-length Bcl-2 (Bcl-2-GFP) was impaired in WAVE1-depleted cells; and 3). Bcl-2 did not inhibit ADM-induced cell death in WAVE1-deficient leukemia cells.
In response to diverse stimuli including treatment with chemotherapeutic taxanes, Bcl-2 is phosphorylated on specific residues within an unstructured loop, and is associated with poor survival in leukemia patient 6-9. Bcl-2 may be phosphorylated by kinases of the JNK signaling pathway, and involved in the regulation of ER Ca2+ homeostasis and cell death during apoptosis and autophagy 27, 28. Thus, Bcl-2 regulates ER-to-mitochondrion communication by Ca2+ and thereby triggers mitochondrial dysfunction and cell death 37. Our experimental data suggest WAVE1 may be involved in the regulation of Bcl-2 phosphorylation, because depletion of WAVE1 expression led to an increase in thapsigargin-induced phosphorylation of JNK1/2, and an upstream signaling kinase ASK1. Indeed, the JNK and ASK1 have been reported to be activated by an ER-resident complex following ER stress 39.
Notably, the ability of the GFP tag to dimerize may potentially cause dimerization of GFP-Bcl-2 at the expense of interaction with other natural counterparts (e.g., Bad or Bcl-XL), which may partly explain why WAVE1 fails to interact with other Bcl-2-binding proteins (e.g., Bad or Bcl-XL). In addition, other adaptor proteins (such as Pancortin-2) are needed for WAVE1 to interact with Bcl-XL in neuronal cells, because disruption of pancortin-2 expression prevents WAVE1/Bcl-XL interaction in these cells 16. We do not know, however, whether such adaptor proteins (e.g., Pancortin-2) are expressed in human leukemia cells, and whether a potential lack of pancortin-2 expression accounts for the failure of WAVE1 to interact with Bcl-XL in human leukemia cells.
In summary, here we showed that WAVE1 was over-expressed in blood cancer cell lines, and functioned as a negative regulator of apoptosis. Enhanced expression of WAVE1 by gene transfection rendered leukemia cells more resistant to anti-cancer drug-induced apoptosis; whereas suppression of WAVE1 expression by RNA interference restored leukemia cells' sensitivity to anti-drug-induced apoptosis. WAVE1 formed complexes with Bcl-2 in the mitochondria, and regulated Bcl-2 localization and phosphorylation, thereby regulating mitochondrial ROS levels and ER Ca2+ homeostasis (Figure 7G). In light of the therapeutic promise of gene therapy to render tumor cells more susceptible to conventional therapies 40, our discovery of WAVE1 as a key Bcl-2 regulator in leukemia cells may lead to the development of novel drugs for treatment of human leukemia.
MATERIALS AND METHODS
Materials and plasmids
The antibodies to WAVE1, WAVE2, and monoamine oxidase B (MAO-B) were obtained from Santa Cruz Biotechnology (USA); The antibodies to GAPDH, and Tubulin were from sigma (USA); The antibodies to Bcl-2, Phospho-Bcl-2 (Ser70), Bcl-XL, Bad, Bid, H3, COXIV, Phospho-SAPK/JNK (Thr183/Tyr185), Phospho-p38 (Thr180/Tyr182), Phospho-ASK1 (Thr845), Phospho-Rac1/cdc42 (Ser71), GFP-Tag and Myc-Tag were from Cell Signaling Technology (USA); The antibody to Caspase 12 was from Millipore (USA); The antibody to mitochondria HSP70 (mHSP70) was from Abcam (USA); The antibody to PDI and Calnexin was from NOVUS (USA).
Vincristine (VCR), arsenic trioxide (AS2O3), adriamycin (ADM), thapsigargin, JNK inhibitor (SP600125), p38 inhibitor (SB203580), H2O2, and FCCP were from sigma (USA). JNK, ASK1, and p38 kinase were from Cell Signaling Technology (USA).
pEFBOS-WAVE1 or pEFBOS-WAVE2 expression vectors 32 was a gift from Dr. Tadaomi Takenawa (University of Tokyo). pcDNA3.1-Myc-WAVE1 was constructed from pEFBOS-WAVE1. WAVE1-siRNA was obtained from Santa Cruz Biotechnology. WAVE1 shRNA vector was from Sigma. Nonphosphorylatable of pcDNA3-Bcl-2AAA (replace Ser70, Ser87, or Thr69 with Ala), pcDNA3-Bcl-2 WT, GFP-Bcl-2, GFP-Mit-Bcl-2 [the mitochondrial targeting domain (amino acids 492–520) of monoamine oxidase B was used to replace amino acids 217–239 of human Bcl-2 gene] and GFP-ER-Bcl-2 [the ER-targeting domain of cytochrome b5 (amino acids 100–134) was used to replace amino acids 217–239 of human Bcl-2 gene] expression vectors were kind gifts from Dr. Stanley J. Korsmeyer and Dr. Clark W. Distelhorst as described previously 9, 29.
Cell culture
The human Jurkat T leukemia cells, Burkitt's lymphoma cell Raji, acute promyelocytic leukemia cell HL-60, chronic myelogenous leukemia cell K562 and multidrug-resistant cell HL-60/MX2, human lung A549 cancer cells, Hela cervical cancer cells, umbilical vein endothelial cell UVEC, lung fibroblast cell WI-38, liver cell QSG7701, MG-63 osteosarcoma cells, and CNE2 nasopharyngeal carcinoma cells (Xiangya School of Medicine Type Culture Collection, China, or ATCC, USA) were cultured in RPMI 1640 or DMEM medium (Life Technologies, USA) with 10% heat-inactivated FBS, 2 mM glutamine, and antibiotic-antimycotic mix in a humidified incubator with 5% CO2 and 95% air.
Gene transfection and RNAi
Expression vector transfection or co-transfection with WAVE1 shRNA using FuGENE® HD Transfection Reagent (Roche Applied Science, Sweden) or Lipofectamine 2000 reagent (Life Technologies, USA) according the manufacturer's instructions. Human WAVE1-siRNA or control-siRNA was transfected into cells using X-tremeGENE siRNA reagent (Roche Applied Science, Sweden) according to the manufacturer's instructions.
Cell viability assay
After drug treatment, cell viability was evaluated with Cell Counting Kit-8 (CCK8) (Dojindo, Japan) according to manufacturer's instructions.
Assessment of apoptosis
After drug treatment, cytocentrifuge preparations were stained with Hoechst 33258 and viewed by fluorescence microscopy to evaluate the extent of apoptosis as described previously 41. For each condition, 5 randomly selected fields, encompassing more than 500 cells, were scored. To confirm the results of morphologic analysis, flow cytometric analysis of annexin V/PI-stained cells was performed. In all studies, the results of morphologic assessment and annexin V/PI staining yielded highly concordant results.
Measurement of intracellular ROS and calcium
ROS was assessed with cell permeable dye CM-H2DCFDA (Molecular Probes), cytosolic-free Ca2+ using CaGreen-1 (Molecular Probes) and mitochondrial Ca2+ using Rhod-2 (Molecular Probes) according to manufacturer's instructions. Labeled cells were washed twice with PBS or calcium-free Krebs-Henseleit buffer (Sigma, USA). Analysis was performed using flow cytometry.
Preparation of cellular extracts
At the designated time after the treatment, cells were harvested. Mitochondrial and cytoplasmic purification was performed with Optiprep™ Mitochondria Isolation Kit (Pierce, USA) according to manufacturer's instructions. Crude ER purification was performed with Endoplasmic Reticulum Isolation Kit (Sigma,USA) according to manufacturer's instructions.
Patients and cell separation
We obtained diagnostic childhood acute lymphoblastic leukemia (ALL) and acute nonlymphocytic leukemia (ANLL) bone marrow samples after informed consent and with the approval of research ethics committee at Central South University. The diagnoses of ALL and ANLL were based on morphology and flow cytometric analysis of immunophenotype. BMMCs or PBMCs were isolated by Ficoll density gradient centrifugation.
Western blotting analysis
Proteins in whole-cell lysate or subcellular fractions were resolved on 10% SDS-PAGE gel, and transferred to a polyvinylidene fluoride membrane. After blocking the membrane at room temperature for 3 h, the membrane was incubated overnight at 4 °C with various primary antibodies. After incubation with peroxidase-conjugated secondary antibodies for 1 h at 25°C, the signals were visualized using enhanced chemiluminescence (Pierce, USA) 42, 43.
Immunoprecipitation analysis
Cells or cell fractionation were lysed at 4°C in ice-cold lysis buffer (50 mM Tris-HCl, pH 7.4, containing 150 mM NaCl, 1% NP-40, 0.5% Na-deoxycholate, 0.1% SDS, protease inhibitors cocktail), and cell lysates were cleared by a brief centrifugation (12000 g, 10 min). Concentrations of proteins in the supernatant were determined by the BCA assay. Prior to immunoprecipitation, samples containing equal amount of proteins were pre-cleared with Protein A or protein G agarose/sepharose (Amersham Biosciences) (4°C, 3 h), and subsequently incubated with various irrelevant IgG or specific antibodies (5 μg/mL) in the presence of protein A or G agarose/sepharose beads for 2 h or overnight at 4°C with gently shaking 42, 43. Following incubation, agarose/sepharose beads were washed extensively with PBS, and proteins eluted by boiling in 2 × SDS sample buffer before SDS-PAGE electrophoresis.
Caspase activity assay
Caspase-3 activity was assayed using the Caspase-3 Colorimetric Assay Kit (Calbiochem, Germany) according to manufacturer's instructions.
Immunocytochemical analysis the mitochondrial localization of WAVE1
Cells were fixed in 4% formaldehyde for 30 min at room temperature prior to cell permeabilization with 0.1% Triton X-100 (4°C, 10 min). Cells were saturated with PBS containing 2% BSA for 1 h at room temperature and processed for immunofluorescence with anti-WAVE1 antibody followed by Alexa Fluor 488-conjugated Ig and MitoTracker (Molecular Probes, USA). Between all incubation steps, cells were washed three times for 3 min with PBS containing 0.2% BSA. Fluorescence signals were analyzed using a confocal fluorescence microscope (Olympus, Japan).
Statistical analysis
Data are expressed as the mean±SEM. Significance of differences between groups was determined by two-tailed Student's t test or Fisher's LSD test. P<0.05 was considered significant.
ACKNOWLEDGMENTS
This work was supported by grants from The National Natural Sciences Foundation of China (30571982 and 30772353 to L.C., 30500485 to D.T.), Doctoral Program of Higher Education of China (20070533042 to L.C.), and supported in part by the National Institutes of Health grants (NIH/NIGMS, R01GM063075 and R01GM070817, to H.W.).
REFERENCES
- 1.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 2.Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–2614. doi: 10.1182/blood.v98.9.2603. [DOI] [PubMed] [Google Scholar]
- 3.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 4.Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 5.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 6.Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 7.Rudner J, Lepple-Wienhues A, Budach W, Berschauer J, Friedrich B, Wesselborg S, et al. Wild-type, mitochondrial and ER-restricted Bcl-2 inhibit DNA damage-induced apoptosis but do not affect death receptor-induced apoptosis. J Cell Sci. 2001;114:4161–4172. doi: 10.1242/jcs.114.23.4161. [DOI] [PubMed] [Google Scholar]
- 8.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Wang NS, Unkila MT, Reineks EZ, Distelhorst CW. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J Biol Chem. 2001;276:44117–44128. doi: 10.1074/jbc.M101958200. [DOI] [PubMed] [Google Scholar]
- 10.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 11.Gourlay CW, Carpp LN, Timpson P, Winder SJ, Ayscough KR. A role for the actin cytoskeleton in cell death and aging in yeast. J Cell Biol. 2004;164:803–809. doi: 10.1083/jcb.200310148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suetsugu S, Miki H, Takenawa T. Identification of two human WAVE/SCAR homologues as general actin regulatory molecules which associate with the Arp2/3 complex. Biochem Biophys Res Commun. 1999;260:296–302. doi: 10.1006/bbrc.1999.0894. [DOI] [PubMed] [Google Scholar]
- 13.Dahl JP, Wang-Dunlop J, Gonzales C, Goad ME, Mark RJ, Kwak SP. Characterization of the WAVE1 knock-out mouse: implications for CNS development. J Neurosci. 2003;23:3343–3352. doi: 10.1523/JNEUROSCI.23-08-03343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawe VY, Payne C, Navara C, Schatten G. WAVE1 intranuclear trafficking is essential for genomic and cytoskeletal dynamics during fertilization: cell-cycle-dependent shuttling between M-phase and interphase nuclei. Dev Biol. 2004;276:253–267. doi: 10.1016/j.ydbio.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 15.Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 16.Cheng A, Arumugam TV, Liu D, Khatri RG, Mustafa K, Kwak S, et al. Pancortin-2 interacts with WAVE1 and Bcl-xL in a mitochondria-associated protein complex that mediates ischemic neuronal death. J Neurosci. 2007;27:1519–1528. doi: 10.1523/JNEUROSCI.5154-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sung JY, Engmann O, Teylan MA, Nairn AC, Greengard P, Kim Y. WAVE1 controls neuronal activity-induced mitochondrial distribution in dendritic spines. Proc Natl Acad Sci U S A. 2008;105:3112–3116. doi: 10.1073/pnas.0712180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang R, Cao LZ, Yu Y, Hu T, Wang Z, Xu WQ, et al. Role of WAVE1 in drug resistance of K562/A02 leukemia cells. Zhonghua Xue Ye Xue Za Zhi. 2007;28:379–382. [PubMed] [Google Scholar]
- 19.He YL, Cao LZ, Yang J, Yang MH, Xu WQ, Xie M, et al. Expression of WAVE1 and p22phox in children with acute lymphocytic leukemia and the relationship of WAVE1 with oxidative stress. Zhongguo Dang Dai Er Ke Za Zhi. 2009;11:88–92. [PubMed] [Google Scholar]
- 20.Wang Z, Hu T, Cao LZ, Kang R, Zhao MY, Yu Y, et al. Expression of WAVE1 in childhood acute lymphocytic leukemia and in the apoptosis of Jurkat cells induced by adriamycin. Zhongguo Dang Dai Er Ke Za Zhi. 2008;10:620–624. [PubMed] [Google Scholar]
- 21.Lesser MP. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol. 2006;68:253–278. doi: 10.1146/annurev.physiol.68.040104.110001. [DOI] [PubMed] [Google Scholar]
- 22.Arai M, Yoguchi A, Takizawa T, Yokoyama T, Kanda T, Kurabayashi M, et al. Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca(2+)-ATPase gene transcription. Circ Res. 2000;86:8–14. doi: 10.1161/01.res.86.1.8. [DOI] [PubMed] [Google Scholar]
- 23.Nilsen J, Diaz Brinton R. Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci U S A. 2003;100:2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, et al. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu S, Eguchi Y, Kosaka H, Kamiike W, Matsuda H, Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995;374:811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- 26.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 27.Bassik MC, Scorrano L, Oakes SA, Pozzan T, Korsmeyer SJ. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. EMBO J. 2004;23:1207–1216. doi: 10.1038/sj.emboj.7600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanchoin L, Amann KJ, Higgs HN, Marchand JB, Kaiser DA, Pollard TD. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature. 2000;404:1007–1011. doi: 10.1038/35010008. [DOI] [PubMed] [Google Scholar]
- 31.Westphal RS, Soderling SH, Alto NM, Langeberg LK, Scott JD. Scar/WAVE-1, a Wiskott-Aldrich syndrome protein, assembles an actin-associated multi-kinase scaffold. Embo J. 2000;19:4589–4600. doi: 10.1093/emboj/19.17.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oda A, Miki H, Wada I, Yamaguchi H, Yamazaki D, Suetsugu S, et al. WAVE/Scars in platelets. Blood. 2005;105:3141–3148. doi: 10.1182/blood-2003-04-1319. [DOI] [PubMed] [Google Scholar]
- 33.Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96:2181–2196. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood. 2003;101:4098–4104. doi: 10.1182/blood-2002-08-2512. [DOI] [PubMed] [Google Scholar]
- 35.Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- 36.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 37.Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13:1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- 38.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 39.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brenner MK. Gene transfer and the treatment of haematological malignancy. J Intern Med. 2001;249:345–358. doi: 10.1046/j.1365-2796.2001.00807.x. [DOI] [PubMed] [Google Scholar]
- 41.Cuvillier O, Mayhew E, Janoff AS, Spiegel S. Liposomal ET-18-OCH(3) induces cytochrome c-mediated apoptosis independently of CD95 (APO-1/Fas) signaling. Blood. 1999;94:3583–3592. [PubMed] [Google Scholar]
- 42.Tang D, Kang R, Xiao W, Wang H, Calderwood SK, Xiao X. The Anti-inflammatory Effects of Heat Shock Protein 72 Involve Inhibition of High-Mobility-Group Box 1 Release and Proinflammatory Function in Macrophages. J Immunol. 2007;179:1236–1244. doi: 10.4049/jimmunol.179.2.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang D, Kang R, Xiao W, Jiang L, Liu M, Shi Y, et al. Nuclear Heat Shock Protein 72 as a Negative Regulator of Oxidative Stress (Hydrogen Peroxide)-Induced HMGB1 Cytoplasmic Translocation and Release. J Immunol. 2007;178:7376–7384. doi: 10.4049/jimmunol.178.11.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]