Abstract
Bone marrow endothelial cells (ECs) are essential for reconstitution of hematopoiesis, but their role in self-renewal of long term-hematopoietic stem cells (LT-HSCs) is unknown. We have developed angiogenic models to demonstrate that EC-derived angiocrine growth factors support in vitro self-renewal and in vivo repopulation of authentic LT-HSCs. In serum/cytokine-free co-cultures, ECs through direct cellular contact, stimulated incremental expansion of repopulating CD34−Flt3−cKit+Lineage−Sca1+ LT-HSCs, which retained their self-renewal ability, as determined by single cell and serial transplantation assays. Angiocrine expression of Notch-ligands by ECs promoted proliferation and prevented exhaustion of LT-HSCs derived from wild-type, but not Notch1/Notch2 deficient mice. In transgenic notch-reporter (TNR.Gfp) mice, regenerating TNR.Gfp+ LT-HSCs were detected in cellular contact with sinusoidal ECs and interfering with angiocrine, but not perfusion function, of SECs impaired repopulation of TNR.Gfp+ LT-HSCs. ECs establish an instructive vascular niche for clinical scale expansion of LT-HSCs and a cellular platform to identify stem cell-active trophogens.
Introduction
Maintenance of long-term hematopoietic stem cells (LT-HSCs) at steady state conditions and reconstitution after myeloablation are two distinct physiological processes that are dependent on extrinsic signals conveyed by the bone marrow (BM) niches (Till et al., 1964). Initial studies attempting to study the interaction of HSCs with their niches were centered on identifying soluble factors released by the BM stromal cells that support the expansion of LT-HSCs. Using serum-free cultures, it was demonstrated that a combination of soluble kit-ligand (sKitL), thrombopoietin, Flk-2, and angiopoietin-like factors could augment HSC expansion, with one study demonstrating a 24-fold expansion of LT-HSCs (Krosl et al., 2003; Miller and Eaves, 1997; Willert et al., 2003; Zhang et al., 2006). Others have shown that the introduction of HoxB4 and Notch-1, increases HSC numbers, as well (Antonchuk et al., 2002; Varnum-Finney et al., 2000). However, in these stroma-free cultures there is a rapid attrition of HSCs, suggesting that the interaction of the HSCs with BM stromal or vascular cells may be necessary to support long-term self-renewal and maintenance of HSCs.
Among the known BM stromal cells, osteoblasts/osteoclasts, “the osteogenic niche”, is believed to mediate the quiescent state and size of the phenotypically marked putative HSCs (Arai et al., 2004; Calvi et al., 2003; Heissig et al., 2002; Kollet et al., 2005, Zhang et al., 2003). Similarly, phenotypically marked HSCs, including CD150+CD48−CD41−KLS cells, localize to the BM endothelial cells (ECs), “the vascular niche” (Avecilla et al., 2004; Kiel et al., 2005; Kopp et al., 2006), raising the possibility that ECs might modulate HSC homeostasis. We have shown that BM sinusoidal ECs (SECs), demarcated by the VEGFR3+Sca1−VE-cadherin+VEGFR2+ SECs, constitute the predominant surface area of the BM vascular niche (BMVN) (Hooper et al., 2009) and are essential for engraftment and reconstitution of hematopoiesis. However, in this study the role of ECs in replenishing bona fide LT-HSCs was not evaluated. Thus, it remains to be determined whether ECs are essential for the self-renewal of LT-HSCs in vitro or BM repopulation in vivo. Furthermore, the mechanism by which ECs might support long-term expansion and lineage-specific differentiation of LT-HSCs remains unknown.
To define the role of ECs in LT-HSC self-renewal and maintenance, we have devised a model in which ECs are co-cultured with hematopoietic cells for weeks in serum- and cytokine-free conditions without diminishing their angiogenic potential or promoting cellular attrition (Ramalingam et al., 1999; Seandel et al., 2008; Zhang et al., 2004; Zhang et al., 2005). Herein, we demonstrate that co-culture of putative mouse HSCs in direct cellular contact with ECs in serum-free cultures, only supplemented with soluble Kit ligand (sKitL), is required for incremental self-renewal of LT-HSCs beyond 21 days, with 1 of every 4 cells representing an authentic repopulating LT-HSCs. ECs support self-renewal of LT-HSCs through elaboration of EC-derived stem-cell active paracrine growth factors and trophogens, referred to as angiocrine factors (Butler et al., 2010). Using the transgenic notch reporter (TNR.Gfp) mice, in which stimulation of the Notch signaling pathway results in GFP expression, we demonstrate that ECs support long-term expansion of TNR.Gfp+cKit+Sca-1+Lineage− (TNR.Gfp+KLS), but not Notch1-/- Notch2-/- CD34−Flt-3−KLS LT-HSCs. Blocking the angiocrine function of the SECs without interfering with the perfusion capacity of the SECs, results in impaired replenishment of TNR.Gfp+KLS cells. These results suggest that the vascular niche is an essential component of the BM microenvironment that balances the expansion and differentiation of LT-HSCs through expression of angiocrine factors that prevent the exhaustion of the repopulating LT- HSC pool.
Results
E4ORF1+ ECs maintain and expand LT-HSCs in serum- and cytokine-free conditions
The use of ECs to support long-term self-renewal of HSCs has been hindered by a lack of serum- and cytokine-free co-culture models in which ECs maintaining their angiogenic potential could be cultivated in vitro or selectively targeted in vivo. Depriving EC cultures of serum or essential EC-growth factors, results in apoptosis, thereby making it impractical to study the role of ECs in sustaining long-term HSC self-renewal. Furthermore, the requirement for supplementation of EC cultures with exogenous recombinant growth factors, such as FGF-2 and IGF, may mask the true potential of ECs in promoting LT-HSC expansion, as these growth factors could directly influence the expansion of stem cells. To circumvent these hurdles, we have developed a reproducible culture system, in which introduction of the adenoviral E4ORF1 gene into human primary ECs (E4ORF1+ ECs) allows for long-term cultures of ECs in serum- and cytokine-free conditions without compromising their proliferative and angiogenic properties (Seandel et al., 2008; Zhang et al., 2004; Zhang et al., 2005). To elaborate on the unique capability of E4ORF1+ ECs to survive under serum- and cytokine-free culture conditions and the potential to support HSCs, we compared E4ORF1+ ECs to other EC lines, including freshly isolated ECs (HUVECs), immortalized SV40 large T BM ECs (BMECs), and hTERT BMECs (Supplemental Figure 1A). Only E4ORF1+ ECs maintained their integrity in serum- and cytokine-free cultures, while other primary and immortalized EC cell lines underwent apoptosis (Supplemental Fig. 1 A,B). Therefore, E4ORF1+ ECs provide an ideal cellular platform to interrogate the role of ECs in regulating LT-HSCs homeostasis.
To study the role of ECs in expanding and maintaining LT-HSCs, lineage negative (Lin−) mouse BM-derived hematopoietic stem and progenitor cells (HSPCs) were co-cultured with E4ORF1+ ECs in serum- and cytokine-free conditions, supplemented only with mouse sKitL (Supplemental Fig. 2 A, B). Cultures were fed by replacing the serum- and cytokine-free media with sKitL every other day. Hematopoietic cells were then analyzed every 7 days by separating the Lin− from lineage positive (Lin+) and ECs (Supplemental Fig. 2 A, B). There was an increase in total number of Lin− and Lin+ hematopoietic cells over time (Supplemental Figure 2C,D). Within the Lin− population, there was a substantial increase in phenotypically marked KLS and Lin+ mature CD11b+Gr1+ myeloid and CD41a+ megakaryocytic precursor cells (Supplemental Fig. 2C,E), suggesting that E4ORF1+ ECs could support expansion of immature HSPCs as well as differentiation of these cells into mature precursor cells.
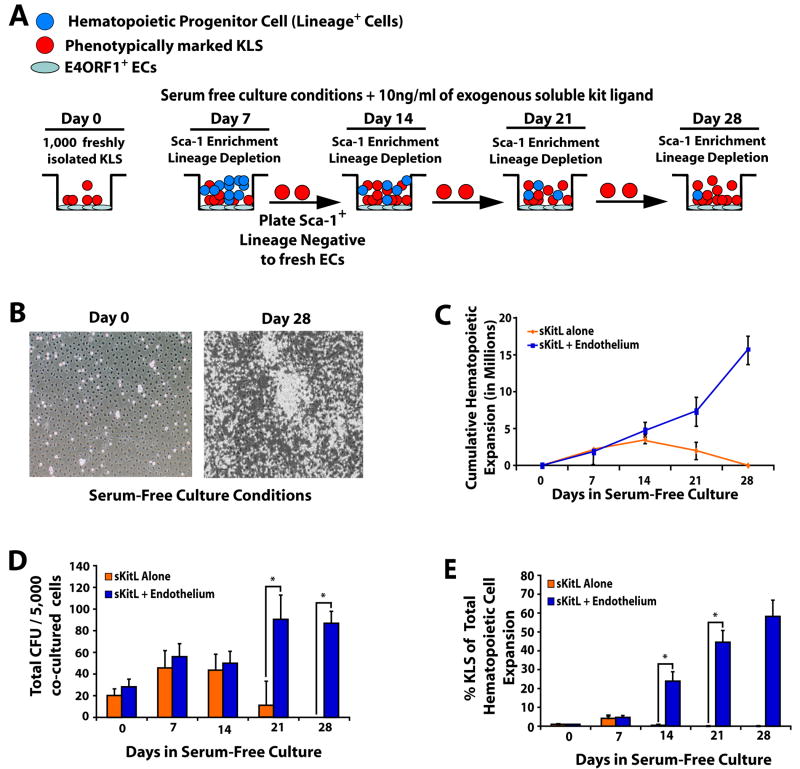
As generation of large numbers of Lin+ cells deplete the nutrients thereby compromising the true potential of E4ORF1+ ECs to self-renew LT-HSC, we developed a model to remove the expanding Lin+ mature cells during the co-culture period. To this end, 1000 KLS cells freshly isolated from the BM of the wild-type (WT) CD45.2+ mice were co-cultured with either E4ORF1+ ECs + sKitL or sKitL alone. Subsequently, every week the expanding Sca-1+Lineage− cells were separated from mature Lin+ cells and replated on E4ORF1+ ECs (Fig. 1A). Co-culture of KLS cells with E4ORF1+ ECs and sKitL resulted in a dramatic hematopoietic cell expansion for beyond 21 days without attrition of the EC monolayers (Fig. 1 B, C). Within the expanded hematopoietic cell population, we found an increase in progenitor cell numbers as determined by a colony forming cell assay (CFU, Fig. 1D) and a significant expansion of phenotypically marked KLS cells by four weeks of co-culture (Fig. 1E and Supplemental Fig. 3). Notably, KLS cells cultured in serum-free media and sKitL alone were unable to survive by four weeks (Fig. 1C).
Figure 1. Serum- and cytokine- free E4ORF1+ EC co-cultures expand phenotypically marked HSCs.
A) Schematic representing assay for serial ex vivo expansion of hematopoietic cells. B) Representative phase contrast micrographs of proliferating hematopoietic cells co-cultured with E4ORF1+ EC + sKitL at Day 0 and Day 28. C) Comparison of total lineage negative hematopoietic cell expansion from culture systems that included the conditions E4ORF1+ EC + sKitL or sKitL alone. D) Five thousand enriched Sca1+Lineage− cells from both culture conditions and were mixed with methylcellulose containing cytokines to generate total colony forming units (CFU). E) Total lineage negative hematopoietic cells were compared from culture conditions that included E4ORF1+ EC + sKitL or sKitL alone for HSC phenotypic expression of KLS. Error Bars represent SD based on three to four independent experiments. (*p<0.05, n = 10)
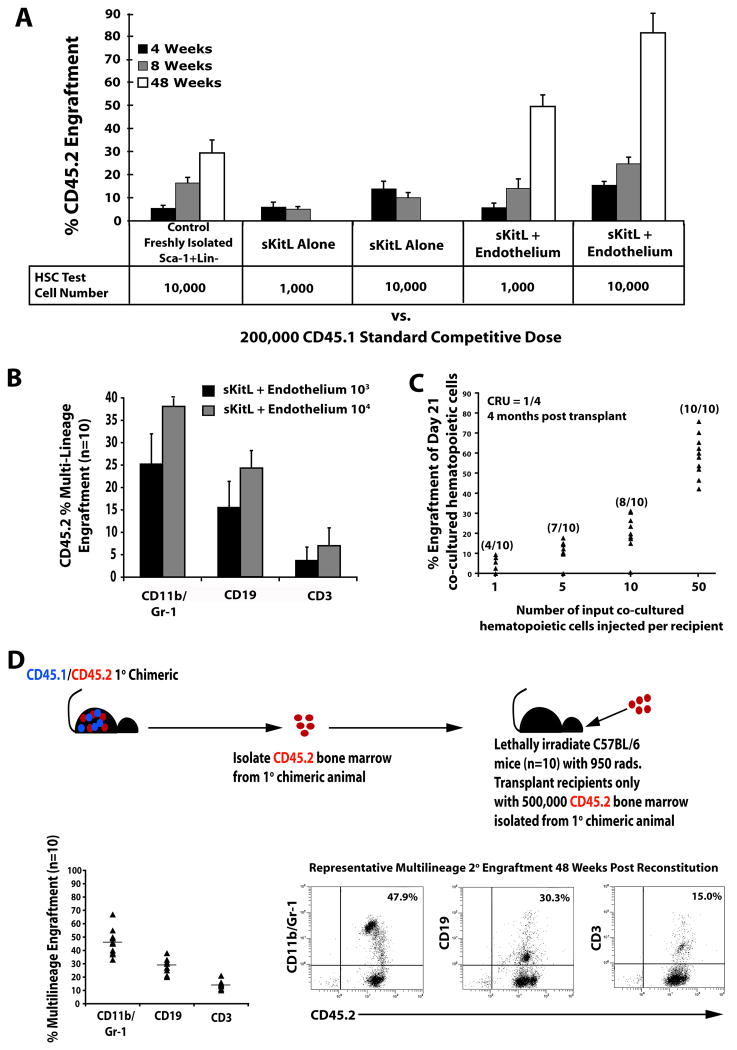
The increase in phenotypically marked KLS cells in EC co-cultures does not accurately reflect the number of repopulating LT-HSCs. Therefore, we examined the long-term repopulation capacity of expanded KLS cells in BM transplantation assays. First, we examined the stem cell potential of co-cultured KLS cells by performing a two-dose competitive repopulation assay, which revealed that as little as 1,000 KLS cells co-cultured with E4ORF1+ ECs + sKitL for 3 weeks supported long-term (> 12 months), multi-lineage engraftment in all transplanted mice (Fig. 2A,B,D). By contrast, KLS cells cultured with sKitL alone for three weeks only supported short-term engraftment (< 2 months) (Fig. 2A). To test the self-renewal potential of the LT-HSC co-cultured with E4ORF1+ ECs, we performed serial transplantation assays. Whole BM mononuclear cells were isolated from long-term, multi-lineage engrafted mice (Fig. 2A) and CD45.2+ hematopoietic cells, (progeny of cells co-cultured with ECs), were sorted and transplanted into lethally irradiated secondary recipients. By transplanting only CD45.2+ cells, it allowed us to demonstrate that the hematopoietic cells that were co-cultured with ECs were able to sustain long-term, multi-lineage engraftment in all secondary recipients (Fig. 2D).
Figure 2. Hematopoietic cells co-cultured with E4ORF1+ ECs and sKitL give rise to long-term multi-lineage engraftment and maintain self-renewal capacity.
A) Comparison of engraftment efficiency between 10,000 freshly isolated Sca1+Lineage− (Control) (n=10) vs. hematopoietic cells co-cultured for 3 weeks with E4ORF1+ ECs + sKitL (n=10) vs hematopoietic cells cultured for 3 weeks with sKitL alone (n=10), using a two dose competitive repopulation assay. As little as 1,000 and 10,000 hematopoietic cells co-cultured with E4ORF1+ ECs + sKitL gave rise to long-term multi-lineage engraftment and out competed the standard competitive dose of 200,000 freshly isolated CD45.1 whole BM. B) The percentage of long-term multi-lineage engraftment in both test cell numbers in the hematopoietic cells cocultured with E4ORF1+ ECs and sKitL. C) Limiting dilution assay to determine the frequency of LT-HSC in day 21 co-cultured hematopoietic cells. CRU of 1 in 4 was determined using Poisson statistics by L-calc software (Stem Cell Technologies). D) Whole BM was isolated from mice that had been long-term engrafted (Figure 2A) and CD45.2 cells were sorted and were transplanted into lethaly irradiated (950 Rads) secondary recipients (n=10), as depicted in schematic. The primary cells that were co-cultured with E4ORF1+ ECs and sKitL were able to sustain self-renewal, as is shown in the representative FACS dot plot showing long-term multi-lineage engraftment in all secondary recipients. Error bars represent SD.
To accurately quantify the frequency of true LT-HSC expanding in cellular contact with E4ORF1+ ECs, we performed a limiting dilution assay in which Day 21 co-cultured proliferating hematopoietic cells were injected at different doses of 1, 5, 10, and 50 Sca1+Lin− hematopoietic cells into lethally irradiated recipient mice along with a competitive dose of recipient BM. The frequency of long-term reconstituting cells (CRU) was calculated according to Poisson statistics and was found to be 1 out of every 4 cells (Fig. 2C). Thus, E4ORF1+ ECs provide a unique vascular platform that not only maintains, but also stimulates long-term expansion of true repopulating LT-HSCs under a serum- and cytokine-free microenvironment.
LT-HSCs derived from EC co-cultures do not exhibit signs of leukemia
One concern of being able to expand such a proliferative population of LT-HSCs is that KLS cells co-cultured with E4ORF1+ ECs may have acquired aberrant properties of leukemic cells. However, all of the mice transplanted with co-cultured LT-HSCs and those that had undergone secondary or single cell transplantation survived beyond one year and the circulating blood counts of primary, secondary, and single cell transplanted mice were comparable to non-transplanted control mice, manifesting normal white blood cell, hematocrit and platelet counts (Supplemental Fig. 4). These data suggest that co-cultured KLS cells have not undergone any leukemogenic transformation and ECs through secretion of stem cell active angiocrine factors sustain self-renewal of KLS cells.
ECs activate Notch signaling on co-cultured KLS cells
Endothelial cells have been speculated to modulate the homeostasis of normal and malignant organ-specific stem cells through elaboration of specific angiocrine factors, such as Notch ligands (Butler et al., 2010). In agreement with published reports (Fernandez et al., 2008; Karanu et al., 2000), we show by qPCR (Supplemental Fig. 5A) and protein expression (Supplemental Fig. 5C) that ECs express Jagged-1, Jagged-2 and Dll4 and Dll1 (data not shown), suggesting that activation of Notch may contribute to expansion of the co-cultured HSCs. Notch-1 is expressed on LT-HSCs and maintains HSC in an undifferentiated state (Calvi et al., 2003; Duncan et al., 2005; Wu et al., 2007). Over expression of the intracellular domain of Notch enhances HSC self-renewal (Varnum-Finney et al., 2000), while loss of Notch signaling impairs the maintenance of HSC both in vitro and in vivo (Calvi et al., 2003; Hadland et al., 2004). However, at steady state conditions maintenance of the HSCs has been shown to be independent of Notch signaling (Maillard et al., 2008; Mancini et al., 2005), but its role in reconstitution of HSCs after myeloablation remains unknown. Indeed, the role of Notch signaling after severe irradiation-induced hemangiogenic regression or repetitive myelosuppressive insults as is instituted in patients undergoing chronic chemotherapy is unknown. Therefore, we hypothesized that angiocrine expression of Notch-ligands by ECs promote self-renewal of HSCs through reducing the exhaustive differentiation into lineage-committed progenitors.
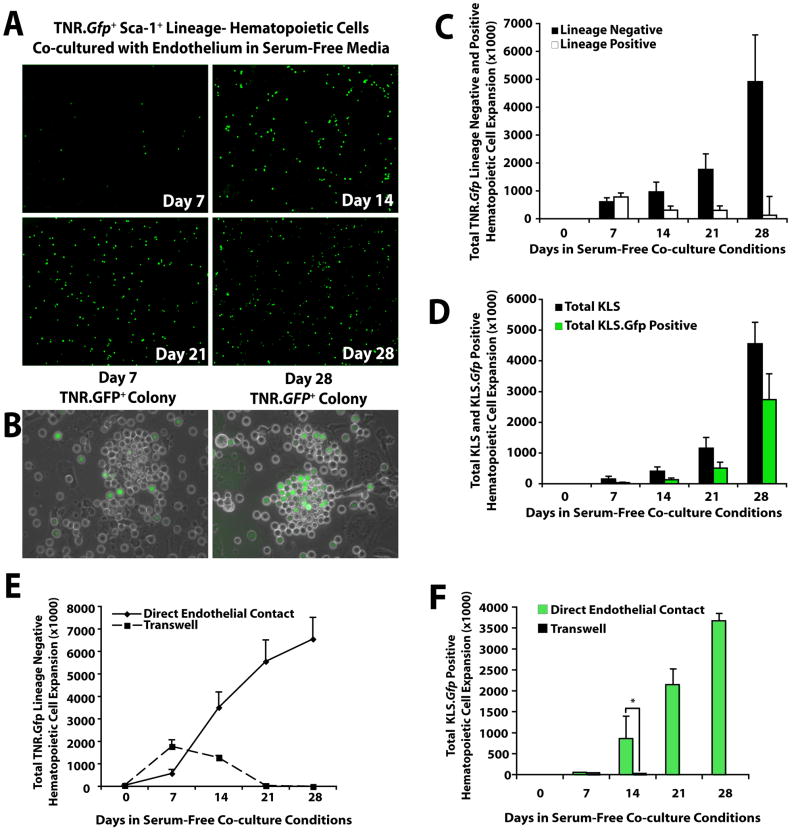
To test this hypothesis, we used a transgenic mouse that expresses the GFP reporter gene in cells that are actively signaling through Notch (transgenic Notch reporter mouse, TNR.Gfp) (Duncan et al., 2005; Mizutani et al., 2007). We found that hematopoietic cells derived from the BM of TNR.Gfp mice (TNR.Gfp hematopoietic cells) co-cultured with E4ORF1+ ECs increase Gfp expression over time (Fig. 3A), suggesting that ECs stimulate Notch signaling on hematopoietic cells. Furthermore, the majority of the hematopoietic cells isolated from TNR.Gfp mice that form colonies in co-culture with ECs were activated through Notch pathway expressing Gfp (TNR.Gfp+ hematopoietic cells) (Fig. 3B). Phenotypic analysis of the TNR.Gfp cells co-cultured with ECs for 4 weeks showed that 90% of the expanding cells comprised of Lin− hematopoietic cells (Fig. 3C,D) of which 60% comprised of TNR.Gfp+ HSPCs (Fig. 3D). Thus, Notch expression supports proliferation of immature hematopoietic cells in EC co-cultures by inhibiting excessive differentiation of HSPCs.
Figure 3. Notch signaling is activated in hematopoietic cells co-cultured with E4ORF1+ ECs and sKitL.
A) One thousand freshly isolated KLS hematopoietic cells from the BM of TNR.Gfp mice were co-cultured with E4ORF1+ ECs and sKitL. The degree of Notch signaling in the culture increased over time as indicated by enhanced Gfp expression (TNR.Gfp+ cells) in the expanding hematopoietic cells. B) Hematopoietic colonies that attach to ECs increase Notch signaling over time. C) Quantification of total number of TNR.Gfp+ Lin− versus Lin+ hematopoietic cell expansion. TNR.Gfp+ hematopoietic cells cultured in the absence of endothelium died by three weeks in culture. D) Phenotypic expression comparing the expansion of total KLS versus TNR.Gfp+ KLS cells expanded in the presence of E4ORF1+ ECs and sKitL. E,F) TNR.Gfp hematopoietic cells cultured in direct cellular contact with E4ORF1+ EC + sKitL or placed on the upper chamber of the transwells, physically separated from E4ORF1+ ECs. Lack of cellular contact of the TNR.Gfp hematopoietic cells with the E4ORF1+ EC monolayers, even in the presence of sKitL, results in a severe impairment of survival and diminished proliferation of the Lin− TNR.Gfp hematopoietic cells (E) and impaired expansion of TNR.Gfp+ KLS cells (F). By contrast, TNR.Gfp hematopoietic cells that were cultured in direct cellular contact with E4ORF1+ ECs and sKitL underwent significant expansion over a 28 day period, generating large numbers of Lin− TNR.Gfp (E) and TNR.Gfp+ KLS cells (F). Error bars represent SD. (*p<0.05, n = 6)
Expansion of TNR.Gfp+ KLS cells requires direct cellular contact with ECs
During co-culturing of TNR.Gfp KLS cells on E4ORF1+ ECs, a large number of Notch-activated TNR.Gfp+ hematopoietic cells forming cobblestone HSPC colonies were expanding attached to the ECs, (Fig. 3B), suggesting that hematopoietic cells required direct cellular contact with ECs to undergo expansion. To corroborate this finding, freshly isolated TNR.Gfp KLS cells were either co-cultured in direct cellular contact or physically separated from the E4ORF1+ EC monolayers by plating them on the upper chamber of 0.4 micron transwell plates. TNR.Gfp hematopoietic cells that were placed on the transwells were unable to expand (Fig. 3E, F), while the cells plated in direct cellular contact with E4ORF1+ ECs underwent significant expansion generating abundant colonies of both TNR.Gfp Lin− and TNR.Gfp+KLS cells (Fig. 3E, F). These data suggest that direct cellular interaction between ECs and HSPC is essential for Notch-activation supporting the expansion of LT-HSCs.
Blocking angiogenic pathways inhibits Notch-ligand expression and decreases expansion of LT-HSCs
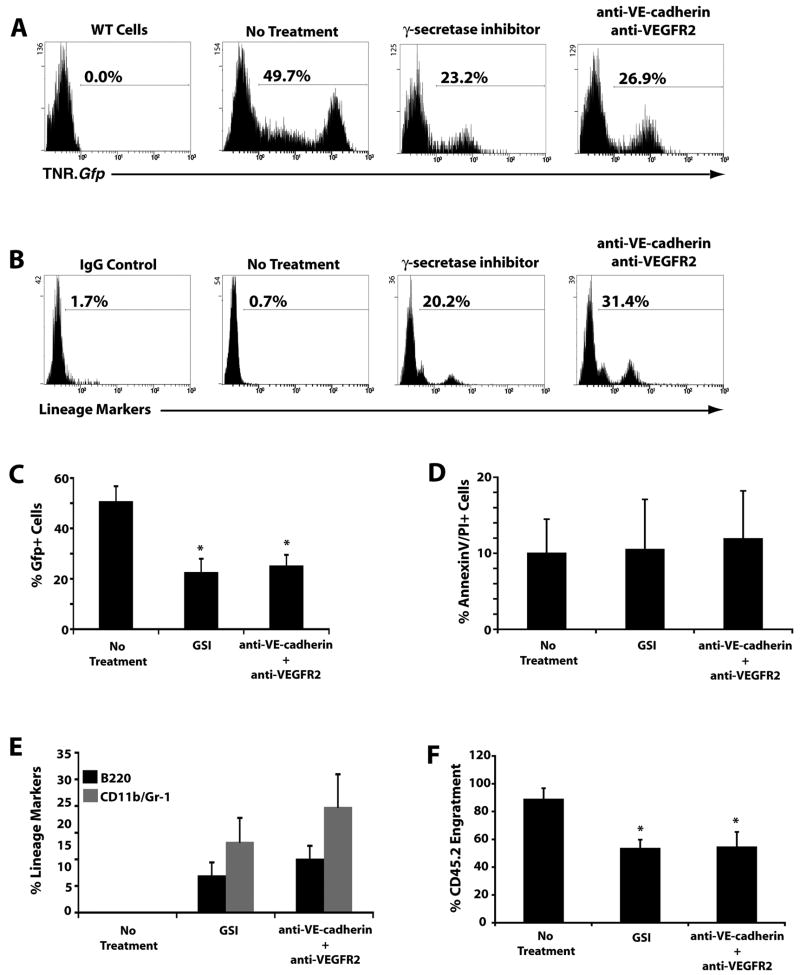
To formally determine whether ECs support the proliferation of LT-HSC through Notch signaling, we disrupted signaling pathways that maintain ECs angiogenic activity with neutralizing monoclonal antibodies (mAbs) to Vascular Endothelial-cadherin (VE-cadherin) and vascular endothelial growth factor receptor-2 (VEGFR2) that exert a synergistic effect in maintaining EC survival through Akt activation (Carmeliet et al., 1999). We also evaluated the role of Notch activation in expansion of LT-HSCs, by a γ-secretase inhibitor (Wolfe et al., 1999). Inhibition of the EC function with mAbs to VE-cadherin and VEGFR2 or γ-secretase inhibitor for 3 days induced a marked decrease in the expansion of TNR.Gfp+ hematopoietic cells in culture (Fig. 4A, C), favoring rapid differentiation of the expanding cells (Fig. 4B, E). There was no direct cytotoxicity caused by the mAbs on TNR.Gfp+ hematopoietic cells (Fig. 4D), suggesting that mAbs to angiogenic factors exert their effect by targeting ECs. Moreover, γ-secretase inhibitor did not manifest any hematopoietic toxicity (Fig. 4D). We next determined whether either γ-secretase inhibitor or abrogation of EC angiogenic activity would result in a decrease in engraftment efficiency of expanding co-cultured hematopoietic cells. We treated cocultures of CD45.2+ hematopoietic cells with E4ORF1+ ECs for 3 weeks, with mAbs to VE-cadherin and VEGFR2 or separately with the γ-secretase inhibitor for 3 days. We then transplanted 200,000 treated and untreated CD45.2+ hematopoietic cells co-cultured with ECs along with 200,000 CD45.1+ whole BM into lethally irradiated CD45.1+ recipients. Though all transplanted CD45.2+ (treated and untreated) cells out competed the CD45.1+ hematopoietic cells, the populations treated with mAbs to VE-cadherin and VEGFR2 or γ-secretase inhibitor engrafted poorly, suggesting that inhibition of angiogenic signaling, possibly through the impairment of Notch signaling, decreases the frequency of LT-HSC in co-culture with ECs (Fig. 4F).
Figure 4. Blocking angiogenic pathways in E4ORF1+ ECs decreases Notch signaling favoring hematopoietic cell differentiation.
A) Representative FACS histographs demonstrating a rapid decrease in Notch activity as reflected in a reduction in Gfp expression in TNR.Gfp hematopoietic cell population, when treated with the γ-secretase inhibitor or the vascular targeting antibodies. B) Representative FACS histograms showing a rapid increase in hematopoietic lineage markers when treated with the γ-secretase inhibitor or the vascular targeting antibodies. C) Quantification of Figure 4A (n=3 wells, 3 independent experiments). D) Annexin V/Propidium Iodide staining to determine if the treatment with the γ-secretase inhibitor or the vascular targeting antibodies caused a decrease in Gfp expression due to hematopoietic cell death. There was no change in cell viability after treatment. E) Quantification of Figure 4B (n=3, 3 independent experiments). F) Quantification of percent engraftment of 200,000 CD45.2+ test cells co-cultured with E4ORF1+ ECs and sKitL with or without treatment with the γ-secretase inhibitor or the vascular targeting antibodies against 200,000 standard competitive dose of CD45.1+ freshly isolated whole BM (*p<0.05, n=10 mice per treatment group).
Angiocrine expression of Notch-ligands by ECs reduces exhaustion of TNR.Gfp+ KLS cells
To determine whether angiogenic factors may regulate the expression of Notch-ligands on the ECs, we studied the expression patterns of Jagged-1 and Jagged-2 on the ECs. Using immunohistochemistry, we discovered that Jagged-2 expression was intracellular and did not localize to the cell surface membrane (Supplemental Fig. 5C). Surveying an array of pro-angiogenic cytokines showed that sKitL induces KLS cells to express and secrete VEGF-A164 (Supplemental Fig. 5B). Notably, when ECs were stimulated with VEGF-A164, Jagged-2 translocated to the surface of the ECs. Jagged-1 had no apparent change in protein localization following VEGF-A164 stimulation (Supplemental Fig. 5C). Thus, sKitL, through the induction of VEGF-A expression facilitates recruitment of Jagged-2 to the membrane of ECs thereby supporting the expansion of KLS cells.
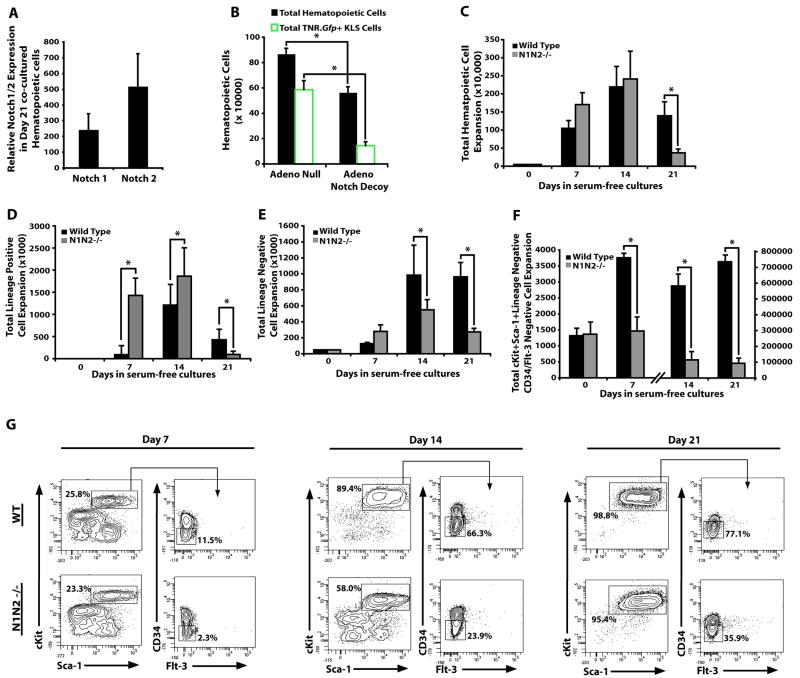
If Notch activation is essential for expansion of LT-HSCs, then expression of Notch receptors should be maintained on expanding immature hematopoietic cells. Using qPCR we found that both Notch1 and Notch2 were expressed on day 21 co-cultured hematopoietic cells (Fig. 5A). To determine the role of Notch signaling in expansion of LT-HSC EC co-cultures, we utilized two experimental approaches to selectively block Notch activation on the expanding hematopoietic cells. The first approach was to block Notch signaling on the hematopoietic cells in the co-culture with E4ORF1+ ECs by a high affinity decoy construct that comprises of the Notch1 receptor fused with IgG-Fc domain, neutralizing all of the potential Notch-ligands elaborated by the ECs (Funahashi et al., 2008). Expansion of TNR.Gfp+ hematopoietic cells that were co-cultured with E4ORF1+ ECs transduced with an adenoviral vector expressing the Notch decoy construct resulted in a profound decrease in proliferation of TNR.Gfp+KLS and total hematopoietic cells. Conversely, E4ORF1+ ECs transduced with a control adenoviral null vector (AdNull) supported the expansion of TNR.Gfp+KLS and other hematopoietic cells (Fig. 5B).
Figure 5. E4ORF1+ ECs prevent attrition of KLS cells through Notch activation.
A) Quantitative RT-PCR for assessing Notch 1 and Notch 2 expression on day 21 co-cultured hematopoietic cells. B) E4ORF1+ ECs were infected with adenovirus that overexpress a Notch decoy construct or an empty adenovirus. The Notch decoy significantly decreased the ability of E4ORF1+ ECs to expand total hematopoietic cells and total TNR.Gfp+KLS cells. C-F) Notch1-/-Notch2-/- lineage− (N1N2-/-) BM cells (25,000 cells) were co-cultured with E4ORF1+ ECs. BM cells were analyzed on weekly time points and were demi-depopulated each week. C) Total hematopoietic cell expansion was significantly decreased by day 21 in co-culture with Notch1-/-Notch2-/- versus WT BM cells. D) Total lineage− hematopoietic cell expansion was significantly decreased by day 21 in co-culture of Notch1-/- Notch2-/- versus WT BM cells with E4ORF1+ ECs. E) Total lineage+ hematopoietic cell expansion was significantly decreased by day 21 in co-culture of Notch1-/-Notch2-/- BM cells versus WT cells E4ORF1+ ECs. F) Total CD34-Flt3-KLS cells (containing a large number of phenotypically marked HSCs) were significantly decreased by day 14 in co-cultures of E4ORF1+ ECs with Notch1-/-Notch2-/-versus WT BM cells. G) Representative contour plots of phenotypic analysis of Notch1-/-Notch2-/-CD34− Flt3−KLS versus WT BM CD34−Flt3−KLS cells co-cultured for 7, 14, and 21 days with E4ORF1+ ECs. (*p<0.05, n = 6).
In a second approach, we determined whether selective deletion of Notch1 and Notch2 in undifferentiated LT-HSCs diminishes their expansion in co-cultures with ECs. Lineage− hematopoietic cells purified from the BM of Mx1-Cre-floxed Notch1/Notch2 mice, were treated with Poly I/C to generate Notch1-/-Notch2-/- mice. As control, Lin− hematopoietic cells were also obtained from the syngenic control WT mice. Equal numbers of WT control and Notch1-/-Notch2-/- Lin− BM cells were co-cultured on E4ORF1+ ECs with sKitL (Figure 5). Every 7 days, WT and Notch1-/-Notch2-/- hematopoietic cells were demi-depopulated and Lin− cells were replated on fresh E4ORF1+ ECs.
Analysis at day 21 demonstrated a significant decrease in the total number of hematopoietic cells, and phenotypically marked CD34−Flt-3−KLS HSCs within the expanding Notch1-/-Notch2-/- co-cultured cells (Fig. 5C-G). Notably, when the remaining Notch1-/-Notch2-/- KLS cells on day 21 were transplanted into lethally irradiated mice, the hematopoietic cells were able to engraft and support long-term hematopoiesis (data not shown). These data suggest that Notch1,2 has no affect on the functionality of the HSCs, but promotes expansion of the LT-HSCs by preventing the exhaustion of CD34−Flt-3−KLS LT-HSCs in the E4ORF1+ EC co-culture model. This finding sets forth the notion that angiocrine expression of Notch-ligands by ECs promotes long-term expansion of LT-HSCs.
Vascular targeting blocks angiocrine expression of Notch-ligands on perfused SECs
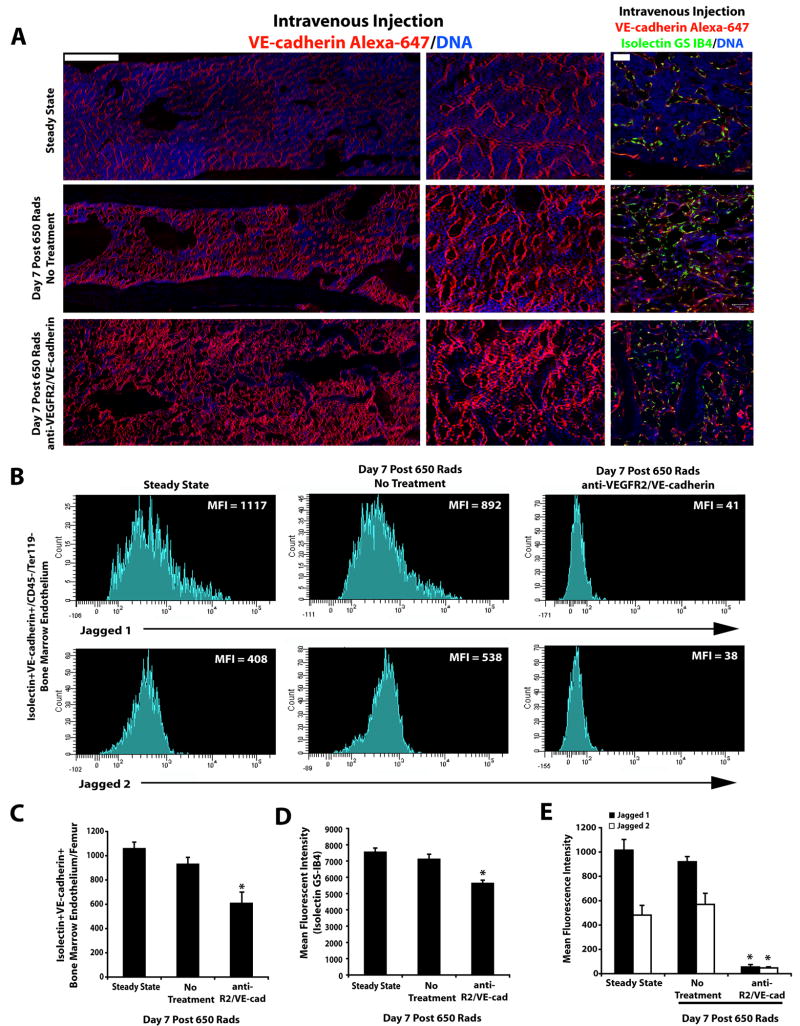
Although the angiocrine function of the ECs could readily be assessed in vitro, it is more cumbersome to determine whether myeloablation afforded by irradiation primarily impairs the angiocrine or the perfusion capacity of the SECs. Integrity of the SECs during regeneration from irradiation injury is dependent on proper signaling through EC-specific molecules; VEGFR2 and VE-cadherin. Activation of VEGFR2 is essential for reconstitution of hematopoiesis after sublethal irradiation (Hooper et al., 2009). However, in these studies the angiocrine role of the SECs and vascular functionality of the SECs in mediating the regeneration of repopulating LT-HSCs were not assessed. Thus, to determine the distribution of Notch-ligands on the functional perfused SECs, the control and vascular targeted 650-irradiated mice were intravenously injected with low doses of Alexa 647-VE-cadherin and Alexa 488-Isolectin 10 minutes before sacrificing the mice to visualize the number of perfused Type I vessels (Hooper et al 2009) (Figure 6A). Subsequently, polyvariate flow cytometry was used to quantify the number of functionally patent Isolectin+VE-cadherin+Ter119−CD45− SECs (Fig. 6C, D and Supplemental Fig. 6) that co-express either Jagged-1 or Jagged-2 in control and 650-irradiated mice following vascular targeting (Fig. 6B, E). Using this approach, we demonstrate that in untreated 650-irradiated mice (Figure 6A, middle panel) and strikingly, even in mice treated with both VEGFR2 and VE-cadherin mAbs (Figure 6A, lower panel), there was still a significant number of functional perfused Isolectin+VE-cadherin+Ter119−CD45− SECs present throughout the femur (Figure 6A, C and Supplemental Fig. 6). Jagged-1 and Jagged-2 were co-expressed on SECs and their expression levels were maintained on the functional SECs of the untreated and irradiated mice (Figure 6B,E). By contrast, treatment of the irradiated TNR.Gfp mice with mAbs to VEGFR2 and VE-cadherin downregulated the expression of Jagged-1 and Jagged-2 on the functional Isolectin+VE-cadherin+ SECs (Fig. 6B, E).
Figure 6. Selective vascular targeting diminishes the expression of Notch-ligands on the functional patent SECs.
A) Full length montage Z-stacks of femurs of mice under steady state conditions and 650 irradiated mice with and without vascular targeting that received intravenous injection of low dose VE-cadherin Alexa 647 (Red fluorescence) and Isolectin GS-IB4 Alexa 488 (Green fluorescence) to identify functional perfused vessels. B) Mean fluorescence intensity of Jagged-1 and Jagged-2 expression on the functional Isolectin+VE-cadherin+CD45−Ter119− BM endothelium at Steady State and Day 7 Post 650 irradiation with and without treatment with blocking mAbs to VEGFR2 and VE-cadherin. Note the dramatic decrease in mean fluorescence intensity of the Jagged-1 and Jagged-2 expression on the perfused patent Isolectin+VE-cadherin+ vessels in the cohort of mice that were treated with the neutralizing mAbs to VEGFR2 and VE-cadherin (as compared to Steady State and Day 7 650 irradiated No Treatment cohorts). C) Quantification of functional patent SECs. D) Mean fluorescent intensity of Isolectin GS-IB4 gated on VE-cadherin+ BM endothelium. Error bars represent SD. p≤0.05 as compared to No Treatment controls. E) Quantification of the mean fluorescence intensity of the Notch-ligands Jagged-1 and Jagged-2 expression. Error bars represent SD. (*p≤0.05 as compared to No Treatment controls). Scale Bars: Left and Middle Panels 500μm; Right Panels 50μm.
In the 650-irradiated mice treated with vascular targeting agents, the total number of individual ECs exposed to intravenously injected Isolectin and VE-cadherin was decreased (Figure 6C). However, a significant number of structurally disrupted type I dilated (Hooper et al 2009) but perfused Isolectin+VE-cadherin+ vessels were still present in the BM of these mice (Figure 6A, lower panel). Although the vascular-targeted mice maintained a large number of patent remodeling SECs, their instructive functions were impaired due to downregulation of Jagged-1 and Jagged-2 expression, suggesting that anti-angiogenic agents impair the angiocrine expression of Notch-ligands of perfused SECs.
Blocking the angiocrine function of SECs inhibits recovery of the repopulating HSC pool
To determine whether vascular targeting by downregulating the expression of Notch-ligands might directly block reconstitution of LT-HSCs, we used TNR.Gfp mice to spatially and temporally image the regeneration of the repopulating TNR.Gfp+ LT-HSCs during BM recovery model after 650 irradiation. Similar to WT mice, the TNR.Gfp mice had full hemangiogenic recovery (Fig. 7 A, B), while the TNR.Gfp mice treated with the neutralizing mAbs to both VE-cadherin and VEGFR2 had impaired restoration of the repopulating cells leading to the demise of the treated animals (Fig. 7A-C). Analysis of the BM ECs in the treated group showed the paucity of the regenerating TNR.Gfp+ hematopoietic cells (Fig. 6A and Fig.7A), while BM of control group manifested regeneration of the TNR.Gfp+ hematopoietic cells (Fig. 6A and Fig.7A). The difference in mouse survival was significant between the two cohorts, with the entire cohort of vascular-targeted TNR.Gfp mice dying by day 14 after sublethal radiation (Fig. 6 B, C). Examination of organs of the irradiated mice treated with mAbs to VE-cadherin and VEGFR2 did not show any apparent vascular damage, suggesting that the death of these mAb treated mice was due to persistent pancytopenia.
Figure 7. Selective disruption of SECs angiocrine function inhibits reconstitution of TNR.Gfp+ LT-HSCs.
A) Analysis of TNR.Gfp+ hematopoietic cells following 650 sublethally irradiation with or without administration of vascular targeting agents. There is a profound inhibition of TNR.Gfp+ hematopoietic cell regeneration and the impaired remodeling of BM ECs manifesting as disrupted and dilated ECs (red stain) in the vascular-targeted cohort (white arrowheads). (Scale Bar 50mm). TNR.Gfp+ cells reside in close proximity to the VE-cadherin+ SECs, and are often cellularly positioned in between the bone (osteoblastic cells) and vascular cells (white arrows and inserts in right panel of 650 rads treated alone mice). VE-cadherin+ ECs could be detected in the close proximity of the GFP+ osteoblasts in the Col2.3GFP mice (Insets), in which the expression of the GFP is restricted to the osteoblasts. B) Circulating blood counts in the control cohort vs. the vascular-targeted cohort. C) Survival curve of both cohorts. D) Comparison of total hematopoietic cell numbers and populations between the control cohort and the vascular targeted cohort at day 7 post sublethal irradiation. E) Total TNR.Gfp+ and TNR.Gfp− KLS cells per femur. Targeting the SECs inhibits the regeneration of Notch-dependent phenotypically marked LT-HSC. F) TNR.Gfp mice were sublethally irradiated with 650 Rads and at day 10 post sublethal irradiated BM was isolated and TNR.Gfp+Sca-1+Lineage- (TNR.Gfp+) and TNR.Gfp−Sca-1+Lineage− (TNR.Gfp−) were sorted. One hundred cells isolated from each of the sorted hematopoietic population were transplanted along with 300,000 NOD-SCID whole BM into irradiated NOD-SCID recipients (n=5). TNR.Gfp+Sca-1+Lineage− hematopoietic cells are highly enriched for LT-HSC. Note the majority of the TNR.Gfp-Sca-1+Lineage− hematopoietic cells were unable to give rise to long-term engraftment. Only one out of 5 mice showed long-term engraftment. Error bars represent SD. (*p<0.05, n = 10).
Since the VEGFR2/VE-cadherin vascular-targeted cohort began dying at day 10 post sublethal irradiation, we quantified the total and TNR.Gfp+ hematopoietic cells per femur at Day 7 post irradiation (Fig. 7A, D), which showed a significant decrease in total cell number and a complete abrogation in the recovery of TNR.Gfp+ hematopoietic cells (Fig 7D). It has been shown that TNR.Gfp+KLS population contains the LT-HSC, while the TNR.Gfp−KLS cells represents the short term repopulating-HSCs (ST-HSCs) (Wu et al., 2007). We found that approximately 7% of the regenerating hematopoietic cells at this time point were TNR.Gfp+KLS cells (Fig. 7E). Importantly, the vascular-targeted cohort failed to show any evidence of TNR.Gfp+ KLS cell expansion, but did contain TNR.Gfp−KLS cells at lower frequency as compared to the control group (Figure 7E). Upon transplantation of TNR.Gfp+Sca-1+Lineage− and TNR.Gfp−Sca-1+Lineage− cells, we found that the TNR.Gfp+Sca-1+Lineage− subpopulation was highly enriched for LT-HSC, while the majority of the TNR.Gfp-Sca-1+Lineage− cells were unable to sustain long-term hematopoietic engraftment (Fig. 7F).
In 650-irradiated mice treated with mAbs to either VE-cadherin or VEGFR2, there was also a trend towards a decrease in the number of TNR.Gfp+KLS population (Fig. 7E), suggesting that interfering with SEC function delays regeneration of repopulating hematopoietic cells. Inhibition of VE-cadherin was more effective than blocking the extracellular domain of VEGFR2 in diminishing reconstitution of TNR.Gfp+KLS cells. VE-cadherin inhibition through impairing the VEGFR2 intracellular signaling enhances the anti-VEGFR2 mAb effect (Carmeliet et al, 1999), which primarily inhibits the VEGFR2 extracellular-mediated signaling. Hence, the combination of VE-cadherin and VEGFR2 inhibition exerts a synergistic effect in impairing angiocrine function of SECs and regeneration of TNR.Gfp+KLS cells.
Since the majority of the TNR.Gfp+ cells comprise of LT-HSCs at day 7 post-sublethal irradiation (Fig 7D,E), the localization of the GFP+ regenerating hematopoietic cells could identify the specific niches that support the expansion of the repopulating hematopoietic cells. Thus, in vivo tracking of TNR.Gfp+ cells serves to precisely locate regenerating LT-HSC and ST-HSCs in the regenerating BM. We found that TNR.Gfp+ hematopoietic cells were closely associated with the VE-cadherin+ SECs (Fig 7A, 650 rads treated alone mice). Notably, every regenerating TNR.Gfp+ hematopoietic cells that were detected in close physical association with the osteoblastic zone were also detected in direct apposition to the SECs (Figure 7A, demarcated by the white arrows and inserts in right panel of 650 rads treated alone mice) and (Supplemental Figure 7). These data suggest that direct cellular interaction of repopulating LT-HSC with SECs, is critical for the expansion of the LT-HSCs following myelosuppression.
Discussion
The self-renewal and differentiation of LT-HSCs is regulated by interaction with niche cells. We have previously shown that SECs is essential for reconstitution of hematopoiesis after myeloablation (Hooper et al., 2009). However, whether ECs have the capacity to elaborate stem-cell active angiocrine factors to support the self-renewal of LT-HSCs and restore repopulating HSC pool were not explored. Here, we have devised physiologically relevant angiogenic models to demonstrate that ECs, through release of angiocrine factors, stimulate long-term in vitro self-renewal of LT-HSCs and in vivo reconstitution of the LT-HSC pool following myeloablation. Activation of Notch and c-Kit reduces the attrition of TNR.Gfp+ LT-HSCs that undergo coordinated self-renewal and differentiation in direct cellular contact with ECs. We show that Notch1-/-Notch2-/-CD34−Flt-3−KLS HSCs are functionally normal, but failed to expand in co-culture with ECs. Therefore, within the vascular niche, release of sKitL (Heissig et al., 2002) and angiocrine expression of Notch-ligands by structurally intact perfused ECs establishes an instructive niche for the restoration of LT-HCS pool. Collectively, we demonstrate that E4ORF1+ ECs establish an ideal vascular model not only to expand LT-HSCs for therapeutic BM transplantation, but also provides for an unlimited source of ECs to identify as yet unrecognized factors that collaborate with Notch and c-Kit signaling to balance LT-HSC expansion and lineage-specific differentiation.
To interrogate the role of the vascular niche in vivo, we disrupted the function of SECs with neutralizing mAbs to both VE-cadherin and extracellular domain of VEGFR2. Inhibiting both VE-cadherin and VEGFR2 was essential to impair the pro-hematopoietic function of the SECs after myeloablation, as compared to single antibody treatment with neutralizing mAb to either VE-cadherin or VEGFR2, which only resulted in a partial delay in hematopoietic recovery. The necessity to utilize both neutralizing mAbs was due to the inability of the VEGFR2 mAb to block the intracellular tyrosine kinase activity of VEGFR2. Indeed, as intrakine activity of VEGFR2 (Gerber et al., 2002) could still impart pro-angiogenic signals to SECs, we employed mAb to VE-cadherin to significantly diminish VEGFR2 signaling (Carmeliet et al., 1999). Using this approach, we demonstrated that inhibition of both VEGFR2 and VE-cadherin resulted in a profound decrease in the instructive functionality of SECs leading to the down-regulation of Notch-ligands, impaired reconstitution of TNR.Gfp+ cells, and demise of the treated mice (Supplemental Fig. 6 and Fig. 6). These data suggest that during hematopoietic recovery from myelodepletion, angiocrine expression of stem-cell active cytokines, such as Notch-ligands, by SECs replenish the TNR.Gfp+ LT-HSCs population restoring hematopoiesis.
The present study indicates that ECs are not passive conduits that support the delivery of oxygen and nutrients but also through secretion of EC-derived angiocrine factors restore LT-HSC population. We show that intact perfused SECs or in vitro cultured ECs, through expression of Notch-ligands, prevent exhaustion of LT-HSCs thereby replenishing the stem cell mass within the myeloablated BM. The remarkable decrease in the number of the TNR.Gfp+ HSCs in 650-irradiated mice treated with vascular-targeting agents suggests that Notch-ligand+ SECs directly promote the expansion of the TNR.Gfp+ HSCs. We show that even in the 650-irradiated mice treated with vascular targeting agents, a large number of structurally disrupted type I vessels (Hooper et al 2009) had maintained their functional perfusion patency but had lost their capacity to express Notch-ligands leading to a significant decrease in the regeneration of TNR.Gfp+ LT-HSCs and TNR.Gfp+ ST-HSCs. However, we cannot rule out the possibility that targeting SECs will not affect non-EC stromal cells that could also express Notch-ligands. Nonetheless, the finding that selective targeting of ECs interferes with regeneration of TNR.Gfp+ LT-HSCs and TNR.Gfp+ ST-HSCs indicates that SECs directly, independent of the non-EC stromal cells, modulate the balance between expansion and lineage-specific differentiation.
Then how do ECs by elaboration of specific angiocrine factors could support both self-renewal and differentiation of the LT-HSC and progenitors? It is plausible that activation state of the ECs might regulate the balance between expansion and lineage-specific differentiation of the LT-HSCs. We show here that upregulation of angiogenic factors favors the expression of Notch-ligands that shifts the balance towards expansion of LT-HSC at the expense of decelerating differentiation. By contrast, expression of other cytokines, such as SDF-1 and FGF-4 (Avecilla et al., 2004), which promote vascular-dependent thrombopoiesis in TPO-deficient mice favors differentiation of LT-HSCs. The precise mechanism by which activation state of the ECs through angiocrine release of cytokines balances LT-HSC expansion and differentiation remains to be studied.
We have used human ECs to support expansion of murine HSCs, suggesting that many stem cell active cytokines, such as Notch signaling pathway can function in a cross species manner. However, in the case of sKitL, lack of cross species reactivity of this cytokine necessitates supplementation of the medium with murine sKitL to facilitate murine HSC expansion. Soluble KitL synergizes with E4ORF1+ ECs to expand HSCs by two mechanisms, one by increasing the survival of HSC and also by inducing the release of VEGF-A (Heissig et al., 2002), which subsequently induces surface expression of Jagged-2 on the ECs. Therefore, E4ORF1+ ECs constitute an efficient vascular platform to cultivate Notch and cKit dependent subsets of LT-HSC in serum-free conditions that could be used to screen for instructive factors that support self-renewal of LT-HSCs.
Our study also sets forth the notion that angiocrine factors released by ECs in vivo contribute to the replenishment of the LT-HSC pool thereby reconstituting hematopoiesis. It is plausible that other as of yet unknown EC-derived angiocrine factors might collaborate with sKitL to orchestrate self-renewal of LT-HSCs, while Notch activation primarily prevent the exhaustion of LT-HSCs. The establishment of serum- and cytokine-free E4ORF1+ ECs provides for an ideal vascular niche model to identify angiocrine factors that foster long-term self-renewal and lineage-specific differentiation of HSCs. Factors that promote vascular regeneration and modulate angiocrine function of SECs offer novel therapeutic approaches to accelerate reconstitution of LT-HSCs after myeloablation as well as to expand plentiful of transplantable repopulating HSCs in vitro, thereby ameliorating life-threatening pancytopenia associated with chemo-irradiation. Furthermore, identification of angiocrine factors elaborated by SECs may also shed light on the mechanism by which myeloproliferative disorders evolve, thereby opening new avenues of research to employ anti-angiogenic agents to target pre-leukemic syndromes.
Experimental Procedures
Mice
All animal experiments were performed under the approval of Weill Medical College of Cornell University's Institutional Animal Care and Use Committee. C57BL/6J (CD45.2) and TNR.Gfp mice were used at 8 weeks of age. B6.SJL-Ptprca Pepcb/BoyJ mice were used as transplant recipients (CD45.1) and were 10 weeks of age. NOD.CB17-Prkdcscid/J mice were used at 10 weeks of age for transplantation of TNR.Gfp bone marrow (BM). Col2.3Gfp mice were obtained from Dr. David Rowe at the University of Connecticut.
Generation and maintenance of E4ORF1+ ECs
The Adenoviral E4ORF1 gene and/or Gfp derived from the serotype 5 adenoviruses were cloned into the lentivirus vector (Ramalingam et al., 1999; Seandel et al., 2008; Zhang et al., 2004; Zhang et al., 2005). Lentiviruses were generated by co-transfecting 15mg of lentiviral vector, 3mg of pENV/VSV-G, 5mg of pRRE and 2.5mg of pRSVREV in 293T cells (passage 8-10; subconfluent) by the calcium precipitation method. Medium was changed 24h after transfection, and supernatants were collected 40h and 64 hr after transfection. Supernatants were immediately sterile-filtered using surfactant-free cellulose acetate membranes, aliquoted and stored at -80°C.
Bone marrow (BM) endothelial cell (EC) isolation and flow cytometry
For quantification of the SECs (Figure 6A-C), mice were injected by retro-orbital sinus with 50 μg Isolectin GS IB4 conjugated to Alexa Fluor 488 (Alexa 488) and 10μg of VE-cadherin (clone BV13, ImClone) conjugated to Alexa Fluor 647 (Alexa 647). After 10 minutes, mice were sacrificed and whole BM from femur was mechanically denuded of muscle and any connective tissue and were then crushed in a sterile mortar. After 30 minutes of collagenase/dispase digestion at 37 C with agitation to release SEC from the BM microenvironment, the single cells were the number of SEC were quantified and by co-staining with conjugated antibodies to CD45 (30F-11 BD Pharmingen) and Ter119 (TER119 BD Pharmingen). The number of functional SECs was quantified by the number of Isolectin+VE-cadherin+CD45−Ter119− cells per femur. The intensity of the Isolectin expression was determined by calculating the Mean Fluorescent Intensity (MFI) with the FACSDiva software. Analysis of co-expression of Jagged-1 (R&D) or Jagged-2 (Biolegend) on the functional Isolectin+VE-cadherin+CD45− Ter119− SECs was determined by intracellular staining. Isolectin+VE-cadherin+CD45−Ter119− cells were fixed with 2% paraformaldehyde, permeabilized, and stained with either Jagged-1 or Jagged-2 directly conjugated to PE. BM SECs were analyzed using a LSRII SORP (BD Biosciences). All antibody staining was done at room temperature for 30 minutes.
Hematopoietic cell isolation, hematopoietic cell culture, and flow cytometry
Whole BM cells from C57BL/6J or TNR.Gfp mice were isolated and subjected to Ficoll-Paque (GE Healthcare) gradient separation to enrich for mononuclear cells. Cells were then enriched by three rounds of Lineage Cell Depletion (Miltenyi biotech) to ensure >95% purity. Cells underwent positive selection for Sca-1 (Miltenyi biotech) and were then stained with Sca-1 PE and cKit APC and sorted using a Aria II SORP (BD Biosciences). One thousand KLS cells were plated in a 12-well plate with or without E4ORF1+ ECs and both culture conditions consisted of X-Vivo serum-free media (Lonza) supplemented with 10ng/ml of soluble Kit Ligand (sKitL) (Biosource). Total hematopoietic cell expansion, total Sca1+cKit+Lineage− (KLS) and total CFU-C were analyzed at weekly time points, where another Sca1+Lineage− hematopoietic enrichment was performed. Total cell number was determined using an Advia 120 (Bayer) automated hematological analyzer. Colony assays were performed by placing 5,000 cultured hematopoietic cells into methycellulose based media (Methocult GF M3434 from Stem Cell Technologies) with cytokines and analyzed at Day 7 for total colony number. Hematopoietic cells were analyzed based on surface marker expression of c-Kit APC, Sca-1 PE-Cy7, CD34 FITC, Flt-3 PE and Lineage− Alexa 350 expression. The combination of the following antibodies defined the lineage markers: HM48-1 (anti-CD48), MWReg30 (anti-CD41), 145-2C11 (antibody to CD3 (anti-CD3)), 53-7.3 (anti-CD5), GK1.5 (anti-CD4), 53-6.7 (anti-CD8), RB6-8C5 Ly-6G (anti-Gr-1), M1/70 (anti-CD11b, Mac-1), Ter119 (anti-erythrocyte specific antigen) and 6B2 (anti-B220). Other antibodies used included clones 2B8 (anti-CD117, anti-c-Kit), D7 (anti-Ly-6A/E, anti-Sca-1), RAM34 (anti-CD34), and A2F10.1 (anti-CD135, Flt-3). All antibodies were purchased from Pharmingen or eBioscience. Hematopoietic cells were analyzed using a LSRII SORP (BD Biosciences).
HSC transplantation and LT-HSC repopulation studies
Freshly isolated CD45.2 Sca1+Lineage− hematopoietic cells were cocultured with or without E4ORF1+ ECs in X-Vivo serum-free media supplemented with 10ng/ml sKitL for three weeks. CD45.1 recipients were lethally irradiated (950 Rads) and transplanted with test limiting dilution cell numbers, as indicated, along with 200,000 CD45.1 standard competitive whole BM and hematopoietic engraftment was monitored at 4, 8, 16, 24, and 48 weeks by FACS analysis of peripheral blood for CD45.2/CD45.1 content (n=6 for control; n=10 for all test cell dilutions). Secondary transplants were performed by isolating whole BM from CD45.2/CD45.1 chimeric long-term multi-lineage engrafted mice. CD45.2 cells were sorted and transplanted into lethally irradiated CD45.1 recipients and engraftment was monitored up to 48 weeks. Single cell transplants were performed in three independent experiments (n=9, n=19, n=19 for each culture condition). CD45.2 hematopoietic cells were co-cultured with or without E4ORF1+ ECs in X-Vivo serum-free media supplemented with 10ng/ml sKitL for three weeks. Hematopoietic cells were Sca-1 enriched and lineage depleted (Lineage−, Lin−) prior to use. One, 5, 10, or 50 hematopoietic cells were mouth pipetted and mixed with 200,000 CD45.1 whole BM. The cell mixture was transplanted into CD45.1 lethally irradiated recipients. To test the repopulating ability of TNR.Gfp+KLS cells, 1000 TNR.Gfp+KLS cells were transplanted into irradiated NOD-SCID mice (3.5 Gy) with 300,000 NOD-SCID BM cells. Immunodeficient recipient mice were used because the TNR.Gfp mice are on an outbred CD1 background. Transplanted mice were bled every 4 weeks and peripheral blood analyzed to determine the level of donor-derived chimerism.
In vitro inhibition of Notch signaling
TNR.Gfp+ hematopoietic cells were cultured with E4ORF1+ ECs and sKitL for three weeks and then were treated for three days with a combination of monoclonal human specific neutralizing mAbs to VEGFR2 (1C11, 10μg/ml, ImClone) and VE-cadherin (BV9, 10μg/ml, ImClone) to selectively disrupt the E4ORF1+ ECs, 20μM of a γ-secretase inhibitor (Sigma) in 10% DMSO or 10% DMSO to serve as a no treatment control, or E4ORF1+ ECs were infected with adenoviruses (50 MOI) that expressed a Notch decoy or AdNull for control. Media (X-Vivo + 10ng/ml sKitL) and antibodies were replaced every day. Percentage of TNR.Gfp+ hematopoietic cells was analyzed by flow cytometry. The degree of differentiation of cultured cells was monitored by staining of hematopoietic cells for expression of lineage cell surface markers and was analyzed by flow cytometry.
Generation and co-culture of Notch1-/-Notch2-/- BM with E4ORF1+ ECs
Mice bearing both the floxed Notch1 and Notch2 genes were maintained and bred with transgenic mice bearing the interferon inducible Mx1-Cre gene at the Fred Hutchinson Cancer Research Center. To generate Notch1-/-Notch2-/- Mice the Mx1-Cre-Notch1fl/fl Notch2fl/fl 2-3 wk old mice were injected with 25-mg/kg solution of Poly I/C (InvivoGen) over a 3-week period. They first received a series of 5 ip injections every two to three days, followed by a 7- day break. Subsequently, Lin− hematopoietic cells were purified from the BM Notch1-/-Notch2-/- mice. As control, Lin− hematopoietic cells were also obtained from the syngenic control wild-type mice. Genotype analyses confirmed knock down of Notch1 and Notch2 on the hematopoietic cells. 25,000 Notch1-/-Notch2-/- lineage depleted BM cells were co-cultured on E4ORF1+ ECs with 50ng/mL of sKitL in twelve well plates. Both WT as well as the Notch1-/-Notch2-/- hematopoietic cells were analyzed every 7 days by demi-depopulating the expanding hematopoietic populations and replating 25,000 Lin− hematopoietic cells on fresh E4ORF1+ ECs. Total cell hematopoietic cell, lineage positive, lineage negative, and CD34−Flt-3−KLS cell expansion was quantified on a weekly basis.
Quantitative real-time PCR analysis
RNA was freshly isolated from sKitL-stimulated cKit+Lineage-Sca1+ (KLS) HSCs, E4ORF1+ ECs, or day 21 co-cultured hematopoietic cells using RNeasy (Qaigen) and was converted to cDNA using Superscript II (Invitrogen). Concentrations of cDNA were measured with a Nanodrop. Quantitative realtime PCR was done using a 7500 Fast Real-Time PCR System (Applied Biosystems) by mixing equal amounts of cDNAs, iQ SYBR Green Supermix (BioRad) and mouse VEGF-A164 (Fwd: AACAAAGCCAGAAAATCACTGTGA and Rev: CGGATCTTGGACAAACAAATGC) and β-actin (FWD: CGTGCGTGACATCAAAGAGAA and Rev: GGCCATCTCCTGCTCGAA). Oligo sequences for qRT-PCR of E4ORF1+ ECs are as follows: Dll1 (Fwd: CTACACGGGCAGGAACTGCAG and Rev: CGCCTTCTTGTTGGTGTTCTTG), Dll4 (Fwd: CGGGTCATCTGCAGTGACAAC and Rev: AGTTGAGATCTTGGTCACAAAACAG), Jagged1 (Fwd: GCTTGGATCTGTTGCTTGGTGAC and Rev: ACTTTCCAAGTCTCTGTTGTCCTG), and Jagged2 (Fwd: GCTATTTCGAGCTGCAGCTGAG and Rev: GCGGCAGGTAGAAGGAGTTG). Oligo sequences for qRT-PCR of day 21 co-cultured cells: Notch 1 (Fwd: GGATCACATGGACCGATTGC and Rev: ATCCAAAAGCCGCACGATAT) and Notch 2 (Fwd: CCCCTTGCCCTCTATGTACCA and Rev: GGTAGGTGGGAAAGCCACACT).
Selective targeting of BM vascular niche in vivo and HSC function
TNR.Gfp mice were sublethally irradiated (650 Rads) and were divided into two cohorts (n=20/cohort). One cohort was allowed to fully recover. The other cohort was treated with monoclonal mouse-specific neutralizing antibodies (mAb) to VEGFR2 (DC101, 800μg/mouse, ImClone) and VE-cadherin (BV13, 50μg/mouse, ImClone) to selectively target the vascular niche. Mice were treated every other day for a total of 5 intraperitoneal injections. In a separate experiment, the hematopoietic compartment (n=5/cohort) was analyzed at Day 7. Total hematopoietic cell number per femur was determined by flushing out femurs and counting using an hemacytometer. Total hematopoietic cells derived from TNR.Gfp mice and total TNR.Gfp+ and TNR.Gfp−KLS cells were determined by flow cytometry analysis and the percentage positive was applied to the total cell count per femur in order to calculate the total number per femur.
Immunostaining of bone sections and E4ORF1+ ECs
Freshly obtained bone specimens from TNR.Gfp that were sublethally irradiated (650 Rads), with or without anti-VEGFR2/anti-VE-cadherin at indicated time points, were fixed overnight in 4°C with 4% paraformaldehyde and were decalcified for two hours at room temperature. The bones were then washed with 1× PBS for 30 minutes and either prepared for frozen sections or paraffin sections. For frozen sections, bones were placed in 30% sucrose at 4°C O/N and were then embedded in Tissue-Tek Optimal Cutting Temperature (OCT). For paraffin sections, bones were placed in 30% ethanol and then shipped to Histoserv, Inc. Histoserv, Inc cut 4-6μm sections and performed hemotoxylin and eosin staining. For immunofluorescence, sections/cells were post-fixed for 15 minutes with 4% paraformaldehyde and then were blocked with 10% normal donkey serum/5% BSA/0.1% Triton-X 100 for 1 hour. Bones were then incubated with purified goat anti-mouse VE-cadherin antibody (R&D Systems, 1:50) O/N at 4°C, followed by 1 hour incubation at room temperature with anti-goat Cy3 fluorocrome (Jackson Immunoreasearch, 1:250). Primary ECs were grown to 70% confluency and then were either stimulated or unstimulated with mouse VEGF-A164 for 24 hours or three days. E4ORF1+ ECs were post-fixed for 15 minutes in 4% paraformaldehyde and blocked as described. E4ORF1+ ECs were then stained with a rabbit anti-human/mouse Jagged-2 or Jagged-1 antibody (SantaCruz Biotechnology) at 4°C overnight, followed by one hour incubation at room temperature with anti-rabbit Cy3 fluorocrome (Jackson Immunoreasearch, 1:250). Sections/cells were stained for 20 minutes at room temperature with TOPRO-3 nuclear counterstain (Molecular Probes, 1:2000). Samples were mounted using VectaShield (Vector Laboratories) and were viewed with appropriate filters. For immunohistochemistry, paraffin slides were deparaffinized, rehydrated and heat-induced antigen retrieved using Target Retrieval Solution (DAKO, USA). Slides were treated with 0.1% H2O2 for 10 minutes. Slides were blocked with 10% normal donkey serum/5% BSA/0.1% Triton-X 100 for 1 hour. Slides were Avidin/Biotin blocked (Vector Laboratories). Slides were stained with purified goat anti-mouse VE-cadherin antibody (R&D Systems, 1:100) O/N at 4°C. Following primary antibody incubation, sections were incubated with appropriate secondary pAbs as follows: biotinylated donkey anti-rat immunoglobulin G (IgG) or biotinylated donkey anti-goat IgG (Jackson Immunoresearch, 1:100). Staining was developed with horseradish peroxidase (HRP)-conjugated streptavidin (Jackson Immunoresearch) followed by development in AEC Ready-to-use (DAKO, USA) and brief counterstaining in Mayer's hematoxylin (DAKO
Peripheral-blood analysis
Retro-orbital blood was collected on indicated days after sublethal irradiation with capillary pipettes. Differential blood counts were obtained using an automated Advia 120 (Bayer). Error bars represent SD.
Supplementary Material
Acknowledgments
J.M.B. is supported by Starr Stem Cell Scholars Fellowship and Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant. M.S. is a Fiona and Stanley Druckenmiller Fellow of the New York Stem Cell Foundation. S.R. is supported by Howard Hughes Medical Institute, Ansary Stem Cell Institute, Anbinder and Newmans Own Foundation, National Institute of Health grants, HL66592, HL097797 and AI080309, Qatar National Priorities Research Program, Empire State Stem Cell Board and the New York State Department of Health grant NYS C024180.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- Butler JM, Kobayahsi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10(2):138–46. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Fernandez L, Rodriguez S, Huang H, Chora A, Fernandes J, Mumaw C, Cruz E, Pollok K, Cristina F, Price JE, et al. Tumor necrosis factor-alpha and endothelial cells modulate Notch signaling in the bone marrow microenvironment during inflammation. Exp Hematol. 2008;36:545–558. doi: 10.1016/j.exphem.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi Y, Hernandez SL, Das I, Ahn A, Huang J, Vorontchikhina M, Sharma A, Kanamaru E, Borisenko V, Desilva DM, et al. A notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesis. Cancer Res. 2008;68:4727–4735. doi: 10.1158/0008-5472.CAN-07-6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- Hadland BK, Huppert SS, Kanungo J, Xue Y, Jiang R, Gridley T, Conlon RA, Cheng AM, Kopan R, Longmore GD. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood. 2004;104:3097–3105. doi: 10.1182/blood-2004-03-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanu FN, Murdoch B, Gallacher L, Wu DM, Koremoto M, Sakano S, Bhatia M. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. 2000;192:1365–1372. doi: 10.1084/jem.192.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- Kopp HG, Hooper AT, Broekman MJ, Avecilla ST, Petit I, Luo M, Milde T, Ramos CA, Zhang F, Kopp T, et al. Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization. J Clin Invest. 2006;116:3277–3291. doi: 10.1172/JCI29314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krosl J, Austin P, Beslu N, Kroon E, Humphries RK, Sauvageau G. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat Med. 2003;9:1428–1432. doi: 10.1038/nm951. [DOI] [PubMed] [Google Scholar]
- Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, Pross SE, Aster JC, Bhandoola A, Radtke F, et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2:356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini SJ, Mantei N, Dumortier A, Suter U, MacDonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105:2340–2342. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- Miller CL, Eaves CJ. Expansion in vitro of adult murine hematopoietic stem cells with transplantable lympho-myeloid reconstituting ability. Proc Natl Acad Sci U S A. 1997;94:13648–13653. doi: 10.1073/pnas.94.25.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Rafii S, Shapiro F, Pettengell R, Ferris B, Nachman RL, Moore MA, Asch AS. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353–3363. [PubMed] [Google Scholar]
- Ramalingam R, Rafii S, Worgall S, Brough DE, Crystal RG. E1(-)E4(+) adenoviral gene transfer vectors function as a “pro-life” signal to promote survival of primary human endothelial cells. Blood. 1999;93:2936–2944. [PubMed] [Google Scholar]
- Seandel M, Butler JM, Kobayashi H, Hooper AT, White IA, Zhang F, Vertes EL, Kobayashi M, Zhang Y, Shmelkov SV, et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc Natl Acad Sci U S A. 2008;105:19288–19293. doi: 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till JE, McCulloch EA, Siminovitch L. A Stochastic Model of Stem Cell Proliferation, Based on the Growth of Spleen Colony-Forming Cells. Proc Natl Acad Sci U S A. 1964;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Moore CL, Leatherwood DD, Ostaszewski B, Rahmati T, Donkor IO, Selkoe DJ. Peptidomimetic probes and molecular modeling suggest that Alzheimer's gamma-secretase is an intramembrane-cleaving aspartyl protease. Biochemistry. 1999;38:4720–4727. doi: 10.1021/bi982562p. [DOI] [PubMed] [Google Scholar]
- Wu M, Kwon HY, Rattis F, Blum J, Zhao C, Ashkenazi R, Jackson TL, Gaiano N, Oliver T, Reya T. Imaging hematopoietic precursor division in real time. Cell Stem Cell. 2007;1:541–554. doi: 10.1016/j.stem.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug C, Lodish HF. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Cheng J, Hackett NR, Lam G, Shido K, Pergolizzi R, Jin DK, Crystal RG, Rafii S. Adenovirus E4 gene promotes selective endothelial cell survival and angiogenesis via activation of the vascular endothelial-cadherin/Akt signaling pathway. J Biol Chem. 2004;279:11760–11766. doi: 10.1074/jbc.M312221200. [DOI] [PubMed] [Google Scholar]
- Zhang F, Cheng J, Lam G, Jin DK, Vincent L, Hackett NR, Wang S, Young LM, Hempstead B, Crystal RG, et al. Adenovirus vector E4 gene regulates connexin 40 and 43 expression in endothelial cells via PKA and PI3K signal pathways. Circ Res. 2005;96:950–957. doi: 10.1161/01.RES.0000165867.95291.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.