Abstract
Microparticle drug delivery systems have been used for over twenty years to deliver a variety of drugs and therapeutics. However, effective microencapsulation of proteins has been limited by low encapsulation efficiencies, large required amounts of protein, and risk of protein denaturation. In this work, we have adapted a widely used immobilized metal affinity protein purification strategy to non-covalently attach proteins to the surface of microparticles. Polyketal microparticles were surface modified with nitrilotriacetic acid-nickel complexes which have a high affinity for sequential histidine tags on proteins. We demonstrate that this high affinity interaction can efficiently capture proteins from dilute solutions with little risk of protein denaturation. Proteins that bound to the Ni-NTA complex retain activity and can diffuse away from the microparticles to activate cells from a distance. In addition, this surface modification can also be used for microparticle targeting by tethering cell-specific ligands to the surface of the particles, using VE-Cadherin and endothelial cells as a model. In summary, we show that immobilized metal affinity strategies have the potential to improve targeting and protein delivery via degradable polymer microparticles.
Keywords: Affinity, Drug delivery, Growth factors, Microsphere, Nickel, Surface modification
1. Introduction
Hydrophobic, biodegradable polymers, such as poly(lactic-co-glycolic acid) and polyketals, have been widely used to deliver drugs to diseased tissues. There is currently great interest in using these polymers for the delivery of proteins, however protein-based therapeutics present unique challenges for drug delivery vehicle design. Microencapsulation of traditional pharmaceuticals (small molecules) has been straightforward in hydrophobic biodegradable polymers due to good drug solubility in organic solvents and stability over a wide range of temperatures. However, the secondary and tertiary structures of proteins strongly dictate bioactivity and are sensitive to processing conditions such as temperature and exposure to organic solvent. These properties have made it more difficult to microencapsulate proteins and limited the widespread use of protein delivery via microparticles.
A common procedure used to encapsulate proteins in hydrophobic polymer microparticle systems employs a double emulsion, solvent evaporation procedure [1-3]. In this process, the protein is typically dissolved at a high concentration in aqueous medium and emulsified into an oil phase that includes the polymer matrix. This first emulsion is then homogenized in a second water phase, forming the second emulsion that gives rise to the microparticle shape. Polymers such as PLGA and the polyketal, poly(cyclohexane 1,4-diylacetone dimethylene ketal) (PCADK), have been used successfully to deliver proteins in vivo, but the techniques used to encapsulate the proteins are not optimal [4-7]. During the drying process, the protein is exposed to organic solvent, which frequently damages its structure/activity. Similarly, the protein can also diffuse out of the microparticle while organic solvent evaporates, reducing encapsulation efficiency. Approaches such as solid/oil/water emulsions have attempted to address these issues, but they require additional processing steps and use hydrophilic polymer carriers, such as poly(ethylene glycol) (PEG) and sodium alginate, to protect proteins from denaturation [8, 9].
In an effort to avoid denaturation due to organic solvents, alternative protein delivery systems have focused on hydrophilic materials to achieve sustained delivery of proteins. Most of these approaches use crosslinked biologically derived polymers, such a gelatin or alginate, or synthetic polymers, such as PEG, to form a matrix around polymers, thus reducing diffusivity. A variety of crosslinking strategies, including covalent [10-12], ionic/electrostatic [13], and mechanical [14] strategies have been used to form drug delivery vehicles with these polymers. While these strategies have shown success in reducing protein diffusion and have even seen some success in vivo, the larger pore sizes limit their use with small molecule therapeutics.
Immobilized metal affinity chromatography (IMAC) techniques have been used to purify recombinant proteins for over thirty years and are well suited for protein delivery since they rely on non-covalent, reversible interactions with proteins[15, 16]. IMAC works by immobilizing chelating chemical groups on a substrate, which in turn chelates a bivalent metal ion (typically Ni+2, Cu+2, or Co+2). The use of nitrilotriacetic acid (NTA) for IMAC was reported in 1987 by Hochuli, et al. and has become one of the more widely used chemistry in commercially available products [16]. The bond strength for these types of interactions have been estimated to be in the 200-400 pN range by using single molecule AFM studies [17]. Studies have been performed varying the number of histidine residues on the protein as well as the valency of NTA on the capture substrate in order to tune dissociation constants and thus provide a pathway for changing release kinetics [18, 19]. This metal complex binds sequential histidine residues on proteins and interacts with a high affinity ranging from 13 mM to 1.2 nM depending on NTA valency [20, 21].
Recently, NTA chemistry has been employed for a variety of new applications outside chromatography. Polystyrene microparticles have been modified with NTA for flow cytometric analysis of proteins [22]. NTA has also been used in lipid bilayers to examine protein interactions; 1,2-dioleoyl-sn-glycero-3- [N (5-amino-1-carboxypentyl) iminodiacetic acid] succinyl (DOGS-NTA), a lipid conjugate of NTA, was used to create two dimensional protein crystals and study protein-protein interactions on supported lipid bilayers [23-25]. Despite these new applications, NTA has seen limited use for drug delivery purposes. Researchers have functionalized liposomes with DOGS-NTA for capturing and presenting His6-tagged proteins [26-29]. In addition, poly(ethylene glycol) hydrogels have been functionalized with NTA in order to retard the release rates of proteins encapsulated in the hydrogels [30-32].
The work presented here focuses on applying IMAC chemistry to surface functionalize polyketal microparticles in order to non-covalently tether proteins to polyketal microparticle surfaces. We have adapted NTA, which is traditionally used to purify recombinant proteins, in order to achieve completely aqueous loading of proteins onto the surface of the microparticle. This reduces the risk of denaturation as well as provides a platform for microparticle targeting and dual delivery.
2. Materials and Methods
2.1 PCADK synthesis and microparticle fabrication
PCADK was synthesized as described previously[4, 33-35]. Microparticles were made in 100 mg batches via a single emulsion procedure. 1,2-dioleoyl-sn-glycero-3- [(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (DOGS-NTA, Avanti Polar Lipids) was dissolved in dichloromethane at 10 mg/ml. The following procedure was used to produce 10 wt% NTA microparticles; other concentrations were obtained by varying the amount of DOGS-NTA stock solution and neat dichloromethane. All NTA percentages in this manuscript refer to weight percentage of the microparticles. Ninety milligrams of PCADK was added to 1 ml of DOGS-NTA stock solution and gently heated to dissolve the polymer. Five milliliters of 4% poly(vinyl alcohol) (PVA) in water was added to the polymer solution and the mixture was homogenized using a Powergen 500 (ThermoFisher) homogenizer for 60 seconds. The resulting emulsion was transferred to 30 ml of 1% PVA and stirred for 4-6 h to evaporate the dichloromethane. Particles were then centrifuged and washed with deionized water before being frozen in liquid nitrogen and lyophilized to produce a free flowing powder. Microparticles were subsequently loaded with Ni+2 by incubating overnight in a 0.05 M NiCl2 solution at a concentration of 1 mg particles/ml.
2.2 Cell culture
RAW 264.7 macrophages were cultured according to a modification of ATCC protocols. Cells were raised in Dulbecco’s Modified Eagle Medium (DMEM, Cellgro) supplemented with 10% fetal bovine serum (Hyclone) and penicillin/streptomycin/l-glutamine (Gibco). Cells were subcultured by scraping when they reached approximately 80-90% confluence. Human umbilical vein endothelial cells (HUVEC) were maintained in M199 media (Hyclone) supplemented with 20% fetal bovine serum (FBS, Hyclone), penicillin/streptomycin/l-glutamine (Gibco), heparin (20 U/ml, BD biosciences), and endothelial cell growth supplement (BD Biosciences). Cells were passaged with trypsin/EDTA at 80-90% confluence. Tissue culture flasks were coated with a 1 mg/ml solution of gelatin for 20 min at room temperature before plating cells.
Rat neonatal cardiac myocytes were obtained from 1-2 day old Sprague-Dawley pups (Charles River Labs). Pups were sacrificed and ventricles isolated and minced. The tissue was minced and suspended in a 1 mg/ml solution of collagenase (Worthington) in Hank’s buffered saline solution (HBSS) for 4-6 h at 4°C. The suspension was filtered through a 70 μm screen and centrifuged 10 min at 500g. The supernatant was discarded and pellet resuspended and washed in Hanks’ buffered saline solution (HBSS) three times before being plated in DMEM supplemented with 10% FBS. Tissue culture flasks were treated with a 1 μg/ml solution of fibronectin before cells were plated.
Prior to all experiments, cells were quiesced overnight in serum-free media. All cells were incubated at 37°C with 5% CO2 and 100% humidity.
2.3 Green fluorescent protein (GFP) quantification
Green fluorescent protein (GFP) bearing a His6-tag was obtained from Millipore. Microparticles were loaded with GFP by incubating them in GFP solutions made with phosphate buffered saline (PBS) at a concentration of 1 mg particles/ml overnight at 4°C. Particles were centrifuged and washed three t imes with PBS. Following washing, microparticles were incubated with a horseradish peroxidase-conjugated antibody against GFP (1:5000 dilution in PBS-T with 1% goat serum, Rockland) for 2 h at 4°C. Particles were again washed with PBS three times and resuspended in PBS at a concentration of 1 mg particles/ml. Five microliters of the particle suspension were aliquotted into wells of a 96-well plate and amounts of GFP quantified colorimetrically via tetramentylbenzidine (1-Step Slow TMB, Pierce) conversion in a plate reader (Biotek Synergy 2). Kinetic reads were compared to standard curves and supernatants that have been immobilized on HisGrab 96-well plates (Pierce).
2.4 Release studies
Microparticles containing 2 wt% rhodamine-B (TCI Chemicals) and 10 wt% NTA were made using a modification to the above procedure. Microparticles were loaded in a 1 μg/ml solution of GFP overnight at 4°C. After washi ng, microparticles were suspended at 1 mg/ml in PBS and aliquotted into separate samples. The release study was conducted at 37°C. At specified time points, p articles were centrifuged, supernatant collected for analysis, and fresh buffer used to resuspend the particles. GFP was quantified via ELISA and rhodamine-B via fluorescence measurements. Total rhodamine-B was measured by hydrolyzing the particles in 1 N HCl and measuring fluorescence. SB239063 release data was obtained from Sy et al. [35]
2.5 MTT assay
Cells were plated at confluence in 6 well plates and quiesced with serum-free media overnight. Cells were then washed with PBS and treated with microparticles suspended in serum free media (1 ml per well) for 6 h. 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) was dissolved in phenol red-free DMEM at a concentration of 0.5 mg/ml. Following microparticle treatment, cells were washed three times with PBS to remove microparticles and incubated with MTT media for 2h. Cells were then washed three times with PBS and 0.5 ml of MTT solvent (0.1 N HCl in isopropanol) was added to each well to dissolve formazan crystals. This solution was centrifuged to pellet cell debris and measured spectrophotometrically at 570 nm.
2.6 VEGF studies and Western Blotting
His-tagged VEGF (His-VEGF) was expressed as described in [36]. HUVECs were plated on gelatin coated 6 well plates at confluence and quiesced overnight in serum-free media. Ten percent NTA microparticles were loaded with nickel as described above, washed, and incubated with a solution of 1 μg His-VEGF/ml in PBS overnight at 4°C. The microparticles were then was hed three times with PBS and resuspended in a serum-free media at various concentrations. One milliliter of media with microparticles was added to each well and incubated at 37°C for 20 minutes. Controls of Ni-NTA microparticles (no His-VEGF) and free VEGF (0-100 ng/ml) were also tested. Following incubation, cells were washed three times with ice cold PBS with phosphatase inhibitors (Sigma). Protein was harvested in 75 μl of ice cold RIPA buffer with protease and phosphatase inhibitors (Sigma). Lysates were centrifuged to pellet cell debris and microBCA assays (Pierce) were used to determine protein concentrations for each sample. Protein lysates were run under denaturing conditions (Laemmli Buffer system) on 7% polyacrylamide gels and transferred to PVDF membranes. Membranes were blocked and probed for phospho- and total- VEGFR2 antibodies (Cell Signaling) using manufacturer protocols. Band intensities were determined by exposing films using a HRP-ECL (Amersham) system.
2.7 Cell targeting studies
HUVECs were plated on gelatin-coated coverslips at 30% confluence and quiesced overnight in serum-free media. Ten percent NTA-microparticles with 1% coumarin-6 or rhodamine-B were loaded with nickel as described above, washed three times and incubated with a 100 nM solution of rhVE-Cadherin (R&D Systems) and 2 mM CaCl2 overnight. Particles were then washed and suspended in serum-free media at a concentration of 0.1 mg microparticle/ml. Cells were washed once with PBS and incubated with microparticles for 3h at 37°C. Foll owing incubation, media was aspirated and washed once with PBS, followed by a 30-min incubation with 4% paraformaldehyde in PBS at room temperature. Cells were then rinsed three times with PBS+0.1% BSA before coverslips were mounted on glass slides and imaged using fluorescent microscopy.
3. Results
3.1 Surface functionalized PCADK microparticles
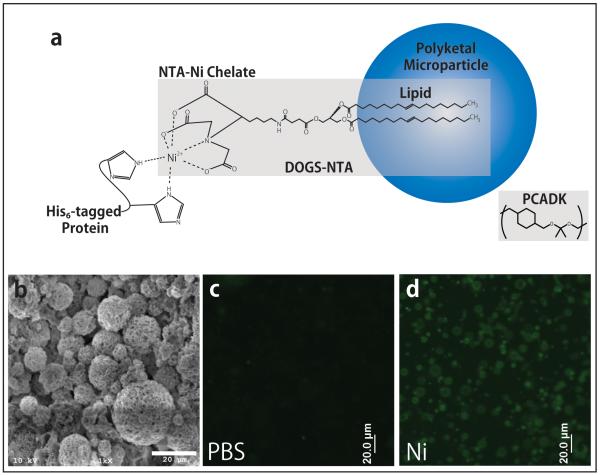
Polyketal microparticles were functionalized by adding DOGS-NTA (shaded box in Figure 1A) to the organic phase of a single-emulsion polyketal microparticle. The lipid tail serves as an anchor for the more hydrophilic NTA moiety, which migrates to the surface of the microparticle. Microparticles were analyzed using scanning electron microscopy (Figure 1B) and found to have diameters that ranged from 10-20 μm. Polyketal particles containing NTA that were pretreated with PBS, followed by washing, and exposure to a 100 nM solution of His6-GFP showed little fluorescence when examined under fluorescence microscopy (Figure 1C). In contrast, particles that were pre-incubated in a 0.05M NiCl2 solution prior to incubation with His6-GFP exhibited extensive green fluorescence around the microparticles (Figure 1D).
Figure 1. The surface of polyketal microparticles can be modified with nitrilotriacetic acid (NTA) to bind His6-tagged proteins.
(A) A cartoon depicting NTA-lipid conjugate added to polyketal microparticles for surface modification. (B) SEM micrograph of NTA-functionalized microparticles. (C) Fluorescence micrograph of NTA polyketal microparticles that were incubated with phosphate buffered saline (PBS) prior to His6-tagged green fluorescent protein (GFP). Microparticles showed little green fluorescence. (D) Fluorescence micrograph of Ni+2-loaded NTA microparticles showed strong green fluorescence after incubation with His6-tagged GFP. (all scale bars 20 μm.)
3.2 Quantitative measurement of His6-GFP loading
The binding capacity of two formulations of NTA-functionalized microparticles was determined quantitatively using a modified ELISA assay. Both 1 wt% and 10 wt% Ni-NTA microparticles showed similar His6-GFP binding properties (Figure 2). Microparticles that were loaded at concentrations of less than 1 μg His6-GFP/ml showed a linear loading profile with a slope corresponding to approximately 40% loading efficiency. The 1% NTA formulation saturated at a loading concentration of 800 ng His6-GFP /ml, while the 10% NTA formulation had a higher saturation point at 1200 ng His6-GFP /ml. These loading capacities correspond to a mass dose of 330 ng GFP/mg particle and 440 ng His6-GFP /mg particle, respectively.
Figure 2. Quantitative analysis of GFP binding to Ni-NTA functionalized microparticles.
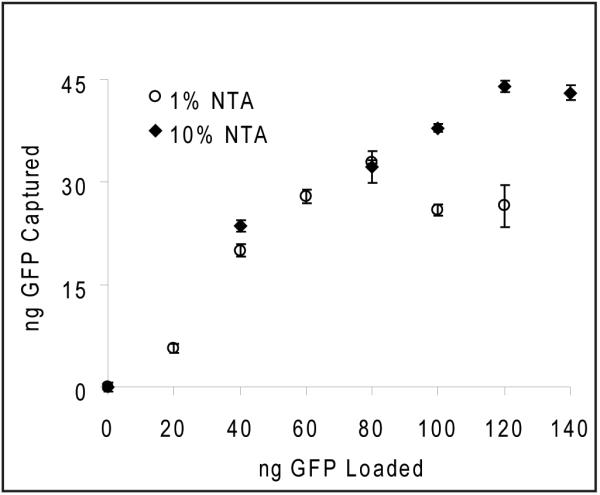
Surface-bound His6-GFP was determined by using a modified ELISA technique. Two different formulations (1% and 10% NTA) were incubated with His6-GFP, washed extensively, and assayed for GFP levels. The linear region (<80 ng GFP loaded) corresponds to roughly 40% loading efficiency. The 10% NTA formulation saturated at 440 ng GFP/mg microparticle, whereas the 1% NTA formulation saturated at 330 ng GFP/mg particle. Microparticles were loaded in GFP solutions at concentrations of 1200 ng/ml and 800 ng/ml, respectively. (data shown: mean ± SEM, n=3)
3.3 Particle release studies
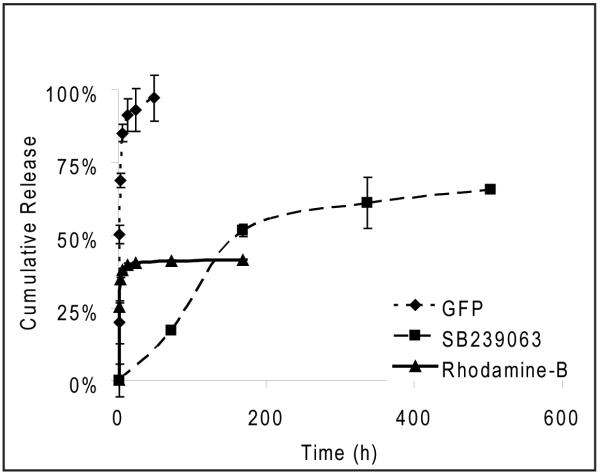
Release studies were conducted from the surface and core of the particles using two model compounds, His6-GFP and rhodamine-B (Figure 3). The core of the microparticles was loaded with 2 wt% rhodamine-B while the surface was loaded with His6-GFP. The release study was conducted under physiological conditions (pH 7.4, 37°C). His 6-GFP was released with a release half life of 2 h with complete release occurring at 48 h. Rhodamine-B showed an initial burst of approximately 39% released in the first 12 h, with slow release occurring over the next week. A more hydrophobic compound, SB239063, was previously shown to have significantly slower release kinetics, with a release half-life of one week[35].
Figure 3. Release studies of proteins and encapsulated compounds from NTA-functionalized microparticles.
His6-GFP was tethered to the surface of the microparticle through the Ni-NTA interaction and release was measured under physiological conditions (pH 7.4, 37°C) using a fluorescence plate reader. Either Rhodamine-B or SB239063, a p38 inhibitor), was encapsulated in the core of the microparticle. (Note: SB239063 data published in Sy et al. [35] GFP was released from the microparticle with a half life of 2h with <95% release at 48h. Rhodamine-B showed a burst release with 38% released within the first 6h and 42% released after one week. SB239063 had a release half life of one week with 66% release at three weeks. (data shown: mean ± SEM, n=3)
3.4 In vitro metabolic assays
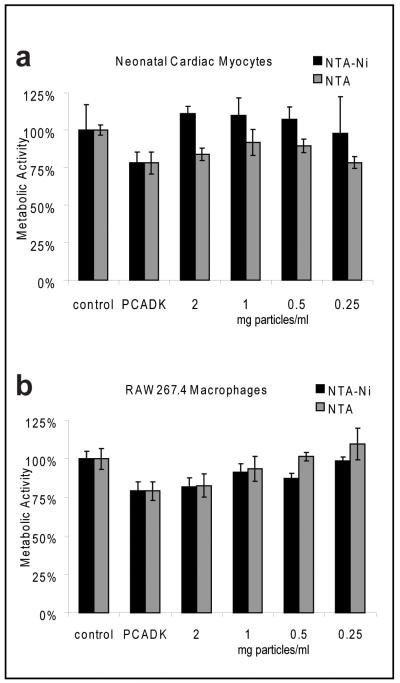
Changes in metabolic activity for two cell lines, neonatal cardiac myocytes (NCM) and RAW264.7 murine macrophages were measured following exposure to microparticles by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay. This assay measures mitochondrial activity of viable cells. NTA-functionalized particles that were pre-incubated with PBS or NiCl2 were tested at doses ranging from 0.25 mg/ml to 2 mg/ml and compared to untreated cells (control) and cells treated with 1 mg/ml of unmodified PCADK microparticles. Neonatal cardiac myocytes treated with NTA microparticles had metabolic activities ranging from 78-92% of control-treated while NCM treated with Ni-NTA microparticles showed no appreciable decrease in metabolic activity compared to control cells (Figure 4A).
Figure 4. Mitochondrial activity assays.
(A) Neonatal cardiac myocytes were treated for 6h before determining mitochondrial activity via MTT assay. A control of unmodified PCADK at 1 mg microparticle/ml was tested in addition to NTA and Ni-NTA modified microparticles. (B) RAW267.4 macrophages were also used to assess toxicity of the modified microparticles similar to (A).
RAW cells treated with 2 mg/ml of either type of modified particle showed similar metabolic activity compared to RAW cells treated with non-modified PCADK microparticles (82-83%). Lower doses of microparticles showed higher metabolic activities, while concentrations of 0.25 mg/ml of NTA and Ni-NTA particles showed similar activity to control-treated cells. No significant difference in metabolic activity between nickel-loaded and nickel-free particles was observed (Figure 4B).
3.5 Delivery of VEGF to HUVEC
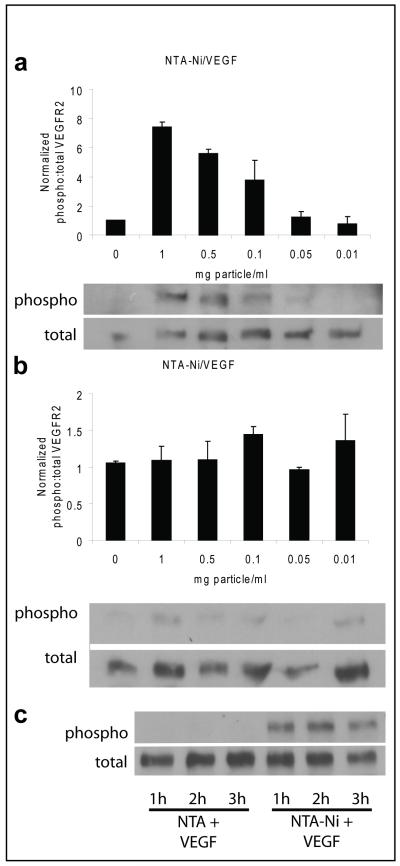
In order to determine whether proteins loaded on the surface retain biological activity, human umbilical vein endothelial cells (HUVECs) were treated with microparticles that were surface loaded with Ni-NTA and His6-tagged vascular endothelial growth factor (PK-VEGF). Cells were incubated with doses ranging from 0.01-1 mg microparticles/ml for a period of 20 min. The bioactivity of the delivered VEGF was measured by Western blot analysis of cell lysates probed with phospho-VEGF receptor-2 (VEGFR2) and total VEGFR2 antibodies. Densitometric measurements of the blots were made and the ratio of phosphorylated:total VEGFR2 was calculated. Cells that were treated with PK-VEGF showed a dose dependent increase in VEGFR2 phosphorylation, with an 8-fold increase at a 2 mg/ml dose (Figure 5A) with no effect of lower doses (0.01 mg particles/ml and 0.05 mg particles/ml). HUVECs treated with Ni-NTA microparticles that had not been loaded with VEGF did not show any change in VEGFR2 phosphorylation compared to control cells (Figure 5B). In order to demonstrate that the VEGF tethered to the microparticle could be released from the surface over time in its active form, cells were treated with PK-VEGF microparticles using a transwell assay with cells on the bottom of the well, and the particles on the top. The pore size chosen was 3 μm to ensure that particles with tethered VEGF could not activate the cells directly. HUVECs did not show activation of VEGFR2 (data not shown) after 20 min of PK-VEGF treatment across a transwell membrane. However, receptor phosphorylation was prevalent after 1, 2, or 3 hrs of treatment (Figure 5C).
Figure 5. Bioactive VEGF is released from microparticles and can phosphorylate VEGFR2 in HUVEC.
(A) Ni-NTA microparticles loaded with His6-tagged vascular endothelial growth factor (VEGF) elicits a dose-dependent response in VEGF-receptor 2 (VEGFR2) phosphorylation following a 20 minute incubation. Representative blots of phospho-VEGFR2 and total-VEGFR2 shown; data are mean ± SEM from 3 separate experiments expressed as a fold-increase. (B) Ni-NTA microparticles that were not loaded with VEGF did not show activation of VEGFR2 at the doses tested. Representative blots of phospho-VEGFR2 and total-VEGFR2 shown. (C) Representative blots of transwell assay with human umbilical vein endothelial cells (HUVECs) in the bottom well. VEGF-loaded microparticles added to the top well were able to activate VEGFR2 across a transwell membrane (3 μm pore size) at 1, 2, and 3h. NTA-functionalized microparticles not loaded with Ni+2 and incubated with His6-tagged VEGF did not activate cells over the same time course.
3.6 Cell surface receptor targeting
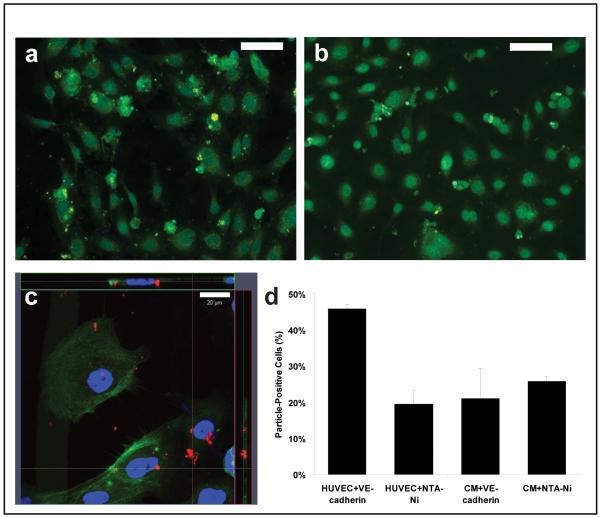
Small microparticles (1~2 μm) were fabricated containing either 1 wt% coumarin-6 or rhodamine-B and 10% NTA in order to assess the potential of targeting microparticles to specific cell types. Polyketal microparticles containing Ni-NTA were surface loaded with His6-rhVE-Cadherin (PK-CAD), a protein specific to endothelial cells that forms homodimers with high affinity, which is important for cell-cell interactions. Two cell lines were tested for targeting, HUVECs (which express VE-Cadherin) and rat NCM (negative control). Cells were incubated with PK-CAD, washed thoroughly, and imaged under fluorescence microscopy. Our data show extensive binding of PK-CAD to endothelial cells (Figure 6A) with little binding of Ni-NTA to HUVECs (Figure 6B). Examination using confocal microscopy suggested that the particles were not internalized by the cells (Figure 6C). Cells that were positive for a fluorescent microparticle were counted and expressed as a total percentage of the cells in the field of view (Figure 6D). Three negative control conditions (HUVECs treated with Ni-NTA particles not loaded with VE-Cadherin and NCMs treated with both particles) showed that approximately 20% of cells had at least one particle attached. HUVECs treated with VE-Cadherin loaded microparticles had a greater than two-fold enhancement of microparticle targeting with nearly half of the cells being positive for at least one particle.
Figure 6. Microparticles can be targeted to HUVEC though VE-Cadherin.
(A) Fluorescence micrograph of HUVECs incubated with VE-Cadherin loaded microparticles. Microparticle cores were loaded with coumarin-6 for tracking. (scale bar 100 μm) (B) Representative fluorescence micrograph of HUVEC incubated with Ni-NTA microparticles. (scale bar 100 μm) (C) Laser scanning confocal micrograph of HUVEC with VE-Cadherin loaded microparticles. Orthogonal projection of z-stack suggests that microparticles are not internalized. (scale bar 20 μm) (D) Percentage of cells positive for at least one microparticle. Negative controls (HUVEC+Ni-NTA, NCM+VE-Cadherin, NCM+Ni-NTA) ranged from 19-26% of cells positive for one microparticle. By contrast, HUVECs incubated with VE-Cadherin loaded microparticles had 45.7% of cells labeled by microparticles, which represents a 2.4-fold increase compared to HUVECs treated with non-targeted microparticles. (data shown: mean ± SEM)
4. Discussion
Biological-based therapeutics, such as proteins and nucleic acids, have the potential to treat a variety of diseases due to their great specificity and high potency. However, delivery of biological compounds presents many challenges for drug delivery vehicles due to their sensitive structural properties and hydrophilicity. The data presented in this manuscript suggest that metal affinity based systems are a simple, yet effective way to functionalize microparticle carriers in order to obtain microparticle targeting as well as delivery of a protein payload with little risk of denaturation.
Other studies examining the use of NTA-modified surfaces have covalently attached NTA to polystyrene microparticles through a condensation reaction [22] for use in flow cytometry. These techniques require several steps to achieve conjugation and may not be compatible with all polymer chemistries. As an example, polyketals are not readily modified due to the difficulty in incorporating reactive chemical handles onto the polymer backbone. However, surface modification using DOGS-NTA is widely applicable, regardless of polymer system due to having both hydrophilic and hydrophobic components. Due to the amphiphilic nature of the DOGS-NTA conjugate, we hypothesized that we could add the lipid to the organic phase of the emulsion and allow the hydrophilic NTA-head groups to migrate to the surface of the microparticle. This one-step procedure does not depend on specific chemical groups and only depends on the emulsion and drying parameters. Thus, we were able to incorporate DOGS-NTA without any major alterations in particles size from our prior studies.
Our data suggest that protein loading has a weak dependence on NTA incorporation at the concentrations used in this study. Ten percent NTA microparticles had a slightly greater capacity with 440 ng GFP/mg microparticle compared to 330 ng GFP/mg microparticle for the 1% NTA formulation (Figure 2), though maximal percent loading remained fairly constant. These loading fractions represent physiologically relevant doses for in vivo applications; growth factors often have effects at nanomolar concentration, which corresponds to nanograms of protein delivered locally [37]. More importantly, protein loading was achieved in purely aqueous conditions using dilute (<2 μg /ml) solutions of protein; typical double emulsion procedures use protein concentrations in excess of 10 mg/ml [34]. Such high concentrations and protein waste may not be acceptable for expensive proteins that are hard to purify or for scaled-up commercial applications. In addition, protein that does not bind to the surface of the microparticle is completely recoverable in our loading procedure by centrifuging the particles. This binding was completely reversible as incubation of particles with saturating concentrations of imidazole-induced immediate release (data not shown). Given the wide popularity of His6-tags for purification of recombinant proteins, a large library of proteins can be used with the system. Surface functionalization is amenable to screening combinatorial therapies without the need to fabricate a new batch of microparticles for a given protein combination; instead, microparticles can be loaded in a complex, aqueous mixture of therapeutic proteins or targeting ligands. Furthermore, the concept of delivering multiple therapeutics can be taken one step further by the encapsulation of small molecules. As illustrated in Figure 3, different release kinetics of an encapsulated compound can be achieved due to the physicochemical properties of the drugs or the polymer [6]. Further tuning of the release rates may potentially be achieved by altering the number of NTA groups as well as the length of the His6-tag [20]. These techniques may offer ways to modulate the release kinetics of metal affinity systems.
One potential concern for metal-affinity delivery systems is potential toxicity from the metal ions. While heavy metals have been shown to be detrimental, especially in environmental contamination situations, the amount of Ni+2 used in this system is far below toxic concentrations. Ten percent Ni-NTA polyketal formulations maximally contain 5.8 ng of nickel per milligram of microparticle. In order to put this into perspective, the average American diet contains 300 μg nickel per day [38]. In addition, chelated metals have been shown to have reduced toxicity compared to their free ion counterparts [39]. Our MTT data suggest that NTA-functionalization, with or without nickel, does not add any additional toxicity compared to PCADK microparticles in cardiac myocytes and macrophages. These particular cell types were chosen due to the susceptibility of the heart to nickel poisoning [40] and the phagocytic nature of immune cells, which engage these types of microspheres in vivo. Both NTA-functionalized and Ni-NTA-functionalized microparticles showed similar levels of toxicity or better when compared to cells treated by non-modified microparticles for both cells lines. Previous work in our lab and others has established biocompatibility of PCADK microparticles in a variety of animal models with mg doses being given to rats and mice [4, 5, 34, 35]. With no increase in toxicity associated with the surface functionalization of these microparticles in vitro, we anticipate safe use of these microparticles for in vivo applications.
After establishing biocompatibility of the Ni-NTA system, we sought to show bioactivity in two potential applications for the non-covalent tethering of proteins. First, we tested the bioactivity of tethered His6-tagged VEGF to activate VEGFR2 with the particle directly in contact with cells or separated by a transwell membrane with 3 μm pores. As expected, protein loaded onto the surface of the microparticle showed a dose dependent response when incubated directly on the cells, indicating the protein activity was still intact after complex formation. In this case, PK-VEGF could have activated its receptor while still bound to the microparticle. In order to separate the effects of tethered protein versus protein that has been released, we tested if VEGF release from microparticles could diffuse across a transwell insert. Our data show that VEGFR2 phosphorylation was seen at 1, 2, and 3 hr but not as early as 20 min suggesting that some time was required for VEGF to be released from the surface of the microparticle and diffuse to the cell layer. Furthermore, these data suggest that sustained activation occurs in our transwell system as VEGF signaling with free ligand is rapid [41].
While proteins were released intact from the surface, our experiments also demonstrated that targeting could be achieved using cell surface receptors; a greater than two-fold improvement in binding was observed. VE-Cadherin acts as a cell-cell junction protein and is a potential target for endothelial cells due to its selective expression in vivo. The targeting system presented here used a His6-VE-Cadherin/Fc chimera that greatly increased the size of the protein, thus reducing the density of targeting ligands on the surface of the microparticle. Diffusion of the VE-Cadherin away from the microparticle could have also played a role in the modest targeting. Furthermore, there was a strong potential for microparticles to interact with each other due to the homodimer formation between particles rather than exclusively with cells. Despite these potential confounding variables, significant improvements in targeting were observed and generate enthusiasm for future targeting studies with unique ligands.
Surface modification of polyketal microparticles to include metal affinity chemistries for proteins show potential for improving the delivery of biological compounds with polymer microspheres. We show here that the Ni-NTA complex is suitable for non-covalent tethering of proteins in their bioactive forms. These proteins can be adapted for use as a therapeutic payload or as targeting ligands for smaller microparticles. Many parameters of the system can potentially be tuned to control features such as release kinetics and warrant further investigation to develop metal affinity delivery systems for future applications.
5. Conclusion
This work demonstrates that PCADK microparticles can be surface functionalized with Ni-NTA. Furthermore, these metal complexes can be used to non-covalently immobilize a variety of proteins in a bioactive form with little risk of denaturation compared to other processing techniques. Using His6-GFP as a model protein, Ni-NTA functionalized microparticles were able to specifically bind 40% of protein from dilute (<1 μg/ml) solutions and release these proteins with a half life of 2 hours, creating a dual delivery system with a slower release of compounds from within the particle. Bioactive VEGF was delivered to HUVECs and activated VEGFR2 in a microparticle dose-dependent manner. VE-Cadherin surface-loaded microparticles were also used to demonstrate the ability of using Ni-NTA functionalization for potentially targeting microparticles to specific cell types. Thus, functionalized PK-Ni-NTA particles may be a clinically useful delivery platform for diseases that require multiple factors, or targeted therapy.
Acknowledgments
Funding
This work was supported by grants from National Institutes of Health (HL089120 and HL090601; MED), UO1 HL80711-01 (MED, NM), EB006418 (NM), the Georgia Tech/Emory Center for the Engineering of Living Tissues (funded by National Science Foundation EEC-9731643; NM), J&J/GT Health Care Innovation Seed Grant Proposal (NM) and the Department of Homeland Security (DHS) Scholarship and Fellowship Program, administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the US Department of Energy (DOE) and DHS (JCS). ORISE is managed by Oak Ridge Associated Universities (ORAU) under DOE contract number DE-AC05-06OR23100. All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of DHS, DOE or ORAU/ORISE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report no conflict of interest.
References
- 1.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm Res. 1991;8(6):713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 3.McGinity JW, O’Donnell PB. Preparation of microspheres by the solvent evaporation technique. Adv Drug Deliv Rev. 1997;28(1):25–42. doi: 10.1016/s0169-409x(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 4.Seshadri G, Sy JC, Brown M, Dikalov S, Yang SC, Murthy N, et al. The delivery of superoxide dismutase encapsulated in polyketal microparticles to rat myocardium and protection from myocardial ischemia-reperfusion injury. Biomaterials. 2010;31(6):1372–1379. doi: 10.1016/j.biomaterials.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiore VF, Lofton MC, Roser-Page S, Yang SC, Roman J, Murthy N, et al. Polyketal microparticles for therapeutic delivery to the lung. Biomaterials. 2010;31(5):810–817. doi: 10.1016/j.biomaterials.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang SC, Bhide M, Crispe IN, Pierce RH, Murthy N. Polyketal copolymers: a new acid-sensitive delivery vehicle for treating acute inflammatory diseases. Bioconjug Chem. 2008;19(6):1164–1169. doi: 10.1021/bc700442g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meinel L, Illi OE, Zapf J, Malfanti M, Merkle HP, Gander B. Stabilizing insulin-like growth factor-I in poly(D,L-lactide-co-glycolide) microspheres. J Control Release. 2001;70(1-2):193–202. doi: 10.1016/s0168-3659(00)00352-7. [DOI] [PubMed] [Google Scholar]
- 8.Morita T, Sakamura Y, Horikiri Y, Suzuki T, Yoshino H. Protein encapsulation into biodegradable microspheres by a novel S/O/W emulsion method using poly(ethylene glycol) as a protein micronization adjuvant. J Control Release. 2000;69(3):435–444. doi: 10.1016/s0168-3659(00)00326-6. [DOI] [PubMed] [Google Scholar]
- 9.Carrasquillo KG, Stanley AM, Aponte-Carro JC, De Jesus P, Costantino HR, Bosques CJ, et al. Non-aqueous encapsulation of excipient-stabilized spray-freeze dried BSA into poly(lactide-co-glycolide) microspheres results in release of native protein. J Control Release. 2001;76(3):199–208. doi: 10.1016/s0168-3659(01)00430-8. [DOI] [PubMed] [Google Scholar]
- 10.Chang CJ. Effects of nerve growth factor from genipin-crosslinked gelatin in polycaprolactone conduit on peripheral nerve regeneration--in vitro and in vivo. J Biomed Mater Res A. 2009;91(2):586–596. doi: 10.1002/jbm.a.32252. [DOI] [PubMed] [Google Scholar]
- 11.Liang HC, Chang WH, Lin KJ, Sung HW. Genipin-crosslinked gelatin microspheres as a drug carrier for intramuscular administration: in vitro and in vivo studies. J Biomed Mater Res A. 2003;65(2):271–282. doi: 10.1002/jbm.a.10476. [DOI] [PubMed] [Google Scholar]
- 12.Vandelli MA, Rivasi F, Guerra P, Forni F, Arletti R. Gelatin microspheres crosslinked with D,L-glyceraldehyde as a potential drug delivery system: preparation, characterisation, in vitro and in vivo studies. Int J Pharm. 2001;215(1-2):175–184. doi: 10.1016/s0378-5173(00)00681-5. [DOI] [PubMed] [Google Scholar]
- 13.Gu F, Amsden B, Neufeld R. Sustained delivery of vascular endothelial growth factor with alginate beads. J Control Release. 2004;96(3):463–472. doi: 10.1016/j.jconrel.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang MQ. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J Control Release. 2005;103(3):609–624. doi: 10.1016/j.jconrel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Porath J, Carlsson J, Olsson I, Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975;258(5536):598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- 16.Hochuli E, Dobeli H, Schacher A. New metal chelate adsorbent selective for proteins and peptides containing neighbouring histidine residues. J Chromatogr. 1987;411:177–184. doi: 10.1016/s0021-9673(00)93969-4. [DOI] [PubMed] [Google Scholar]
- 17.Verbelen C, Gruber HJ, Dufrene YF. The NTA-His6 bond is strong enough for AFM single-molecular recognition studies. J Mol Recognit. 2007;20(6):490–494. doi: 10.1002/jmr.833. [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Valencia CA, Liu RH, Lin WB. Highly-efficient purification of native polyhistidine-tagged proteins by multivalent NTA-modified magnetic nanoparticles. Bioconjugate Chem. 2007;18(2):333–341. doi: 10.1021/bc060195l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai SY, Lin SC, Suen SY, Hsu WH. Effect of number of poly(His) tags on the adsorption of engineered proteins on immobilized metal affinity chromatography adsorbents. Process Biochem. 2006;41(9):2058–2067. [Google Scholar]
- 20.Lata S, Reichel A, Brock R, Tampe R, Piehler J. High-affinity adaptors for switchable recognition of histidine-tagged proteins. J Am Chem Soc. 2005;127(29):10205–10215. doi: 10.1021/ja050690c. [DOI] [PubMed] [Google Scholar]
- 21.Lata S, Piehler J. Stable and functional immobilization of histidine-tagged proteins via multivalent chelator headgroups on a molecular poly(ethylene glycol) brush. Anal Chem. 2005;77(4):1096–1105. doi: 10.1021/ac048813j. [DOI] [PubMed] [Google Scholar]
- 22.Lauer SA, Nolan JP. Development and characterization of Ni-NTA-bearing microspheres. Cytometry. 2002;48(3):136–145. doi: 10.1002/cyto.10124. [DOI] [PubMed] [Google Scholar]
- 23.Bischler N, Balavoine F, Milkereit P, Tschochner H, Mioskowski C, Schultz P. Specific interaction and two-dimensional crystallization of histidine tagged yeast RNA polymerase I on nickel-chelating lipids. Biophys J. 1998;74(3):1522–1532. doi: 10.1016/S0006-3495(98)77864-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubalek EW, Le Grice SF, Brown PO. Two-dimensional crystallization of histidine-tagged, HIV-1 reverse transcriptase promoted by a novel nickel-chelating lipid. J Struct Biol. 1994;113(2):117–123. doi: 10.1006/jsbi.1994.1039. [DOI] [PubMed] [Google Scholar]
- 25.Venien-Bryan C, Lenne PF, Zakri C, Renault A, Brisson A, Legrand JF, et al. Characterization of the growth of 2D protein crystals on a lipid monolayer by ellipsometry and rigidity measurements coupled to electron microscopy. Biophys J. 1998;74(5):2649–2657. doi: 10.1016/S0006-3495(98)77970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruger R, Muller D, Fahr A, Kontermann RE. Generation of immunoliposomes using recombinant single-chain Fv fragments bound to Ni-NTA-liposomes. J Drug Target. 2005;13(7):399–406. doi: 10.1080/10611860500353328. [DOI] [PubMed] [Google Scholar]
- 27.Huang ZH, Park JI, Watson DS, Hwang P, Szoka FC. Facile synthesis of multivalent nitrilotriacetic acid (NTA) and NTA conjugates for analytical and drug delivery applications. Bioconjugate Chem. 2006;17(6):1592–1600. doi: 10.1021/bc0602228. [DOI] [PubMed] [Google Scholar]
- 28.Chikh GG, Li WM, Schutze-Redelmeier MP, Meunier JC, Bally MB. Attaching histidine-tagged peptides and proteins to lipid-based carriers through use of metal-ion-chelating lipids. Biochim Biophys Acta. 2002;1567(1-2):204–212. doi: 10.1016/s0005-2736(02)00618-1. [DOI] [PubMed] [Google Scholar]
- 29.Patel JD, O’Carra R, Jones J, Woodward JG, Mumper RJ. Preparation and characterization of nickel nanoparticles for binding to his-tag proteins and antigens. Pharm Res. 2007;24(2):343–352. doi: 10.1007/s11095-006-9154-7. [DOI] [PubMed] [Google Scholar]
- 30.Lin CC, Metters AT. Enhanced protein delivery from photopolymerized hydrogels using a pseudospecific metal chelating ligand. Pharm Res. 2006;23(3):614–622. doi: 10.1007/s11095-005-9395-x. [DOI] [PubMed] [Google Scholar]
- 31.Lin CC, Metters AT. Metal-chelating affinity hydrogels for sustained protein release. J Biomed Mater Res A. 2007;83A(4):954–964. doi: 10.1002/jbm.a.31282. [DOI] [PubMed] [Google Scholar]
- 32.Lin CC, Metters AT. Bifunctional monolithic affinity hydrogels for dual-protein delivery. Biomacromolecules. 2008;9(3):789–795. doi: 10.1021/bm700940w. [DOI] [PubMed] [Google Scholar]
- 33.Heffernan MJ, Murthy N. Polyketal nanoparticles: a new pH-sensitive biodegradable drug delivery vehicle. Bioconjug Chem. 2005;16(6):1340–1342. doi: 10.1021/bc050176w. [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Yang SC, Heffernan MJ, Taylor WR, Murthy N. Polyketal microparticles: a new delivery vehicle for superoxide dismutase. Bioconjugate Chem. 2007;18(1):4–7. doi: 10.1021/bc060259s. [DOI] [PubMed] [Google Scholar]
- 35.Sy JC, Seshadri G, Yang SC, Brown M, Oh T, Dikalov S, et al. Sustained release of a p38 inhibitor from non-inflammatory microspheres inhibits cardiac dysfunction. Nat Mater. 2008;7(11):863–868. doi: 10.1038/nmat2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phelps EA, Landazuri N, Thule PM, Taylor WR, Garcia AJ. Regenerative Medicine Special Feature: Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103(21):8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barceloux DG. Nickel. J Toxicol Clin Toxicol. 1999;37(2):239–258. doi: 10.1081/clt-100102423. [DOI] [PubMed] [Google Scholar]
- 39.Borenfreund E, Puerner JA. Cytotoxicity of metals, metal-metal and metal-chelator combinations assayed in vitro. Toxicology. 1986;39(2):121–134. doi: 10.1016/0300-483x(86)90130-7. [DOI] [PubMed] [Google Scholar]
- 40.Novelli ELB, Rodrigues NL, Sforcin JM, Ribas BO. Toxic effects of nickel exposure on heart and liver of rats. Tox Subst Mech. 1997;16(3):251–258. [Google Scholar]
- 41.Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Beliveau R. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell. 2003;14(1):334–347. doi: 10.1091/mbc.E02-07-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]