Summary
Inflammatory responses play decisive roles at different stages of tumor development, including initiation, promotion, malignant conversion, invasion, and metastasis. Inflammation also affects immune surveillance and responses to therapy. Immune cells that infiltrate tumors engage in an extensive and dynamic crosstalk with cancer cells and some of the molecular events that mediate this dialog have been revealed. This review outlines the principal mechanisms that govern the effects of inflammation and immunity on tumor development and discusses attractive new targets for cancer therapy and prevention.
Keywords: Cancer, inflammation, immunity, cytokines, NF-κB, STAT3
Introduction
The presence of leukocytes within tumors, observed in the 19th century by Rudolf Virchow, provided the first indication of a possible link between between inflammation and cancer. Yet, it is only during the last decade that clear evidence has been obtained that inflammation plays a critical role in tumorigenesis, and some of the underlying molecular mechanisms have been elucidated (Karin, 2006). A role for inflammation in tumorigenesis is now generally accepted, and it has become evident that an inflammatory microenvironment is an essential component of all tumors, including some in which a direct causal relationship with inflammation is not yet proven (Mantovani et al., 2008). Only a minority of all cancers are caused by germline mutations, whereas the vast majority (90%) are linked to somatic mutations and environmental factors. Many environmental causes of cancer and risk factors are associated with some form of chronic inflammation. Up to 20% of cancers are linked to chronic infections, 30% can be attributed to tobacco smoking and inhaled pollutants (such as silica and asbestos), and 35% to dietary factors (20% of cancer burden is linked to obesity) (Aggarwal et al., 2009).
Although it is now well-established that the induction of inflammation by bacterial and viral infections increases cancer risk (de Martel and Franceschi, 2009), recent work has shown that in addition to being a tumor initiator by virtue of its high carcinogen content, tobacco smoke is also a tumor promoter due to its ability to trigger chronic inflammation (Takahashi et al., 2010). Likewise, obesity, whose prevalence is growing at an alarming rate, promotes tumorigenesis in the liver (Park et al., 2010) and pancreas (Khasawneh et al., 2009). Most solid malignancies appear in older individuals and even old age (Ershler and Keller, 2000) and cell senescence (Rodier et al., 2009) are postulated to be tumor promoters that act through inflammatory mechanisms. Along with its pro-tumorigenic effects, inflammation also influences the host immune response to tumors and can be used in cancer immunotherapy (Dougan and Dranoff, 2009) and to augment the response to chemotherapy (Zitvogel et al., 2008). Yet, in some cases, inflammation can diminish the beneficial effects of therapy (Ammirante et al., 2010). This review is mainly focused on the pro-tumorigenic effects of inflammation but also touches on the relationship between inflammation and anti-tumor immunity,
Types of inflammation and general mechanisms
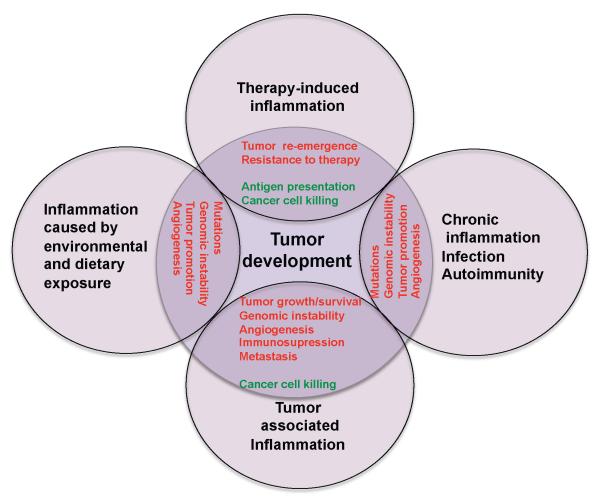
Several types of inflammation—differing by cause, mechanism, outcome, and intensity—can promote cancer development and progression (Figure 1). Persistent Helicobacter pylori infection is associated with gastric cancer and MALT (mucosa-associated lymphoid tissue) lymphoma. Infections with hepatitis B (HBV) or C (HCV) viruses increase the risk of hepatocellular carcinoma (HCC) and infections with Schistosoma or Bacteroides species are linked to bladder and colon cancer, respectively (Karin, 2006; Wu et al., 2009a). The inflammatory response triggered by infection precedes tumor development and is a part of normal host defense, whose goal is pathogen elimination. However, tumorigenic pathogens subvert host immunity and establish persistent infections associated with low grade but chronic inflammation. By contrast, acute inflammation induced by certain microbial preparations was used by Coley with some success to treat cancer in the 1890s and one such preparation is currently used in the treatment of bladder cancer (Rakoff-Nahoum and Medzhitov, 2009). What makes bladder carcinoma uniquely sensitive to acute inflammation, even though it is promoted by chronic inflammation, is currently unknown. This is an important problem whose solution should reveal how to successfully deploy inflammation in cancer therapy. Another type of chronic inflammation that precedes tumor development is caused by immune deregulation and autoimmunity. An example is inflammatory bowel disease, which greatly increases the risk of colorectal cancer (Waldner and Neurath, 2009).
Figure 1.
Types of inflammation in tumorigenesis and cancer.
Chronic inflammation associated with infections or autoimmune disease precedes tumor development and can contribute to it through induction of oncogenic mutations, genomic instability, early tumor promotion, and enhanced angiogenesis. Prolonged exposure to environmental irritants or obesity can also result in low-grade chronic inflammation that precedes tumor development and contributes to it through the mechanisms mentioned above. Tumor-associated inflammation goes hand in hand with tumor development. This inflammatory response can enhance neo-angiogenesis, promote tumor progression and metastatic spread, cause local immunosuppression, and further augment genomic instability. Cancer therapy can also trigger an inflammatory response by causing trauma, necrosis, and tissue injury that stimulate tumor re-emergence and resistance to therapy. However, in some cases, therapy-induced inflammation can enhance antigen presentation, leading to immune-mediated tumor eradication. Tumor promoting mechanisms are in red and anti-tumorigenic mechanisms are in green.
However, not all chronic inflammatory diseases increase cancer risk and some of them, such as psoriasis, may even reduce it (Nickoloff et al., 2005). It is not clear what makes IBD or chronic hepatitis tumor promoting, in comparison with conditions such as rheumatoid arthritis or psoriasis, which do not significantly promote tumorigenesis. One possibility could be related to the exposure of the gastrointestinal tract and liver to dietary and environmental carcinogens, which never make their way into joints or the skin. Chronic inflammation can also be induced by environmental exposure. Particulate material from tobacco smoke and other irritants can precipitate chronic obstructive pulmonary disease, a condition associated with higher lung cancer risk (Punturieri et al., 2009). Inflammatory mechanisms account for the tumor promoting effect of exposure to tobacco smoke on lung cancer in mice (Takahashi et al., 2010). Inhaled asbestos or silica particles also give rise to lung cancer but have no obvious mutagenic activity. Such particles, however, can trigger inflammation through effects on pro-interluekin-1β (IL-1β) processing by the inflammasome (Dostert et al., 2008) and this may mediate their tumorigenic activity. Even obesity, which increases cancer risk by 1.6-fold (Calle, 2007), can lead to chronic inflammation (Tuncman et al., 2006) that promotes development of hepatocellular carcinoma (Park et al., 2010). Accumulation of damaged DNA and cell senescence can also give rise to tumor promoting chronic inflammation (Rodier et al., 2009; Zheng et al., 2007).
A completely different type of inflammation is the one that follows tumor development. Most, if not all, solid malignancies trigger an intrinsic inflammatory response that builds up a pro-tumorigenic microenvironment (Mantovani et al., 2008). In addition to cell-autonomous proliferation, certain oncogenes, such as RAS and MYC family members, induce a transcriptional program that leads to remodeling of the tumor microenvironment through recruitment of leukocytes and lymphocytes, expression of tumor-promoting chemokines and cytokines, and induction of an angiogenic switch (Soucek et al., 2007; Sparmann and Bar-Sagi, 2004). All solid malignancies, at some point outpace their blood supply and become oxygen and nutrient deprived. This results in necrotic cell death at the tumor’s core and the release of pro-inflammatory mediators, such as IL-1 and HMGB1 (Vakkila and Lotze, 2004). The ensuing inflammatory response promotes neo-angiogenesis and provides surviving cancer cells with additional growth factors, produced by newly recruited inflammatory and immune cells (Karin, 2006).
Other tumors, for instance lung cancer, can promote inflammation through active secretion of molecules, such as the extracellular matrix component versican that activates macrophages through Toll-like receptor (TLR) 2 (Kim et al., 2009). Based on the continuous cell renewal and proliferation induced by tumor-associated inflammation, tumors have been referred to as “wounds, which never heal” (Dvorak, 1986). This type of inflammation is largely a subverted wound healing and tissue regenerative response. Even dominant oncogenes such as v-Src or K-Ras are unable to induce cancer in adult animals unless accompanied by injury and subsequent tissue regeneration (Guerra et al., 2007; Sieweke et al., 1990).
Lastly, a strong tumor-associated inflammatory response can be initiated by cancer therapy. Radiation and chemotherapy cause massive necrotic death of cancer cells and surrounding tissues, which in turn trigger an inflammatory reaction analogous to a wound-healing response (Zong and Thompson, 2006). The net outcome of therapy-induced inflammation is controversial, as on one hand it can have tumor-promoting functions just like the necrosis that accompanies rapid tumor growth (Ammirante et al., 2010; Vakkila and Lotze, 2004), but on the other hand it can enhance the cross-presentation of tumor antigens and subsequent induction of an anti-tumor immune response (Zitvogel et al., 2008). The latter and its importance will be discussed below.
Immune cells in tumorigenesis
As a result of these different forms of inflammation, the tumor microenvironment contains innate immune cells (including macrophages, neutrophils, mast cells, myeloid derived suppressor cells, dendritic cells, and natural killer cells) and adaptive immune cells (T and B lymphocytes) in addition to the cancer cells and their surrounding stroma (which consists of fibroblasts, endothelial cells, pericytes, and mesenchymal cells) (de Visser et al., 2006) (Table 1). These diverse cells communicate with each other by means of direct contact or cytokine and chemokine production and act in autocrine and paracrine manners to control and shape tumor growth. It is the expression of various immune mediators and modulators as well as the abundance and activation state of different cell types in the tumor microenvironment that dictate in which direction the balance is tipped and whether inflammation-promotes tumor growth or anti-tumor immunity will ensue (Lin and Karin, 2007; Smyth et al., 2006). In established tumors this balance is profoundly tilted towards pro-tumor inflammation, as without therapeutic intervention advanced tumors rarely regress. Yet, it is difficult to unequivocally assess the overall impact of immunity and inflammation on early tumorigenic events, because direct in vivo models for evaluating the effects of these phenomena on initial tumor growth are missing. In addition, our current knowledge is based on measurement of tumor load at a point where malignant cells may have already escaped early surveillance mechanisms. However, it is safe to assume that tumor promoting inflammation and anti-tumor immunity co-exist at different points along the path of tumor progression (Figure 2) and that environmental and microenvironmental conditions dictate the balance between the two (Bui and Schreiber, 2007; Swann et al., 2008).
Table 1.
Roles of different subtypes of immune and inflammatory cells in anti-tumor immunity and tumor-promoting inflammation
| Cell types | Anti-tumor | Tumor-promoting |
|---|---|---|
| Macrophages, dendritic cells, myeloid-derived suppressor cells |
Antigen presentation Production of cytokines (IL- 12 and type I IFN) |
Immunosuppression Production of cytokines, chemokines, proteases. growth factors, and angiogenic factors |
| Mast cells | Production of cytokines | |
| B cells | Production of tumor specific antibodies? |
Production of cytokines Activation of mast cells Immunosuppression |
| CD8+ T cells | Direct lysis of cancer cells Production of cytotoxic cytokines |
Production of cytokines? |
| CD4+ Th2 cells | Education of macrophages Production of cytokines B cell activation |
|
| CD4+ Th1 cells | Help to cytotoxic T lymphocytes (CTLs) in tumor rejection |
Production of cytokines |
| Production of cytokines (IFNγ) |
||
| CD4+ Th17 cells | Activation of CTLs | Production of cytokines |
| CD4+ Treg cells | Suppression of inflammation (cytokines and other suppressive mechanisms) |
Immunosuppression Production of cytokines |
| Natural Killer cells | Direct cytotoxicity toward cancer cells Production of cytotoxic cytokines |
|
| Natural Killer T cells | Direct cytotoxicity toward cancer cells Production of cytotoxic cytokines |
|
| Neutrophils | Direct cytotoxicity Regulation of CTL responses |
Production of cytokines, proteases, and ROS |
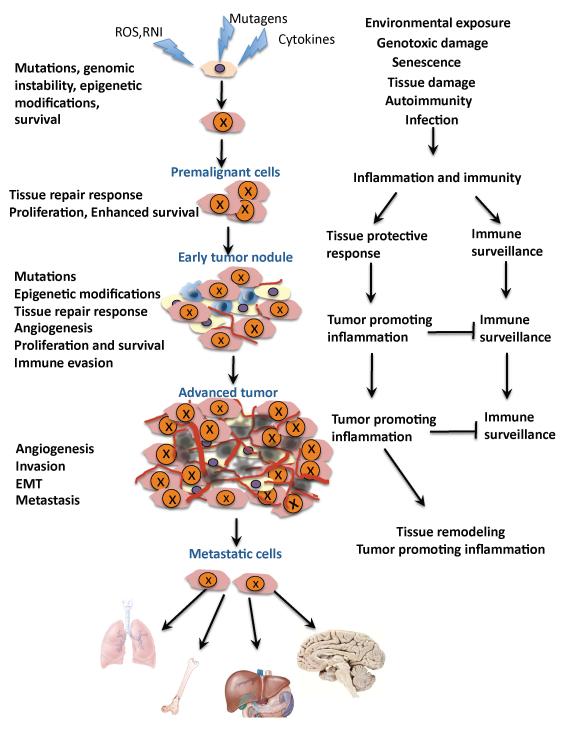
Figure 2.
The multifaceted role of inflammation in cancer
Inflammation acts at all stages of tumorigenesis. It may contribute to tumor initiation through mutations, genomic instability, and epigenetic modifications. Inflammation activates tissue repair responses, induces proliferation of premalignant cells, and enhances their survival. Inflammation also stimulates angiogenesis, causes localized immunosuppression, and promotes the formation of a hospitable microenvironment in which pre-malignant cells can survive, expand, and accumulate additional mutations and epigenetic changes. Eventually, inflammation also promotes metastatic spread. Mutated cells are marked with “X”. Yellow - stromal cells, Brown - malignant cells, Red - blood vessels, Blue - immune and inflammatory cells. Epithelial-mesenchymal transition, EMT; reactive oxygen species, ROS; reactive nitrogen intermediates (RNI)
The most frequently found immune cells within the tumor microenvironment are tumor-associated macrophages (TAMs) and T cells. TAMs mostly promote tumor growth and may be obligatory for angiogenesis, invasion, and metastasis (Condeelis and Pollard, 2006), and high TAM content generally correlates with poor prognosis (Murdoch et al., 2008). Mature T cells are divided into two major groups based on the T cell receptors (TCR) they express: γδ and αβ. αβT cells are further classified according to their effector functions as CD8+ cytotoxic T cells (CTLs) and CD4+ helper T (Th) cells, which include Th1, Th2, Th17 and T regulatory (Treg) cells, as well as natural killer T (NKT) cells. Importantly, T cells can exert both tumor suppressive and promoting effects, as determined by their effector functions (DeNardo et al., 2009; Langowski et al., 2007; Smyth et al., 2006). Increased T cell numbers, specifically activated CTLs and Th cells, correlate with better survival in some cancers, including invasive colon cancer, melanoma, multiple myeloma, and pancreatic cancer (Galon et al., 2006; Laghi et al., 2009; Swann and Smyth, 2007). Correspondingly, T cell deficiency or disruption of specific cytotoxic mechanisms can render experimental animals more susceptible to spontaneous or chemical carcinogenesis (Shankaran et al., 2001; Swann and Smyth, 2007). However, there is also evidence that many of the T cell subsets found in solid tumors are involved in tumor promotion, progression, or metastasis, including CD8+ T cells (Roberts et al., 2007), IFNγ-producing Th1 cells (Hanada et al., 2006), Th2 cells (Aspord et al., 2007; DeNardo et al., 2009) and Th17 cells (Langowski et al., 2006; Wang et al., 2009). The only cells that lack a pro-tumorigenic role, so far, are NK cells. Similar to TAMs, the tumor-promoting functions of T lymphocytes are mediated by cytokines, whereas both cytokines and cytotoxic mechanisms mediate the anti-tumorigenic functions of T lymphocytes (Lin and Karin, 2007; Swann and Smyth, 2007).
Interestingly, Treg cells, which are presumed to act mostly in a pro-tumorigenic fashion through suppression of anti-tumor immune responses (Gallimore and Simon, 2008), may also exert an anti-tumorigenic function under certain circumstances by virtue of their ability to suppress tumor-promoting inflammation (Erdman et al., 2005). In breast cancer, the presence of tumor infiltrating lymphocytes with high CD4+/CD8+ and Th2/Th1 ratio is indicative of poor prognosis (Kohrt et al., 2005). Th2 CD4+ T cells stimulate mammary cancer progression and metastasis by educating TAMs to produce pro-angiogenic and pro-metastatic factors (DeNardo et al., 2009). In colitis associated cancer (CAC), infiltrating T cells also appear to play a tumor promoting function (Waldner and Neurath, 2009). What makes the same T cell subset anti-tumorigenic in one cancer and pro-tumorigenic in another remains largely unknown and may hold the key to the development of successful immunotherapy.
The cytokine and chemokine expression profile of the tumor microenvironment may be more relevant than its specific immune cell content. Different cytokines can either promote or inhibit tumor development and progression, regardless of their source (Lin and Karin, 2007). Through activation of various downstream effectors, such as NF-κB, AP-1, STAT and SMAD transcription factors, as well as caspases, cytokines control the immune and inflammatory milieu to either favor anti-tumor immunity (IL-12, TRAIL, IFNγ) or enhance tumor progression (IL-6, IL-17, IL-23) and also have direct effects on cancer cell growth and survival (TRAIL, FasL, TNF-α, EGFR ligands, TGF-β, IL-6).
TAMs are one of the most important players in the inflammation and cancer arena and an important source of cytokines (Mantovani et al., 2008). In analogy to Th1 and Th2 T cells, macrophages can be classified into M1 and M2 types (Sica et al., 2008). M1 macrophages, activated by IFNγ and microbial products, express high levels of pro-inflammatory cytokines (TNF-α, IL-1, IL-6, IL-12 or IL-23), major histocompatibility complex (MHC) molecules and inducible nitric oxide synthase and are capable of killing pathogens and priming anti-tumor immune responses. By contrast, M2 or “alternatively” activated macrophages, which are induced in vitro by IL-4, IL-10 and IL-13, downregulate MHC class II and IL-12 expression and show increased expression of the anti-inflammatory cytokine IL-10, scavenger receptor A, and arginase. Most TAMs are considered to have an M2 phenotype while promoting tumor angiogenesis and tissue remodeling (Sica et al., 2008). However, most confirmed tumor-promoting cytokines are “M1 cytokines”, whereas IL-10, an M2 cytokine, may be tumor suppressive as shown in in colorectal cancer (Berg et al., 1996; Lin and Karin, 2007). Furthermore, unlike Th1 and Th2 cells, M1 and M2 macrophages are plastic and their phenotype is defined by their gene expression prolife rather than by deterministic differentiation pathways and lineage choices.
Other immune cells also affect tumorigenesis (Table 1). Neutrophils can play both tumor-promoting and tumoricidal functions, depending on their differentiation status and the presence of TGF-β (Fridlender et al., 2009). B lymphocytes and mast cells are also important contributors to immune-mediated tumor growth (Ammirante et al., 2010; de Visser et al., 2006; Soucek et al., 2007) and conventional macrophages and dendritic cells are important for antigen presentation and T cell activation during anti-tumor immunity as well as for cytokine production and immunosuppression in established tumors (Table 1).
Inflammation and tumor initiation
Tumor initiation is a process in which normal cells acquire the first mutational hit that sends them on the tumorigenic track by providing growth and survival advantages over their neighbors. In most cases, however, a single mutation is insufficient and many cancers require at least 4-5 mutations (Fearon and Vogelstein, 1990; Hanahan and Weinberg, 2000). It is also imperative that each mutation will be transmitted to the cell’s progeny, and in cancers that arise within rapidly renewed epithelia (intestinal and skin cancers), oncogenic mutations must occur in either long lived stem cells or transient amplifying cells rather than within differentiated cells, which are rapidly eliminated before the next mutation can strike. Alternatively, oncogenic mutations can occur within differentiated epithelial cells, such as hepatocytes, which are capable of proliferation and are sufficiently long lived to allow subsequent mutational hits.
It has been suggested that an inflammatory microenvironment can increase mutation rates, in addition to enhancing the proliferation of mutated cells. Activated inflammatory cells serve as sources of reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI) that are capable of inducing DNA damage and genomic instability (Figure 3A). However, it is not clear whether ROS and RNI produced and released by neutrophils or macrophages (mainly during acute inflammation) are sufficiently long lived to diffuse through the extracellular matrix, enter epithelial cells, cross their cytoplasm, enter the nucleus and react with DNA packaged into chromatin. Alternatively, inflammatory cells may use cytokines such as TNF-α to stimulate ROS accumulation in neighboring epithelial cells (Figure 3A). It has therefore been debated whether immune-mediated mechanisms as opposed to dietary and environmental mutagens are the critical driving forces behind tumor initiation (Hussain et al., 2003). Nonetheless, p53 mutations, presumably caused by oxidative damage, were found in both cancer cells and in inflamed, but non-dysplastic, epithelium in CAC, suggesting that chronic inflammation causes genomic changes (Kraus and Arber, 2009). Chronic inflammation triggered by the colonic irritant dextran sodium sulfate (DSS) may induce DNA damage that gives rise to colonic adenomas (Meira et al., 2008). However, on its own DSS is a poor carcinogen (Okayasu et al., 1996).
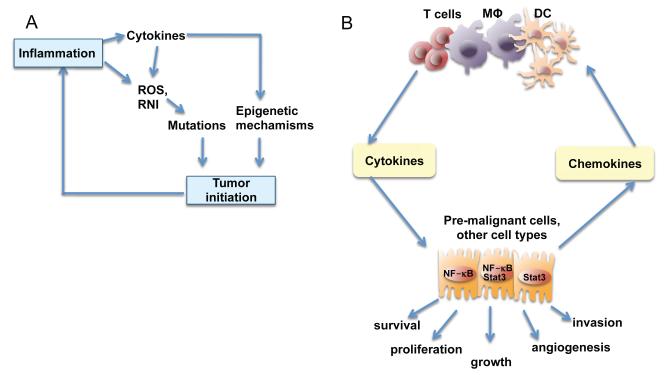
Figure 3.
Role of inflammation in tumor initiation and promotion
A) Tumor initiation. Reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI) produced by inflammatory cells may cause mutations in neighboring epithelial cells. Also, cytokines produced by inflammatory cells can elevate intracellular ROS and RNI in pre-malignant cells. In addition, inflammation can result in epigenetic changes that favor tumor initiation. Tumor-associated inflammation contributes to further ROS, RNI and cytokine production.
B) Tumor promotion. Cytokines produced by tumor infiltrating immune cells activate key transcription factors, such as NF-κB or STAT3, in pre-malignant cells to control numerous pro-tumorigenic processes, including survival, proliferation, growth, angiogenesis, and invasion. As parts of positive feed-forward loops, NF-κB and STAT3 induce production of chemokines that attract additional immune/inflammatory cells to sustain tumor-associated inflammation.
Inflammation-induced mutagenesis may also result in inactivation or repression of mismatch repair response genes and ROS can also cause direct oxidative inactivation of mismatch repair enzymes (Colotta et al., 2009; Hussain et al., 2003). Once the mismatch repair system has been dismantled, inflammation-induced mutagenesis is enhanced and several important tumor suppressors, such as Tgfbr2 and Bax, which harbor microsatellite sequences, may be inactivated (Colotta et al., 2009).
Another mechanism linking inflammation to oncogenic mutations is upregulation of AID (activation-induced cytidine deaminase), an enzyme that promotes immunoglobulin gene class switching by catalyzing deamination of cytosines in DNA (Okazaki et al., 2007). In addition to B cells, where it was discovered, AID is overexpressed in many cancers of diverse origins and its expression is induced by inflammatory cytokines in an NF-κB-dependent manner or by TGFβ (Okazaki et al., 2007). AID induces genomic instability and increases mutation probability during error-prone joining of double-stranded DNA breaks, a process found to introduce mutations into critical cancer genes, including Tp53, c-Myc, and Bcl-6 (Colotta et al., 2009). AID contributes to formation of lymphomas, and gastric and liver cancers (Okazaki et al., 2007; Takai et al., 2009). Other mechanisms of inflammation-induced mutagenesis have also been suggested, including effects of inflammation on non-homologous recombination and NF-κB-mediated inactivation of p53-dependent genome surveillance (Colotta et al., 2009).
In Giα2 knockout mice, which develop spontaneous colonic inflammation and cancer, enterocytes selectively lose expression of components involved in mismatch repair, namely MLH1 and PMS2, due to histone deacetylase- and DEC-1-mediated epigenetic repression of the Mlh1 promoter (Edwards et al., 2009). Other findings implicate epigenetic mechanisms, including microRNA-based silencing and DNA methylation, in inactivation of tumor suppressors, such as INK4a and APC, and other changes that accompany tumor initiation (Cooper and Foster, 2009). Recently, inflammation has been connected to epigenetic reprogramming by the JmjC-domain protein Jmjd3, which is encoded by an NF-κB target gene (De Santa et al., 2007). In inflammation-associated intestinal cancer in Gpx1/2 knockout mice, inflammation induces DNA methyltransferase (DNMT)-dependent DNA methylation and silencing of a large cohort of Polycomb group target genes, some of which are also silenced by methylation in human colon cancer (Hahn et al., 2008). However, it remains to be shown that any of these inflammation-induced epigenetic mechanisms actually makes a critical contribution to tumor initiation, either in a suitable mouse model or through prospective analysis of human specimens.
Another mechanism through which inflammation may enhance tumor initiation is the production of growth factors and cytokines that can confer a stem-cell like phenotype upon tumor progenitors or stimulate stem cell expansion, thereby enlarging the cell pool that is targeted by environmental mutagens. Indeed, STAT3 is linked to both stem cell reprogramming and stem cell renewal (Chen et al., 2008), whereas NF-κB can enhance Wnt/β-catenin signaling in colonic crypts (Umar et al., 2009). The pro-inflammatory cytokine TNF-α promotes nuclear entry of β-catenin during inflammation-associated gastric cancer in the absence of any mutations in Wnt/β-catenin pathway components (Oguma et al., 2008).
The connection between inflammation and tumor initiation is not a one-way street and there is also evidence that DNA damage can lead to inflammation and thereby promote tumorigenesis. One of the best examples is provided by the model of hepatocellular carcinoma induced by the carcinogen diethylnitrosamine (DEN) in which DNA damage contributes to necrotic cell death, resulting in an inflammatory reaction that promotes tumor development (Maeda et al., 2005; Sakurai et al., 2008). A number of oncoproteins (Ras, Myc, RET) can activate signaling pathways that drive production of pro-inflammatory cytokines and chemokines (IL-6, IL-8, IL-1β, CCL2, CCL20) (Mantovani et al., 2008). Genotoxic stress can also induce expression of NKG2D family members, which serve as ligands for NK and γδT cell receptors (Strid et al., 2008) resulting in either elimination of stressed cells or a local inflammatory response. In the same vein, mosaic deletion of the DNA repair gene ATR and Tp53 in the skin results in recruitment of CD11b+Gr1+ myeloid cells, as a part of a prototypical immune response to “altered self” (Ruzankina et al., 2009). Defective DNA repair caused by a deficiency of the Fen1 exonuclease also results in a tumor promoting inflammatory response that is driven by damaged DNA, most likely through activation of a pattern recognition receptor (Zheng et al., 2007).
Inflammation and tumor promotion
Tumor promotion is the process of tumor growth from a single initiated cell into a fully developed primary tumor. Initial tumor growth depends on increased cell proliferation and reduced cell death, both of which are stimulated by inflammation-driven mechanisms. In fact, many of the enhancing effects of inflammation on cancer are exerted at the level of tumor promotion and most known tumor promoters, for instance phorbol esters, are potent inducers of inflammation (Karin, 2006). Inflammation-induced tumor promotion may occur early or late in tumor development and can lead to activation of pre-malignant lesions that were dormant for many years. The mechanisms through which inflammation affects tumor promotion are numerous and in addition to increased proliferation and enhanced survival, can also involve the so-called angiogenic switch, which allows a small dormant tumor to receive the blood supply necessary for the next growth phase (Lewis and Pollard, 2006). Mechanisms of inflammation-driven tumor promotion are discussed below.
Tumor promoting cytokine signaling
Production of tumor promoting cytokines by immune/inflammatory cells that activate transcription factors, such as NF-κB, STAT3 and AP-1, in pre-malignant cells to induce genes that stimulate cell proliferation and survival, is a major tumor promoting mechanism (Figure 3B). Initial evidence for inflammation-mediated tumor promotion came from mouse models of skin, colon, and liver cancer. Although counterintuitive at the time, TNF-α was found to be required for two-stage skin carcinogenesis (Moore et al., 1999). TNF-α activates both AP-1 and NF-κB transcription factors, but in the skin its tumor promoting effects are mediated by AP-1 (Eferl and Wagner, 2003), which was identified as a transcription factor whose activity is stimulated by the classic tumor promoter tetradecanoyl phorbol acetate (TPA) (Angel et al., 1987). By contrast, NF-κB inhibits the development of skin cancer (Zhang et al., 2004). Thus, although a given cytokine may activate several transcription factors, its tumor promoting activity may be mediated by only one of them and antagonized by another. As discussed below, a similar situation may apply to liver cancer. Amongst the different transcription factors that are part of this mechanism, NF-κB and STAT3 are activated in the majority of cancers and act as non-classical oncogenes, whose activation in malignant cells is rarely the result of direct mutations, and instead depends on signals produced by neighboring cells or more rarely on mutational activation of upstream signaling components. NF-κB and STAT3 activate genes that control cell survival, proliferation, and growth, as well as angiogenesis, invasiveness, motility, chemokine, and cytokine production (Grivennikov and Karin, 2009; Yu et al., 2009).
Oncogenic transcription factors can also be activated through pattern recognition receptors by components of bacteria and viruses (Rakoff-Nahoum and Medzhitov, 2009). However, the overall contribution of pattern recognition receptors on epithelial cells versus those expressed by immune/inflammatory cells to tumor promotion is far from being clear and will require the analysis of cell type specific knockout mice. Even the specific agonists that activate these receptors in cancer are not defined. Nonetheless, the role of the cytokines that are produced in response to damage-associated (DAMP) or pathogen-associated (PAMP) molecular patterns in tumor development is more firmly established. For example, AP-1 activation in skin cancer is largely dependent on TNF-TNFR1 signaling (Balkwill, 2009), whereas STAT3 activation in cancer cells is largely dependent on a plethora of growth factors and cytokines including IL-6, IL-11, IL-22, HGF, and EGF, and oncogenic tyrosine kinases, such as c-Met and Src (Bollrath et al., 2009; Grivennikov et al., 2009; Naugler et al., 2007; Yu et al., 2009).
The first critical genetic evidence for inflammatory cells as a source of tumor promoting cytokines was obtained in a mouse model of CAC, where inactivation of NF-κB in myeloid cells reduced tumor growth and blocked production of IL-6 and other cytokines in response to colitis (Greten et al., 2004). Subsequent work demonstrated that the effect of immune cells (macrophages, T cells) on CAC growth is mediated through IL-6, IL-11, TNF-α and IL-1β (Becker et al., 2004; Bollrath et al., 2009; Grivennikov et al., 2009; Popivanova et al., 2008), as well as other cytokines, such as IL-23. IL-11 plays a similar role in gastric cancer (Ernst et al., 2008), in which IL-1β is also a tumor promoter (Tu et al., 2008). TNF-α also promotes HCC in mice lacking the P-glycoprotein Mdr2, which develop cholestatic inflammation followed by hepatocellular carcinoma (HCC) (Pikarsky et al., 2004). HCC can also be promoted by another member of the TNF family, lymphotoxin β(Haybaeck et al., 2009). TNF-α along with IL-6 contributes to obesity-mediated tumor promotion in HCC (Park et al., 2010). The latter effect correlates with the ability of TNF-α and IL-6 to promote hepatosteatosis and steatohepatitis (Park et al., 2010). One of the most critical tumor promoting cytokines in HCC is IL-6. Mice deficient in IL-6 develop much less HCC in response to the chemical pro-carcinogen DEN and the gender-biased production of IL-6 accounts for the much higher HCC load in males (Naugler et al., 2007). High levels of circulating IL-6, are associated with HCC risk factors, including hepatosteatosis, obesity, and liver cirrhosis, and are the best predictors of rapid progression from viral hepatitis to HCC in humans (Wong et al., 2009).
In CAC and HCC, the tumor promoting effect of IL-6 is mainly exerted via STAT3, whose cell type specific inactivation in hepatocytes and enterocytes inhibits the development of these malignancies in mice treated with DEN or azoxymethane (AOM) and DSS, respectively (Bollrath et al., 2009; Grivennikov et al., 2009; Park et al., 2010). Development of CAC in mice is also dependent on IKKβ-mediated NF-κB activation in enterocytes, whose major function in this model is increased survival of pre-malignant cells (Greten et al., 2004). A similar role was proposed for NF-κB in HCC development in mice deficient in Mdr2 and in lymphotoxin-transgenic mice both of which exhibit chronic liver inflammation (Haybaeck et al., 2009). However, in the DEN model of HCC and Helicobacter-driven gastric cancer, NF-κB promotes hepatocyte and epithelial cell survival and acts as an inhibitor of tumor development (Maeda et al., 2005; Shibata et al., 2009). Most likely, the diverse effects of NF-κB in different models are determined by the mechanism of tumor induction and the type of inflammatory response involved in tumor promotion. Mdr2 knockout and lymphotoxin-transgenic mice exhibit a very low level of normal hepatocyte death, which is not enhanced by the absence of NF-κB (Haybaeck et al., 2009; Pikarsky et al., 2004). In these mice, NF-κB in hepatocytes is mainly responsible for propagating inflammation through induction of chemokines, which recruit immune/inflammatory cells into the liver. By contrast, DEN treated mice exhibit an acute inflammatory response triggered by IL-1α release from necrotic hepatocytes (Sakurai et al., 2008). IL-1α induces IL-6 production by Kupffer cells and this response drives the compensatory proliferation of surviving hepatocytes (a type of a wound-healing response); the greater the amount of cell death – the greater the regenerative response. By suppressing accumulation of ROS and preventing hepatocyte necrosis, NF-κB inhibits HCC induction in DEN treated mice (Maeda et al., 2005).
Another tumor-promoting cytokine is IL-23 (Langowski et al., 2006). IL-23 is mostly expressed by TAMs in a manner dependent on STAT3 and NF-κB (Kortylewski et al., 2009). IL-23 blockade with neutralizing antibodies or genetic inactivation of the IL-23p19 gene dramatically decrease tumor multiplicity and growth in the two-step model of skin carcinogenesis (Langowski et al., 2006). In part, the pro-tumorigenic effects of IL-23 may be mediated by IL-17 and IL-22 production by Th17 cells, but other effects of IL-23 on CTLs, Tregs, and myeloid cells should not be discounted. A close relative of IL-23 is IL-12, which shares with IL-23 the IL-12p40 subunit and is involved in Th1 differentiation, IFNγ production, and activation of anti-tumor immunity (Trinchieri et al., 2003). Secretion of IL-23 and IL-12 secretion are reciprocally regulated and the switch from IL-12 to IL-23 production may be an important tumor promoting event. STAT3 activation, PGE2, ATP, and lactic acid increase IL-23 production by TAMs (Kortylewski et al., 2009; Shime et al., 2008). The latter two agonists link cancer cell necrosis (induced by hypoxia or therapy) and the Warburg effect (the switch from oxidative phosphorylation to glycolysis) to IL-23 production, thereby shifting anti-tumor immunity to tumor promotion.
A similar circuit can be executed by myeloid-derived suppressor cells (MDSC) that produce arginase1 and indoleamine-2,3-dioxygenase, which are enzymes that dampen anti-tumor immunity through interference with T cell activation (Gabrilovich and Nagaraj, 2009). Taken together, tumor associated inflammation drives tumor growth, angiogenesis and can perpetuate itself through an extensive network of cytokines and chemokines, which are produced by immune, stromal and malignant cells in response to diverse signals (Figure 3B).
Given that several cytokines (IL-1, TNF, IL-6, IL-23) and transcription factors (AP-1, NF-κB, STAT3) are critical for both inflammation and tumor growth, they control hubs of pro-tumorigenic signaling that may be targeted to curtail both tumor associated inflammation and tumor growth (see below). Pharmacological interference with cytokine signaling decreases tumorigenesis as well as cancer growth (Becker et al., 2004; Grivennikov et al., 2009; Hedvat et al., 2009) and may therefore serve as a basis for preventive and therapeutic approaches. Altogether, cytokine production by immune and inflammatory cells is an important tumor promoting mechanism that provides malignant cells with a continuous supply of growth and survival signals in an initially hostile microenvironment. In most cases, tumor promoting cytokines act in a paracrine manner, yet several types of cancer cells produce their own cytokines, including IL-6, to achieve the same effect (Gao et al., 2007).
Inflammation and angiogenesis
Growth of large tumors requires an increased intratumoral blood supply. This is triggered by tumor hypoxia, which promotes angiogenesis and increases the probability of metastasis. In addition to hypoxia, tumor angiogenesis depends on recruitment of TAMs, which sense hypoxic signals and in turn produce chemokines and pro-angiogenic factors. Recruitment of TAM precursors is largely dependent on angiogenic mediators such as angiopoetin 2 and vascular endothelial growth factor (VEGF). Important pro-angiogenic genes, such as IL-8, CXCL1, CXCL8, VEGF and hypoxia inducible factor 1 alpha (HIF1α), are directly regulated by NF-κB, STAT3 and AP-1 in TAMs, MDSCs, and other cell types (Kujawski et al., 2008; Rius et al., 2008).
Under hypoxic conditions, HIF-1α stimulates expression of CXCL12, which activates and recruits endothelial cells in a CXCR4-dependent manner (Sica et al., 2008). Formation of new lymphatic vessels is regulated by VEGF-C and VEGF-D, whereas VEGF-A facilitates the recruitment of monocytes, which activate lymphoangiogenesis (Murdoch et al., 2008). VEGF-A produced by myeloid cells also inhibits pericyte maturation and endothelial coverage of newly formed blood vessels, and its conditional ablation accelerates tumorigenesis (Stockmann et al., 2008). The recruitment of Gr1+ myeloid cells (presumably MDSC and TAM precursors) into tumors, curtails the effects of anti-VEGF therapy, presumably bypassing the requirement for local VEGF production by cancer cells for recruitment of TAM precursors (Shojaei et al., 2007). As most growing tumors contain some areas of hypoxia, it is not clear whether hypoxia is the direct driver of tumor angiogenesis or whether hypoxic stimuli generate inflammatory signals that drive angiogenesis. Inactivation of NF-κB or STAT3, neutralization of CCL2 or CXCL12, or TAM depletion unequivocally result in disrupted angiogenesis and decreased tumor growth, underscoring the critical role of inflammatory mediators in tumor angiogenesis (Joyce and Pollard, 2009; Kujawski et al., 2008).
Target genes that mediate tumor promotion
Most of the genes that mediate the tumor promoting functions of NF-κB, STAT3, and AP-1 have not been fully defined and most likely the pro-tumorigenic effects of these transcription factors are exerted through multiple effectors. Some targets may be controlled by more than one transcription factor and may be more important in one cell type than in another. The expression of the anti-apoptotic proteins Bcl-2 and Bcl-XL, for instance, are promoted by both NF-κB and STAT3 as are c-IAP1, c-IAP2, Mcl-1, c-FLIP, and survivin (Karin, 2006; Yu et al., 2007). Whereas Bcl-XL may be the most prominent anti-apoptotic gene in enterocytes (Greten et al., 2004), c-FLIP seems to fulfill the same function in hepatocytes (Chang et al., 2006). Both NF-κB and STAT3 interfere with p53 synthesis and attenuate p53-mediated genomic surveillance, representing another potential tumor promoting mechanism (Colotta et al., 2009).
STAT3 controls expression of cyclins D1, D2 and B, as well as the proto-oncogene c-Myc, and through them it may stimulate cell proliferation (Bollrath et al., 2009; Yu et al., 2007). Although cyclin D and c-Myc are also thought to be regulated by NF-κB, inactivation of IKKβ in enterocytes does not interfere with cell proliferation (Greten et al., 2004) and in Rastransformed keratinocytes (Zhang et al., 2004) or DEN-initiated hepatocytes (Maeda et al., 2005) NF-κB inhibition actually enhances cyclin D expression and cell proliferation. The AP-1 protein c-Jun cooperates with STAT3 in repression of Fas expression by tumor cells, thereby attenuating their sensitivity to instructive apoptosis (Eferl and Wagner, 2003). Additional NF-κB and STAT3 targets control cell and tissue resistance to stress and injury and include anti-microbial proteins (RegIIIβ, RegIIIγ, Tff3), heat shock proteins, and anti-oxidants, such as superoxide dismutase 2 (SOD2) and ferritin heavy chain (FHC) (Bollrath et al., 2009; Karin, 2006).
Lastly, another category of target genes that promote tumorigenesis are chemokines and cytokines that act in autocrine or paracrine manners to ensure the continuous recruitment of inflammatory cells into the tumor microenvironment. The perpetuation of chronic inflammation is largely achieved through positive feedback loops, which include inflammatory cells producing cytokines that induce chemokine synthesis in malignant and stromal cells leading to prolonged recruitment of inflammatory cells into the tumor microenvironment (Figure 3). TAMs, MDSCs, Tregs, and Th17 cells are the most critical immune cell subsets in this respect. Recruitment of myeloid cells is governed by multiple pathways, including CCL2-CCR2, CCL1-CXCR2, S100A proteins-RAGE, and IL-1-IL-1R interactions (Bonecchi et al., 2009). Signaling through CCR6 is critical for Th17 infiltration, whereas Treg cells are attracted mostly through CCR4 and CCR7 (Bonecchi et al., 2009). In some cases, the critical chemokines are not produced by cancer cells but are induced in tumor-associated fibroblasts upon interaction with carcinoma cells (Liao et al., 2009; Orimo et al., 2005; Orimo and Weinberg, 2006).
Inflammation and lymphoid malignancies
Chronic inflammatory conditions are also associated with lymphoid malignancies. An excellent example is provided by mucosa-associated lymphoid tissue (MALT) lymphomas, which occur in the context of chronic inflammation caused by infectious agents, such as Helicobacter pylori (the most commonly found gastric lymphoma), Chlamydia psittacii (ocular adnexal MALT lymphoma) and Borrelia burgdorferi (cutaneous MALT lymphoma) (Ferreri et al., 2009). Another example is Epstein-Barr virus (EBV), which is responsible for large B-cell lymphoma in immunocompromised patients, Burkitt’s lymphoma, and Hodgkin’s lymphoma (Ferreri et al., 2009).
It has been proposed that repeated antigenic stimulation, autoimmunity, and inflammation are risk factors for chronic lymphocytic leukemia (CLL), the most common hematopoietic malignancy that accounts for 30% of all leukemias (Chiorazzi et al., 2005). One mechanism through which such stimuli promote CLL development is induction of B cell activating factor (BAFF), a member of the TNF family, recently shown to accelerate development of CLL-like disease in mice (Enzler et al., 2009). Cytokines (such as IL-4 and VEGF), chemokines (such as SDF-1), and interactions with bone marrow stromal cells support CLL expansion and suppress apoptosis through upregulation of Bcl-2, survivin, and MCL-1 (Granziero et al., 2001; Pedersen et al., 2002). This occurs in lymph node pseudofollicles and bone marrow clusters where leukemic cells interact with components of the inflammatory microenvironment that support their survival. Another example for the role of inflammation in lymphoid malignancies are the lymphomas that appear in GM-CSF- and IFNγ-deficient mice, which are caused by infections and regress upon treatment with antibiotics (Enzler et al., 2003).
A similar situation may occur in multiple myeloma. Through secretion of IL-6, IGF-1, VEGF, TNF-α, SDF-1 and BAFF, stromal elements promote the survival and migration of neoplastic plasma cells and also confer drug resistance (Kastritis et al., 2009). IL-6 is of particular importance, as it acts both in paracrine and autocrine manners and IL-6-deficient mice are resistant to induction of multiple myeloma (Hodge et al., 2005). Despite constitutive NF-κB activation, multiple myeloma remains dependent on extrinsic factors, and drugs targeting IL-6 are being evaluated in combination with the proteasome inhibitor bortezomib for the treatment of this malignancy (Kastritis et al., 2009).
Inflammation and metastasis
From a clinical perspective, metastasis is the most critical aspect of tumorigenesis, because over 90% of cancer mortality is caused by metastasis. Recent studies unambiguously show that metastasis requires close collaboration between cancer cells, immune and inflammatory cells, and stromal elements. The process of metastasis can be grossly divided into four major steps. The first step is represented by epithelial-mesenchymal transition, in which cancer cells acquire fibroblastoid characteristics that increase their motility and allow them to invade epithelial linings/basal membranes and reach efferent blood vessels or lymphatics (Kalluri and Weinberg, 2009). Loss of E-cadherin expression is envisioned as a key event in the epithelial-mesenchymal transition. In the second step, cancer cells intravasate into blood vessels and lymphatics. Inflammation may promote this through production of mediators that increase vascular permeability. This is followed by the third step in which metastasis initiating cells survive and travel throughout the circulation. It has been estimated that only about 0.01% of cancer cells that enter the circulation will eventually survive and give rise to micrometastases (Joyce and Pollard, 2009). Next, integrin-mediated arrest allows the extravasation of circulating cancer cells. Finally, single metastatic progenitors interact with immune, inflammatory, and stromal cells and start to proliferate (Polyak and Weinberg, 2009). Some of these cells may already be targeted to the pre-metastatic niche in response to tumor generated inflammatory signals prior to the arrival of metastasis-initiating cancer cells (Kaplan et al., 2005). One of these inflammatory signals is the extracellular matrix component versican, which leads to macrophage activation and production of the metastasis promoting cytokine TNF-α (Kim et al., 2009). However, it has been difficult to determine whether versican production by metastatic cancer cells conditions the future metastatic site prior to their arrival.
TGFβ is an anti-inflammatory cytokine produced by cancer cells, myeloid cells, and T lymphocytes. TGFβ signaling is an important regulator of the epithelial-mesenchymal transition and metastasis, and elevated TGFβ is often associated with poor prognosis (Yang and Weinberg, 2008). TGFβ activates SMAD transcription factors and MAPKs, which control expression of other regulators of the epithelial-mesenchymal transition, such as Slug (Yang and Weinberg, 2008). TGFβ however, also suppresses epithelial cell proliferation and early tumor growth, causing some tumors to acquire inactivating mutations in TGFβ signaling components (Yang and Weinberg, 2008). Despite the defects in TGFβ signaling, such tumors can still metastasize. These opposing effects of TGFβ at different stages of tumor development await mechanistic explanation. Disruption of TGFβ signaling in cancer cells also results in upregulation of the SDF1 (CXCL12)-CXCR4 and CXCL5-CXCR2 chemokine:chemokine receptor pairs and induces rapid recruitment of MDSCs that promote metastasis and dampen anti-tumor immune responses (Yang et al., 2008). Inactivation of TGFβ signaling was proposed to result in elevated local TGFβ concentrations that inhibit anti-tumor T cell responses and induce differentiation of tumor-promoting Th17 cells (Langowski et al., 2007).
Another critical regulator of the epithelial-mesenchymal transition is Snail, a repressor of E-cadherin transcription in epithelial cells. Recent findings suggest that Snail is stabilized in response to TNF-α signaling, a process that is critical for cancer cell migration and metastasis (Wu et al., 2009b). Other mechanisms through which pro-inflammatory cytokines can affect the epithelial-mesenchymal transition is via STAT3-mediated induction of Twist transcription and NF-κB-mediated induction of both Twist and Kiss (Yu et al., 2009), However, these mechanisms remain to be confirmed in vivo, and a recent report suggests that STAT3 is a negative regulator of adenoma-carcinoma transition in colon cancer (Musteanu et al., 2009).
Cancer cell invasion requires extensive proteolysis of the extracellular matrix at the invasive front. Inflammatory cells are important sources of proteases that degrade the extracellular matrix. In a model of invasive colon cancer, CCR1+ myeloid cells, whose recruitment is driven by the chemokine CCL9 produced by cancer cells, promote invasiveness through secretion of the matrix metalloproteinases MMP2 and MMP9 (Kitamura et al., 2007). IL-1, TNF-α and IL-6 promote MMP expression, invasiveness ,and metastasis via NF-κB and STAT3 (Yu et al., 2007).
A different metastatic mechanism dependent on IKKα operates in prostate and breast cancers. As these cancers progress, their malignant cells progressively accumulate activated IKKα in their nuclei (Luo et al., 2007). In prostate cancer, accumulation of activated nuclear IKKα correlates with reduced expression of maspin, an inhibitor of metastasis (Luo et al., 2007). IKKα activation in metastatic prostate and mammary cancer cells is mediated by members of the TNF family, namely lymphotoxin and RANKL and its repressive effects on maspin transcription are NF-κB independent (Luo et al., 2007). How these lymphocytes are recruited into progressing breast and prostate tumors is still unknown. Recruitment of such cells may be a consequence of tumor necrosis, but as mentioned above certain carcinomas actively secrete factors that upregulate fibronectin and cause migration of VEGF receptor 1 (VEGFR1)-positive hematopoietic progenitors to the pre-metastatic niche (Kaplan et al., 2005). However, the pre-metastatic niche concept is somewhat mysterious as it is not clear how primary tumor cells direct inflammatory cells to such sites.
Alternatively, a small number of metastatic cells can interact with and activate different myeloid cell types through secreted factors such as versican (Kim et al., 2009). Breast cancer cells use CSF1 and CXCL12 to induce the recruitment of TAMs, which in turn produce EGF receptor (EGFR) ligands (Joyce and Pollard, 2009). These cytokines may also mediate a physical interaction between TAMs and carcinoma cells (Condeelis and Pollard, 2006). TAMs can be also “programmed” by tumor infiltrating T cells, particularly Th17 cells (Wang et al., 2009) and Th2 cells (DeNardo et al., 2009). IL-13 and IL-4 produced by tumor infiltrating CD4+ T cells stimulate the M1 to M2 transition of TAMs and thereby support pulmonary metastasis of mammary cancer cells (DeNardo et al., 2009). Depletion of TAMs (Joyce and Pollard, 2009) or CD4+ T cells (DeNardo et al., 2009) dramatically reduces metastasis of mouse mammary cancer.
Once metastatic cells enter the circulation, they need to survive in suspension and resist detachment-induced cell death or anoikis. The survival of circulating cancer cells is affected by inflammatory mediators released by immune cells in response to cancer-derived or pathogen-derived stimuli (Kim et al., 2009; Luo et al., 2004). Some of these effects depend on activation of NF-κB in either inflammatory cells or in cancer cells. A variety of cytokines present in the tumor microenvironment, including TNF-α, IL-6, and epiregulin, can promote the survival of circulating metastatic seeds (Nguyen et al., 2009). In addition to NF-κB and STAT3 activation, some of these cytokines can physically link cancer cells to TAMs, allowing them to travel together throughout the circulation (Condeelis and Pollard, 2006). On the other hand, single metastatic cells, which are no longer present within an immunosuppressive environment, may be targeted again by immunosurveillance. Indeed, in some cases, infiltration of tumors by activated T cells decreases the rate of metastasis (Galon et al., 2006; Pages et al., 2005). The interaction of circulating cancer cells with platelets or macrophages may protect them from NK cell-mediated killing, thereby overcoming immunosurveillance (Palumbo et al., 2007).
Intravasation is regulated by prostaglandins (which are produced in a COX2-dependent manner and act on the epithelium), by cytokines (such as epiregulin, which increases cancer cell survival), and by MMPs (which clear the way for the latter to migrate into capillaries (Nguyen et al., 2009)). The migration of metastasis initiating cells is not random and is directed by chemokine gradients sensed via CXCR4, CCR4, CCR7, CCR9 and CCR10 (Bonecchi et al., 2009).
The journey of the circulating metastatic seed ends upon integrin-dependent arrest on the endothelium, followed by extravasation. Molecules like ANGPTL4, which is regulated by TGFβ, facilitate extravasation into lungs by mediating contact between malignant and endothelial cells (Nguyen et al., 2009). Systemic inflammation enhances attachment of circulating cancer cells to hepatic sinusoids and this process is governed by neutrophil-dependent upregulation of adhesion molecules (McDonald et al., 2009). Several proinflammatory cytokines that are elevated in the circulation of cancer patients upregulate expression of adhesion molecules on the endothelium or in target organs and thereby increase the probability of metastatic cell attachment (Mantovani et al., 2008).
Immunity and tumorigenesis
As discussed above, in tumors that arise in the context of underlying inflammation or in advanced tumors containing inflammatory infiltrates, the net effect of the immune system (both innate and adaptive) is stimulation of tumor growth and progression. However, cancer cells represent an “altered self” and express “non-self” antigens in the context of stress and danger signals that can promote antigen presentation. Thus, even growing tumors may be subject to immunosurveillance and killing by activated T and NK cells (Dunn et al., 2004). It is likely that immunosurveillance and tumor-promoting inflammation can coexist even in the same tumor (Bui and Schreiber, 2007) (Figure 4A).
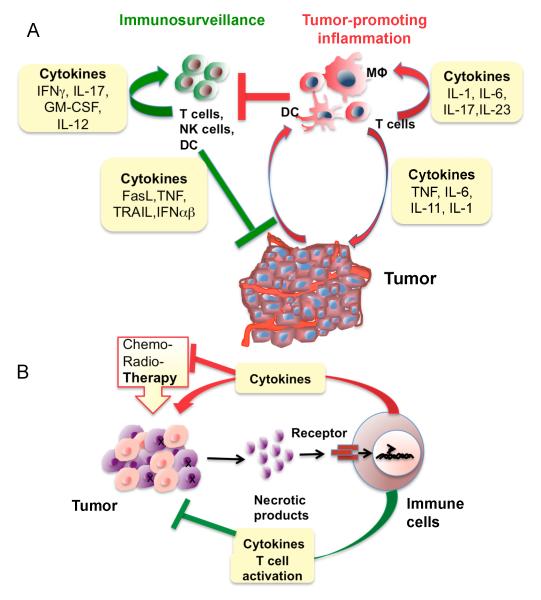
Figure 4.
Immunosurveillance, tumor-promoting and therapy-induced inflammation.
A) Balance between immunosurveillance and tumor promoting inflammation in the tumor microenvironment. Tumor promoting cytokines act on immune and malignant cells to tilt the balance toward tumor promotion. Tumor promoting immunity dampens immunosurveillance, which otherwise inhibits tumor growth. B) Therapy-induced inflammation. Various forms of therapy induce death (necrosis) of malignant cells resulting in the release of necrotic products and damage-associated molecular patterns (DAMPs) that activate cytokine-producing inflammatory cells. These cytokines activate pro-survival genes in residual cancer cells, rendering them resistant to subsequent rounds of therapy. However, in some cases, therapy-induced inflammation augments the presentation of tumor antigens and stimulates an anti-tumor immune response that improves the therapeutic outcome.
According to the immunosurveillance hypothesis, NK cells and CTLs engage in tumor killing (via perforin, granzyme B, TRAIL or FasL dependent mechanisms), whereas Th1 (by virtue of IFNγ production) and in some instances Th17 cells (via production of IL-17A) provide important help that boosts cytotoxic immunity (Dunn et al., 2006; Dunn et al., 2004; Martin-Orozco et al., 2009). On the other hand, Tregs suppress anti-tumor immune responses and are therefore pro-tumorigenic (Dunn et al., 2004). NKT cells can also be involved in surveillance of hematopoietic and chemically-induced tumors (Crowe et al., 2005; Smyth et al., 2000; Swann et al., 2009). Other critical components of this system are dendritic cells and macrophages, which present antigens and respond to danger and stress signals, as well as immunoregulatory and cytotoxic cytokines, such as type I IFN, IFNγ, FasL, TRAIL, GM-CSF and IL-12 (Palucka et al., 2007; Smyth et al., 2006; Swann and Smyth, 2007).
The first experimental demonstration of tumor immunosurveillance came from analysis of Rag2-deficient mice, which lack mature lymphocytes. These mice show enhanced development of a variety of spontaneous cancers by 14-16 months of age (Shankaran et al., 2001). However, even in immunocompromised mice, tumor development occurs in their post-reproductive period, suggesting that the mammalian immune system is not subjected to substantial evolutionary pressure to improve tumor recognition and elimination. Yet, in virallyor bacterially-promoted cancers, the immune system provides considerable protection through its ability to recognize and eliminate microbes (Smyth et al., 2006). Inactivation of various components of the immunosurveillance system, such as perforin, granzyme, and interferon signaling, renders mice susceptible to tumorigenesis (Bui and Schreiber, 2007; Dunn et al., 2004). Mice lacking cytotoxic cytokines, such as membrane-bound forms of FasL or TRAIL also show enhanced development of sarcomas and other tumors (O’ Reilly et al., 2009; Smyth et al., 2003).
More evidence for tumor immunosurveillance and immunoediting comes from the presence of tumor infiltrating lymphocytes (both T and B lymphocytes) that recognize tumor antigens and the favorable prognosis for some patients whose tumors display increased infiltration with activated T cells (Dunn et al., 2004). Such infiltration is even more noticeable in tumors that develop microsatellite instability or have a “mutator” phenotype and therefore express tumor antigens that exhibit greater differences from normal counterparts (Buckowitz et al., 2005; Guidoboni et al., 2001). Additional but indirect evidence for anti-tumor immunity includes various cases of spontaneous tumor regression accompanied by increased infiltration of activated cytotoxic cells and presence of antibodies and T cells that recognize tumor antigens (Swann and Smyth, 2007). The latter suggests that B and T lymphocytes have been activated by tumor-specific antigens but does not necessarily mean that these cells are responsible for tumor regression. Additional evidence is provided by the increased risk of lymphomas (of viral and non-viral etiology) and some solid tumors in immunosuppressed patients (Swann and Smyth, 2007).
Nonetheless, in the vast majority of established tumors the presence of tumor infiltrating lymphocytes is insufficient for curtailing tumor growth. Such considerations have given rise to a revised version of the immunosurveillance theory called immunoediting (Dunn et al., 2004; Smyth et al., 2006). According to this concept, cancer cells constantly edit and modulate the host anti-tumor immune response and the host immune response shapes tumor immunogenicity and clonal selection. During this process the balance between anti-tumor and tumor-promoting immunity can be tilted in favor of tumor growth. Before a tumor undergoes immune escape, it may be maintained at an “equilibrium” between tumor growth and immune destruction, and this may account for decades of tumor dormancy (Koebel et al., 2007). To tilt the balance in its favor, it is proposed that the cancer cell edits its repertoire of tumor antigens towards lower immunogenicity and also re-shapes the tumor microenvironment to become immunosuppressive. Consistent with this hypothesis, cancers that have evolved in alymphocytic mice are more immunogenic than cancers grown in immunocompetent mice (Shankaran et al., 2001).
Therapy induced inflammation – friend or foe?
Surgery, chemotherapy, and radiation are currently the major options for cancer treatment. All three induce local or systemic inflammation triggered by tissue injury and cancer cell death. Surgery results in activation of infectionor stress-sensing pathways, whereas chemo- and radiotherapy kill cancer cells mostly through necrosis, a pro-inflammatory form of cell death (Vakkila and Lotze, 2004). Inflammatory mediators released by necrotic cells include danger associated molecular patterns (DAMPs) such as ATP, nucleic acids, heat shock proteins (Hsp70), HMGB-1, S100 calcium binding proteins, and the cytokine IL-1α. A key question is whether therapy-induced inflammation stimulates the regrowth of residual malignant cells or whether it improves the therapeutic outcome? (Figure 4B). In support of the first possibility, inhibition of autophagy in apoptosis-deficient tumors stimulates tumor growth through induction of necrosis and tumor-associated sterile inflammation (Degenhardt et al., 2006). Tumor growth may also be stimulated in response to hypoxia-induced necrosis in the tumor’s core (Figure 4B). It has also been found that castration-induced death of androgen-dependent prostate cancer, despite resulting in initial tumor regression, triggers an inflammatory response that accelerates the re-growth of castration resistant cancer (Ammirante et al., 2010). Hence, inhibition of therapy-induced inflammation may improve the treatment of prostate cancer and provide the patient with several more years of tumor free survival.
However, in the case of more conventional chemotherapy, therapy-induced inflammation has been found to stimulate antigen presentation by tumor infiltrating dendritic cells and to induce production of cytokines that stimulate adaptive anti-tumor immunity (Apetoh et al., 2007a; Zhang et al., 2007) (Figure 4B). Curiously, the inflammatory trigger for this beneficial response is also the necrotic death of cancer cells, resulting in the release of HMG-B1 and ATP, which together activate TLR4 and the inflammasome to stimulate production of IL-1β, which is critical for adaptive anti-tumor immunity (Ghiringhelli et al., 2009). Interestingly, genetic polymorphisms in the TLR4 and P2X7 (the ATP receptor) loci affect the outcome of chemotherapy (Apetoh et al., 2007a; Apetoh et al., 2007b). What makes tumor necrosis either immunostimulatory or immunosuppressive (Vakkila and Lotze, 2004) is not yet clear. Furthermore, therapy-induced anti-tumor immunity is only seen with certain drugs, including etoposide, oxaliplatin, and doxorubicine but not with others (Apetoh et al., 2007a; Ghiringhelli et al., 2009). As these drugs can also kill infiltrating immune and hematopoietic stem cells, which are necessary for a functional immune response, effective therapy-induced anti-tumor immunity requires the use of small doses of chemotherapy to avoid immunosuppression. Conversely, by causing the death of tumor promoting immune/inflammatory cells, chemo- and radiotherapy may be used to destroy the tumor-promoting inflammatory microenvironment.
Anti-inflammatory drugs in cancer therapy
The findings described above provide an improved understanding of the molecular etiology of cancer and lay the foundations for the use of anti-inflammatory drugs in cancer prevention and therapy. One advantage of targeting the inflammatory microenvironment is that the normal genome of inflammatory/immune cells, which unlike the cancer cell genome, is not subject to mutational and epigenetic changes that result in drug resistance. However, in most cases, anti-inflammatory therapy is not cytocidal on its own and needs to be combined with more conventional therapies that kill cancer cells.
Despite such limitations, several anti-inflammatory drugs have been found to reduce tumor incidence when used as prophylactics, as well as slowing down progression and reducing mortality when used as therapeutics, particularly in the case of sporadic colon cancer (Gupta and Dubois, 2001). Such drugs include COX2 inhibitors, aspirin, and anti-inflammatory steroids, such as dexamethasone. In addition to its well-documented preventive effects in colon cancer, aspirin reduces the incidence of breast cancer (Gierach et al., 2008) and reduces prostate cancer risk, but only in individuals that carry a particular polymorphic allele at the lymphotoxin α locus, which specifies high lymphotoxin production (Liu et al., 2006). Such findings are of general importance because non-steroidal anti-inflammatory drugs (NSAID), such as aspirin, are not very specific and usually have side-effects that preclude their long-term administration except in high risk individuals. Thus, pre-screening for individuals with high cancer risk that are more likely to benefit from such preventive strategies should greatly improve the efficacy and utility of cancer prevention.
Tumor-promoting inflammation can be targeted in several different ways: 1) inhibition of signal transducers and transcription factors that mediate survival and growth of malignant cells in response to inflammatory cytokines; 2) sequestration of chemokines and cytokines that recruit and sustain inflammatory cells in the tumor microenvironment; 3) reducing (or augment) the inflammation that follows anti-cancer therapy; 4) depletion of immune and inflammatory cells that promote tumor development and progression, while sparing cell types and effector functions that support protective immune responses; 5) selective inhibition of tumor promoting cytokines without an effect on expression of anti-tumorigenic cytokines.
In a few cases, a therapy targeting inflammation may be effective as a single agent. For instance, constitutive NF-κB or STAT3 activation in certain lymphoid tumors suggests that inhibitors of these transcription factors can be used as cytocidal agents in such cancers. However in most cases such therapy is likely to be effective only in combination with more conventional approaches. Furthermore, as genotoxic therapies often lead to NF-κB activation in remaining malignant cells, it makes sense to combine genotoxic drugs with NF-κB inhibitors as a way to overcome drug resistance. However, prolonged NF-κB inhibition can result in a severe immune deficiency and may even lead to neutrophilia and greatly enhanced acute inflammation due to enhanced IL-1β secretion (Greten et al., 2007). Such complications as well as increased propensity for liver damage have hindered the clinical development of NF-κB and IKKβ inhibitors. Another attractive target is the STAT3 transcription factor and the signaling pathway that leads to its activation (Kortylewski et al., 2005; Yu et al., 2009). Several STAT3 and JAK2 inhibitors have been described and shown to inhibit the growth of various cancers that exhibit STAT3 activation (Hedvat et al., 2009; Lin et al., 2009). So far, none of the complications associated with NF-κB inhibition have been reported for STAT3 or JAK2 inhibitors.
Even fewer complications should be expected from drugs that inhibit receptor binding of pro-tumorigenic cytokines or chemokines. Several anti-cytokine drugs are already in use for the treatment of chronic inflammatory diseases or are under clinical development for such usage. Although cytokine inhibitors alone are unlikely to cause cancer cell death, several phase I/II clinical trials currently evaluate the efficacy of anti-IL-6 and anti-TNF-α drugs as single agents in various cancers (Balkwill, 2009). The effects obtained so far include disease stabilization and partial responses, but by-and-large the therapeutic effects are modest and underscore the necessity of evaluating such drugs in combination with conventional therapy. Anti-chemokine drugs are also being evaluated, including receptor antagonists and blocking antibodies, targeting CCR2, CCR4, and CXCR4 (Balkwill, 2009). IL-1 inhibition in multiple myeloma slows tumor growth and leads to a chronic disease state, thereby preventing progression to active myeloma (Lust et al., 2009).
Metastasis presents another important application and challenge for drugs that target tumor-associated inflammation. Recently, an anti-RANKL antibody, which was developed for the treatment of osteoporosis, has been found effective in inhibition of bone metastasis in prostate cancer (Hurst et al., 2009). Other experiments done in mice have shown that NF-κB inhibition in metastatic cancer cells or neutralization of TNF-α can convert inflammation promoted metastatic growth to inflammation-induced tumor regression, dependent on IFN-induced TRAIL expression (Luo et al., 2004). Such findings illustrate how manipulation of cytokine expression can be used to convert tumor- and metastasis-promoting inflammation to a strong anti-tumor response.
Conclusions and Prospective
Inflammation can affect every aspect of tumor development and progression as well as the response to therapy. In the past 10 years, we have learned a great deal about the different mechanisms by which cancer and inflammation intersect, and the time is right to translate much of the basic knowledge gained thus far and use it to add new armaments to the arsenal of cancer therapeutics. Only by targeting every single aspect of cancer biology, can we expect to make real gains in the fight against these currently incurable diseases. In addition to a combination of anti-inflammatory approaches that target the tumor microenvironment with more sophisticated and selective tumoricidal drugs, future therapies should also take notice of the natural genetic variation that affects inflammation and immunity. Such considerations are extremely important in the design of new preventive approaches to the reduction of cancer risk that need to be applied to large populations composed of relatively healthy individuals. Indeed, one of the major lessons learned from investigating the relationships between inflammation and cancer, is that most cancers are preventable. Prevention is a much better and more economic way to fight cancer than treating an already advanced and often intractable disease, as is done at the present.
Text Box: Inflammation and cancer-basic facts.
Chronic inflammation increases cancer risk.
Subclinical, often undetectable, inflammation may be as important in increasing cancer risk (for instance, obesity-induced inflammation).
Various types of immune and inflammatory cells are frequently present within tumors.
Immune cells affect malignant cells through production of cytokines, chemokines, growth factors, prostaglandins and reactive oxygen and nitrogen species.
Inflammation impacts every single step of tumorigenesis, from initiation through tumor promotion, all the way to metastatic progression.
In developing tumors anti-tumorigenic and pro-tumorigenic immune and inflammatory mechanisms coexist, but if the tumor is not rejected, the pro-tumorigenic effect dominates.
Signaling pathways that mediate the pro-tumorigenic effects of inflammation are often subject to a feed-forward loop (for example, activation of NF-κB in immune cells induces production of cytokines that activate NF-κB in cancer cells to induce chemokines that attract more inflammatory cells into the tumor).
Certain immune and inflammatory components may be dispensable during one stage of tumorigenesis but absolutely critical in another stage.
Acknowledgements
We thank E. Koltsova for the help in figure preparation. This work was supported by Research Fellowship Award from Crohn’s and Colitis Foundation of America (CCFA #1762) to S.G, by grants from the Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe and the Association for International Cancer Research to F.R.G. and the National Institutes of Health and the American Association for Cancer Research to M.K., who is an American Cancer Society Research Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors declare no competing financial interests.
REFERENCES
- Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- Ammirante M, Luo J-L, Grivennikov S, Nedospasov S, Karin M. B cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010 doi: 10.1038/nature08782. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, Jonat C, Herrlich P, Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007a;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007b;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Bonecchi R, Galliera E, Borroni EM, Corsi MM, Locati M, Mantovani A. Chemokines and chemokine receptors: an overview. Front Biosci. 2009;14:540–551. doi: 10.2741/3261. [DOI] [PubMed] [Google Scholar]
- Buckowitz A, Knaebel HP, Benner A, Blaker H, Gebert J, Kienle P, von Knebel Doeberitz M, Kloor M. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer. 2005;92:1746–1753. doi: 10.1038/sj.bjc.6602534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Calle EE. Obesity and cancer. Br Med J. 2007;335:1107–1108. doi: 10.1136/bmj.39384.472072.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFa-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Cooper CS, Foster CS. Concepts of epigenetics in prostate cancer development. Br J Cancer. 2009;100:240–245. doi: 10.1038/sj.bjc.6604771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martel C, Franceschi S. Infections and cancer: established associations and new hypotheses. Crit Rev Oncol Hematol. 2009;70:183–194. doi: 10.1016/j.critrevonc.2008.07.021. [DOI] [PubMed] [Google Scholar]
- De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gelinas C, Fan Y, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Edwards RA, Witherspoon M, Wang K, Afrasiabi K, Pham T, Birnbaumer L, Lipkin SM. Epigenetic repression of DNA mismatch repair by inflammation and hypoxia in inflammatory bowel disease-associated colorectal cancer. Cancer Res. 2009;69:6423–6429. doi: 10.1158/0008-5472.CAN-09-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- Enzler T, Gillessen S, Manis JP, Ferguson D, Fleming J, Alt FW, Mihm M, Dranoff G. Deficiencies of GM-CSF and interferon gamma link inflammation and cancer. J Exp Med. 2003;197:1213–1219. doi: 10.1084/jem.20021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzler T, Kater AP, Zhang W, Widhopf GF, 2nd, Chuang HY, Lee J, Avery E, Croce CM, Karin M, Kipps TJ. Chronic lymphocytic leukemia of E{micro}-TCL1 transgenic mice undergoes rapid cell turnover that can be offset by extrinsic CD257 to accelerate disease progression. Blood. 2009;114:4469–4476. doi: 10.1182/blood-2009-06-230169. [DOI] [PubMed] [Google Scholar]
- Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, Matthews VB, Armes J, Bhathal PS, Hughes NR, et al. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727–1738. doi: 10.1172/JCI34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Ferreri AJ, Ernberg I, Copie-Bergman C. Infectious agents and lymphoma development: molecular and clinical aspects. J Intern Med. 2009;265:421–438. doi: 10.1111/j.1365-2796.2009.02083.x. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore AM, Simon AK. Positive and negative influences of regulatory T cells on tumour immunity. Oncogene. 2008;27:5886–5893. doi: 10.1038/onc.2008.269. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- Gierach GL, Lacey JV, Jr., Schatzkin A, Leitzmann MF, Richesson D, Hollenbeck AR, Brinton LA. Nonsteroidal anti-inflammatory drugs and breast cancer risk in the National Institutes of Health-AARP Diet and Health Study. Breast Cancer Res. 2008;10:R38. doi: 10.1186/bcr2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granziero L, Ghia P, Circosta P, Gottardi D, Strola G, Geuna M, Montagna L, Piccoli P, Chilosi M, Caligaris-Cappio F. Survivin is expressed on CD40 stimulation and interfaces proliferation and apoptosis in B-cell chronic lymphocytic leukemia. Blood. 2001;97:2777–2783. doi: 10.1182/blood.v97.9.2777. [DOI] [PubMed] [Google Scholar]
- Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKb links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S, Karin M. Dangerous liasons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2009 doi: 10.1016/j.cytogfr.2009.11.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Guidoboni M, Gafa R, Viel A, Doglioni C, Russo A, Santini A, Del Tin L, Macri E, Lanza G, Boiocchi M, et al. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297–304. doi: 10.1016/S0002-9440(10)61695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- Hahn MA, Hahn T, Lee DH, Esworthy RS, Kim BW, Riggs AD, Chu FF, Pfeifer GP. Methylation of polycomb target genes in intestinal cancer is mediated by inflammation. Cancer Res. 2008;68:10280–10289. doi: 10.1158/0008-5472.CAN-08-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada T, Kobayashi T, Chinen T, Saeki K, Takaki H, Koga K, Minoda Y, Sanada T, Yoshioka T, Mimata H, et al. IFNgamma-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J Exp Med. 2006;203:1391–1397. doi: 10.1084/jem.20060436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, Bremer J, Iezzi G, Graf R, Clavien PA, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, et al. The JAK2 Inhibitor AZD1480 Potently Blocks Stat3 Signaling and Oncogenesis in Solid Tumors. Cancer Cell. 2009;16:487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968–979. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Kastritis E, Palumbo A, Dimopoulos MA. Treatment of relapsed/refractory multiple myeloma. Semin Hematol. 2009;46:143–157. doi: 10.1053/j.seminhematol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Khasawneh J, Schulz MD, Walch A, Rozman J, de Angelis M. Hrabe, Klingenspor M, Buck A, Schwaiger M, Saur D, Schmid RM, et al. Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proc Natl Acad Sci U S A. 2009;106:3354–3359. doi: 10.1073/pnas.0802864106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Kometani K, Hashida H, Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M, Takabayashi A, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39:467–475. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005;2:e284. doi: 10.1371/journal.pmed.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mule J, Kerr WG, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9:405–410. doi: 10.1016/j.coph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- Langowski JL, Kastelein RA, Oft M. Swords into plowshares: IL-23 repurposes tumor immune surveillance. Trends Immunol. 2007;28:207–212. doi: 10.1016/j.it.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4:e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Amin R, Gallicano GI, Glasgow E, Jogunoori W, Jessup JM, Zasloff M, Marshall JL, Shetty K, Johnson L, et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene. 2009;28:961–972. doi: 10.1038/onc.2008.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Plummer SJ, Nock NL, Casey G, Witte JS. Nonsteroidal antiinflammatory drugs and decreased risk of advanced prostate cancer: modification by lymphotoxin alpha. Am J Epidemiol. 2006;164:984–989. doi: 10.1093/aje/kwj294. [DOI] [PubMed] [Google Scholar]
- Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- Lust JA, Lacy MQ, Zeldenrust SR, Dispenzieri A, Gertz MA, Witzig TE, Kumar S, Hayman SR, Russell SJ, Buadi FK, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84:114–122. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKb couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2009;125:1298–1305. doi: 10.1002/ijc.24409. [DOI] [PubMed] [Google Scholar]
- Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Musteanu M, Blaas L, Mair M, Schlederer M, Bilban M, Tauber S, Esterbauer H, Mueller M, Casanova E, Kenner L, et al. Stat3 is a negative regulator of intestinal tumor progression in ApcMin mice. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.11.049. in press. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Ben-Neriah Y, Pikarsky E. Inflammation and cancer: is the link as simple as we think? J Invest Dermatol. 2005;124:x–xiv. doi: 10.1111/j.0022-202X.2005.23724.x. [DOI] [PubMed] [Google Scholar]
- O’ Reilly L, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461:659–663. doi: 10.1038/nature08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J. 2008;27:1671–1681. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39:87–92. doi: 10.1136/gut.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki IM, Kotani A, Honjo T. Role of AID in tumorigenesis. Adv Immunol. 2007;94:245–273. doi: 10.1016/S0065-2776(06)94008-5. [DOI] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Hu Z, Barney KA, Degen JL. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133–141. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer R, Osterreicher C, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010 doi: 10.1016/j.cell.2009.12.052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen IM, Kitada S, Leoni LM, Zapata JM, Karras JG, Tsukada N, Kipps TJ, Choi YS, Bennett F, Reed JC. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100:1795–1801. [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punturieri A, Szabo E, Croxton TL, Shapiro SD, Dubinett SM. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst. 2009;101:554–559. doi: 10.1093/jnci/djp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SJ, Ng BY, Filler RB, Lewis J, Glusac EJ, Hayday AC, Tigelaar RE, Girardi M. Characterizing tumor-promoting T cells in chemically induced cutaneous carcinogenesis. Proc Natl Acad Sci U S A. 2007;104:6770–6775. doi: 10.1073/pnas.0604982104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y, Schoppy DW, Asare A, Clark CE, Vonderheide RH, Brown EJ. Tissue regenerative delays and synthetic lethality in adult mice after combined deletion of Atr and Trp53. Nat Genet. 2009;41:1144–1149. doi: 10.1038/ng.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNg and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Shibata W, Takaishi S, Muthupalani S, Pritchard DM, Whary MT, Rogers AB, Fox JG, Betz KS, Kaestner KH, Karin M, et al. Conditional deletion of IkappaB-kinase beta (IKKbeta) accelerates Helicobacter-dependent gastric apoptosis, proliferation and preneoplasia. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.11.054. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shime H, Yabu M, Akazawa T, Kodama K, Matsumoto M, Seya T, Inoue N. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180:7175–7183. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- Sieweke MH, Thompson NL, Sporn MB, Bissell MJ. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-beta. Science. 1990;248:1656–1660. doi: 10.1126/science.2163544. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MR, Yagita H. Nature’s TRAIL--on a path to cancer immunotherapy. Immunity. 2003;18:1–6. doi: 10.1016/s1074-7613(02)00502-2. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, Kaplan DH, Hayday AC, Girardi M. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JB, Uldrich AP, van Dommelen S, Sharkey J, Murray WK, Godfrey DI, Smyth MJ. Type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood. 2009;113:6382–6385. doi: 10.1182/blood-2009-01-198564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, Smyth MJ. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKb and JNK1 dependent inflammation. Cancer Cell. 2010 doi: 10.1016/j.ccr.2009.12.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai A, Toyoshima T, Uemura M, Kitawaki Y, Marusawa H, Hiai H, Yamada S, Okazaki IM, Honjo T, Chiba T, et al. A novel mouse model of hepatocarcinogenesis triggered by AID causing deleterious p53 mutations. Oncogene. 2009;28:469–478. doi: 10.1038/onc.2008.415. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar S, Sarkar S, Wang Y, Singh P. Functional Cross-talk between {beta}-Catenin and NF{kappa}B Signaling Pathways in Colonic Crypts of Mice in Response to Progastrin. J Biol Chem. 2009;284:22274–22284. doi: 10.1074/jbc.M109.020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–648. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- Waldner MJ, Neurath MF. Colitis-associated cancer: the role of T cells in tumor development. Semin Immunopathol. 2009;31:249–256. doi: 10.1007/s00281-009-0161-8. [DOI] [PubMed] [Google Scholar]
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES, Ng EK, Chan FK, Sung JJ, Chan HL. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766–2770. doi: 10.1002/ijc.24281. [DOI] [PubMed] [Google Scholar]
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009a;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009b;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley DA, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Green CL, Tao S, Khavari PA. NF-kappaB RelA opposes epidermal proliferation driven by TNFR1 and JNK. Genes Dev. 2004;18:17–22. doi: 10.1101/gad.1160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Dai H, Zhou M, Li M, Singh P, Qiu J, Tsark W, Huang Q, Kernstine K, Zhang X, et al. Fen1 mutations result in autoimmunity, chronic inflammation and cancers. Nat Med. 2007;13:812–819. doi: 10.1038/nm1599. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]