Abstract
Improvements in performance on many perceptual skills can occur with only a single training session. Of interest here is what aspects of the training experience are being learned during this brief exposure. While there is considerable evidence that learning associated with specific feature values of the stimulus used in training (stimulus learning) contributes to these rapid improvements, there has been little direct investigation of the possibility that other types of learning do so as well. Here we show that not only stimulus learning but also learning of more general aspects of the training experience (procedure learning) contributed to rapid improvements in performance on interaural-time-difference discrimination. However, practice on the type of judgment to be made did not appear to aid performance (no task learning). These results are consistent with physiological reports that different neural mechanisms are engaged at different time points during even a brief training session, and imply that the circuits that are engaged and likely modified vary in the degree of their selectivity to the target condition. Such changes presumably enable further learning.
Keywords: perceptual learning, procedural learning, human, auditory, interaural-time-difference
Introduction
Marked improvements in perceptual ability can result from a single brief training session. Of interest here is what is being learned during this exposure. Our premise is that, because the greatest improvements in performance on perceptual skills typically occur early in an extended course of training, an examination of the learning induced by only a brief training period may reveal aspects of the processes that precede, and perhaps enable, further learning.
In the present investigation, we examined the extent to which improvements in perceptual performance induced by brief training on a basic auditory task could be attributed to each of three possible types of learning: stimulus learning, task learning and procedure learning. By stimulus learning, we mean learning associated with specific feature values of the stimulus used in training (Ahissar & Hochstein, 1996; Robinson & Summerfield, 1996; Rubin, Nakayama, & Shapley, 1997), such as the particular frequency of a tone (Delhommeau, Micheyl, Jouvent, & Collet, 2002; Demany, 1985; Irvine, Martin, Klimkeit, & Smith, 2000) or the particular orientation of a line (Shiu & Pashler, 1992; Vogels & Orban, 1985). We use task learning to denote learning of the specific perceptual judgment to be made (Ahissar & Hochstein, 1993; Karni & Bertini, 1997; Meinhardt & Grabbe, 2002), such as frequency discrimination (Delhommeau, Micheyl, & Jouvent, 2005; Delhommeau et al., 2002; Demany, 1985; Wright, 2001) as opposed to duration discrimination (Wright, 2001; Wright, Buonomano, Mahncke, & Merzenich, 1997). Finally, we use procedure learning to refer to learning of all other components associated with the training experience. These components may include, but are not limited to, the experimental setting (Robinson & Summerfield, 1996), the testing method and response demands (Delhommeau et al., 2002; Demany & Semal, 2002; Hawkey, Amitay, & Moore, 2004; Robinson & Summerfield, 1996), and general strategies for performing the assigned task (Beard, Levi, & Reich, 1995; Demany & Semal, 2002; Fahle, 1994; Fischer, Hallschmid, Elsner, & Born, 2002; Gais, Plihal, Wagner, & Born, 2000; Karni, Tanne, Rubenstien, Askenasy, & et al., 1994; Sowden, Davies, Rose, & Kaye, 1996). Others have applied a different nomenclature to similar concepts. Perceptual learning has been used to refer to our stimulus learning (Demany & Semal, 2002) or to a combination of our task and stimulus learning (Hawkey et al., 2004). Similarly, conceptual or cognitive learning has been used to refer to our procedure learning (Beard et al., 1995; Sireteanu & Rettenbach, 2000) or to a combination of our procedure and task learning (Delhommeau et al., 2002; Demany & Semal, 2002; Recanzone, Schreiner, & Merzenich, 1993; Sowden et al., 1996; Sowden, Rose, & Davies, 2002; Wright & Fitzgerald, 2001).
Of the three learning types discussed here, stimulus learning has been the most frequently examined. Evidence for stimulus learning stems from observations that training often leads to improvements on only the stimulus that was encountered during training (no generalization) or only on stimuli that share a particular feature value in common with the trained stimulus (limited generalization). Thus, this learning is typically revealed by a lack of generalization from a trained stimulus to some or all untrained stimuli. For example, training for 70 or fewer trials on luminance discrimination yielded learning on the trained spatial grating that did not generalize to untrained gratings differing from the trained one in orientation or spatial frequency (Fiorentini & Berardi, 1980). Similarly, approximately one hour of practice discriminating the direction in which vernier stimuli were offset by a given distance yielded learning that generalized to stimuli offset by an untrained distance, but not to stimuli located in a different visual field position (Fahle, Edelman, & Poggio, 1995). Stimulus learning following both multiple-and single-session training has been well documented. In multiple-session training paradigms, it has been reported in the visual (Ahissar & Hochstein, 1996, 1997; Ball & Sekuler, 1982; Doane, Alderton, Sohn, & Pellegrino, 1996; Fahle & Edelman, 1993; Karni & Sagi, 1991; Meinhardt & Grabbe, 2002; Schoups, Vogels, & Orban, 1995; Sowden et al., 2002; Vogels & Orban, 1985), auditory (Delhommeau et al., 2002; Demany & Semal, 2002; Irvine et al., 2000; Mossbridge, Fitzgerald, O'Connor, & Wright, 2006; Wright et al., 1997; Wright & Fitzgerald, 2001) and somatosensory (Nagarajan, Blake, Wright, Byl, & Merzenich, 1998; Sathian & Zangaladze, 1997) systems. Most examples of stimulus learning induced by brief training come from investigations of perceptual learning in the visual system (Casco, Campana, & Gidiuli, 2001; Chou & Vaina, 1995; Fahle et al., 1995; Karni & Sagi, 1993; Poggio, Fahle, & Edelman, 1992; Rubin et al., 1997; Shiu & Pashler, 1992), but there have also been a few reports of similar stimulus-learning patterns in the auditory (Amitay, Irwin, & Moore, 2006; Demany, 1985) and the somatosensory (Harris, Harris, & Diamond, 2001) systems.
In contrast to the numerous cases of stimulus learning following either multiple- or single-session training, nearly all examples of task learning come from experiments incorporating a multiple-session training paradigm. In these experiments, the available evidence for task learning is provided by demonstrations that improvements that occur on a trained task do not generalize to an untrained one, even when the same stimulus is used to assess performance on both (Ahissar & Hochstein, 1993; Ahissar & Hochstein, 1995; Matthews, Liu, Geesaman, & Qian, 1999; Meinhardt, 2002; Meinhardt & Grabbe, 2002; Saffell & Matthews, 2003; Shiu & Pashler, 1992). We are aware of only one test for, and example of, task learning following brief training. In that case, less than 40 minutes of training on auditory intensity discrimination did not yield improvements on auditory frequency discrimination with the same stimulus, whereas the same amount of training on frequency discrimination with that stimulus did (Hawkey et al., 2004).
Finally, little is known about procedure learning regardless of whether training occurs over multiple sessions or a single session. Because we have defined procedure learning as encompassing all aspects of the training experience other than the trained stimulus and task, we do not know what separate components comprise this learning type. Nevertheless, if any of these remaining components were to contribute to training-induced improvements, one manifestation of that influence would be the generalization of learning from the trained stimulus and task to an untrained stimulus and task when all other aspects of the training experience are held constant. Although there is a certain folklore that people who have had prior experience with perceptual experiments perform better in a new experiment than totally naïve participants, we are aware of only two demonstrations of such learning following multiple-session training (Beard et al., 1995; Sireteanu & Rettenbach, 2000), and of only one test for procedure learning using a single practice session (Hawkey et al., 2004). However, in that single-session instance, there was little to no generalization of learning from a visual contrast-discrimination task to an auditory frequency-discrimination task, suggesting that procedure learning had a minimal influence on improvements on frequency discrimination. In other cases in which improvements have been attributed to procedure learning, whether for single- or multiple-session training, task learning could have been a contributing factor because only the stimulus was varied (Ahissar & Hochstein, 1996; Amitay et al., 2006; Delhommeau et al., 2005; Delhommeau et al., 2002; Liu & Weinshall, 2000; Sowden et al., 1996).
Collectively, there is evidence for all three types of learning. However, to date there has been no single experiment in which the contributions of all three learning types to perceptual improvements on a single condition have been simultaneously investigated. We did so in the current experiment. This experiment also differs from the majority of previous investigations in two other respects. First we employed single- rather than multiple-session training because there is so little previous evidence for procedure and task learning early in training. Second, we tested an auditory, rather than a visual, skill as a step toward establishing similarities and differences in perceptual learning across different sensory systems.
Specifically, we investigated the contributions of stimulus, task and procedure learning to early, rapid improvements in interaural-time-difference (ITD) discrimination thresholds. An ITD is the difference in the arrival times of a sound at the two ears, with the sound arriving earlier at the ear closer to the source of the sound (Feddersen, Sandel, Teas, & Jeffress, 1957; Rayleigh, 1907). This between-ear difference is the dominant cue to the horizontal location of a sound source in the free field (Macpherson & Middlebrooks, 2002; Wightman & Kistler, 1992). We chose to evaluate learning on ITD discrimination because we had previously observed that approximately two hours of training induced significant threshold improvements on this discrimination (Wright & Fitzgerald, 2001). However, testing occurred 10 days after training, so the influence of this training after a shorter delay between training and testing has not been established. More importantly, the training in that experiment consisted of exposure to a variety of lateralization conditions, randomly ordered across listeners, making it impossible to determine whether the learning on any one condition resulted from exposure to only that condition or from the generalization of learning from another condition. The relative contributions of stimulus, task and procedure learning to improvements on ITD discrimination are therefore unknown.
As a first step toward establishing the potential contributions of these three learning types to early, rapid improvements on ITD discrimination, we tested listeners on a target ITD-discrimination condition either after no training (naïve listeners) or one day after two hours of training on one of three conditions that shared some similarity with the target ITD condition: (1) a temporal-interval discrimination condition that shared the procedure, but not the task and stimulus, in common with the target condition, (2) an interaural-level-difference (ILD) discrimination condition that shared both the procedure and the lateralization task, but not the stimulus, in common with the target ITD condition, and (3) the target ITD condition itself. Given this hierarchy of similarity between the trained and target conditions, we reasoned that improvements in ITD performance following interval training could be attributed to learning of general, procedural aspects inherent in the training experience, any additional improvements induced by ILD training could be attributed to learning of the specific lateralization task, and beyond that, any additional improvements induced by ITD training could be attributed to learning specific to the stimulus.
Method
Organization of the experiment
All listeners were tested on a target interaural-time-difference (ITD) discrimination condition. Trained listeners completed this target condition as a post-test on the day after training on either (1) the target ITD condition itself (ITD-trained listeners), (2) an interaural-level-difference (ILD) discrimination condition (ILD-trained listeners), or (3) a temporal-interval-discrimination condition (interval-trained listeners). A fourth group performed only the target condition with no prior training (naïve listeners). The three conditions are briefly described below; details can be found in Wright and Fitzgerald (2001; ITD and ILD discrimination) and Wright et al. (1997; temporal-interval discrimination).
Tasks and Stimuli
The ITD condition, which was also the post-test for all listeners, employed a lateralization task and a standard stimulus composed of two 0.5-kHz tones presented simultaneously, one to each ear, over headphones. In one of two observation periods, we presented the two tones with a fixed standard ITD of 0 µs so that the lateral position of the sound image was on or near the median plane inside the head. In the other randomly selected observation period, we presented the tones with a comparison, variable ΔITD that always led at the right ear. The comparison ITD was created by delaying the ongoing phase of the tone to the left ear relative to that to the right ear. Both tones started and ended simultaneously. Listeners were instructed to select the sound that was farther to the right, i.e. the tones with the comparison ITD. The tones were 300 ms in duration, including 10-ms raised cosine rise/fall ramps, and were presented at 70 dB SPL. The two observation intervals were separated by 650 ms.
The ILD-discrimination condition was identical to the target ITD condition in all aspects except the interaural cue and frequency of the stimulus. Similar to an ITD, an ILD is an interaural cue to sound source position on the horizontal plane, but it arises from the difference in the levels, rather than in the arrival times, of a sound at the two ears. The stimuli in the ILD condition were composed of two 300-ms 4-kHz tones, presented one to each ear, with an ITD of 0 µs. For the standard stimulus, the tones were presented at 70 dB SPL to each ear, resulting in a fixed standard ILD of 0 dB so that the lateral position of the sound image was on or near the median plane inside the head. The comparison ILD was equal to the standard ILD plus a variable ΔILD that always favored the right ear, as in the target ITD condition. Tones to the right ear were presented at 70 dB SPL plus 0.5 times the total ΔILD, and tones to the left ear were presented at 70 dB SPL minus 0.5 times the total ΔILD. This technique helped to keep the perceived overall level of the sound image constant across different ILDs.
The temporal-interval-discrimination condition differed from the target ITD condition in both the task and the stimulus. In one of two observation periods, we presented two brief 4-kHz tone pips separated by a standard temporal interval of 100 ms, measured as the time from the onset of the first pip to the onset of the second pip. In the other randomly selected observation period, the two tone pips were separated by a comparison temporal interval equal to 100 ms plus some positive variable interval, Δt. Listeners were instructed to choose the pair of tone pips with the longer (comparison) temporal interval. Tone pips were 15 ms in duration, including 5-ms raised cosine rise/fall ramps, and were presented monaurally at 86 dB SPL. The onsets of the first tone pips in each of the two observation intervals were separated by 900 ms.
All tones were digitally generated using a digital-signal processing board (Tucker-Davis Technologies, AP2). They were delivered through 16-bit digital-to-analog converters (TDT DD1) followed by anti-aliasing filters (8.5-kHz low-pass, TDT FT5), programmable attenuators (TDT PA4) and a headphone buffer (TDT HB6). The sounds were then presented to listeners through Sennheiser HD265 headphones in circumaural cushions. Listeners sat in a sound-attenuated booth.
Threshold Estimation
Discrimination thresholds for all conditions were obtained using an adaptive two-interval, forced-choice (2IFC) paradigm with feedback provided after every trial. Three-down-one-up tracking was used to estimate the 79.4% correct point on the psychometric function (Levitt, 1971). The stimulus level required to obtain this percent correct was called the threshold. Before each block of trials, listeners were presented with samples of the standard sound and of a comparison sound that could be clearly discriminated from the standard sound. Following these samples, on each trial, listeners chose the comparison sound by pressing a key on a computer keyboard. The basis for each threshold estimate was the average value of the greatest even number of reversals (≥ 4) available after excluding the first three or four reversals in a 60-trial block. The step sizes until, and then after, the third reversal were, respectively, 0.2 and 0.05 log-ITD units for the ITD condition, 0.5 and 0.25 dB for the ILD condition, and 10 and 1 ms for the temporal-interval condition. During training, we obtained 20 to 25 threshold estimates from each listener (30 estimates from one). Training lasted about two hours, with breaks occurring approximately every 20 minutes (after every five estimates). Listeners completed the post-test on the day after training. The post-test consisted of four (three trained listeners) to five (45 trained listeners) threshold estimates on the target ITD condition.
Prior to data collection, to confirm that each listener could follow instructions and perform normally on a simple psychoacoustic test, we measured each listener’s detection threshold for a tone presented in a simultaneous noise masker. We obtained one to two threshold estimates from 30-trial blocks. All reported data are from listeners who passed this screening.
Listeners
A total of 138 normal-hearing volunteers (95 females) between the ages of 17 and 38 years (mean: 21.4 years, sd: 4.1) served as listeners. One hundred of these volunteers served as naive ITD listeners, 15 of whom were subsequently trained on the target ITD condition (ITD-trained listeners). An additional 20 participants served as ILD-trained listeners, and 18 as interval-trained listeners. Thirty-four of the listeners received course credit in an undergraduate introductory course in communication sciences and disorders. All other listeners were paid for their participation. None of the listeners had previous experience in any psychoacoustic experiment.
Analyses
Before comparing the performance of different listener groups on the target ITD condition, we removed the data of listeners who performed aberrantly (>1.5 times the interquartile range, beyond the 95% confidence interval) at either the beginning of the experiment or during the post-test. Outliers at the beginning of the experiment were determined by analyzing the first five threshold estimates on ITD-, ILD- or interval discrimination. These data were analyzed as part of large pools of data from naive ITD (n = 100), ILD (n = 106) and interval (n = 28) listeners, ensuring that the values we eliminated were truly unrepresentative of the general population. Post-test outliers were identified by separately analyzing the target ITD post-tests of the ITD-, ILD- and interval-trained groups. Overall, the reported results reflect data from 94 of 100 naïve listeners, 17 of 18 interval-trained listeners, 18 of 20 ILD-trained listeners, and 14 of 15 ITD-trained listeners.
After removing outliers, we compared the ITD discrimination thresholds of the three groups of trained listeners to each other and to naive listeners to determine the effects of the three training regimens. We could not measure the naive ITD-discrimination performance for the ILD- and interval-trained listeners without exposing them to the target ITD condition, and thus potentially influencing their post-training ITD-discrimination thresholds. We therefore assumed that trained and naïve listeners, all of whom were drawn from the same population of normal-hearing young adults, would have had similar ITD pre-training performance. Thus, better ITD performance by any trained group, relative to naïve listeners, was taken as an indication of training-induced improvement, and differences among the various trained groups were used to estimate the degree to which practice on a given condition contributed to overall improvements on the target ITD condition.
Results
Average thresholds on the target ITD condition differed significantly across the listener groups (Fig. 1, filled squares; F[3,139] = 6.71, p < 0.01). The thresholds of ITD-trained listeners (M = 36.67, SD = 17.53) were significantly lower than those of naïve listeners (M = 60.99, SD = 23.18; p < 0.01), replicating previous observations of improvement on ITD threshold with ~2 hours of training (Wright & Fitzgerald, 2001). However, interval- (M = 48.25, SD = 20.22) and ILD-trained (M = 48.47, SD = 18.26) listeners also had ITD thresholds significantly lower than those of naïve listeners (both p ≤ 0.04), and not significantly different from those of ITD-trained listeners (both p ≥ 0.13). Because interval-trained listeners practiced on a condition that differed from the target ITD condition in both the task (temporal-interval vs. lateral-position discrimination) and the stimulus (15-ms 4-kHz tone pips vs. 300-ms, 0.5-kHz tones), the results of these analyses suggest that threshold improvements on ITD discrimination arose solely from procedure learning.
Figure 1.
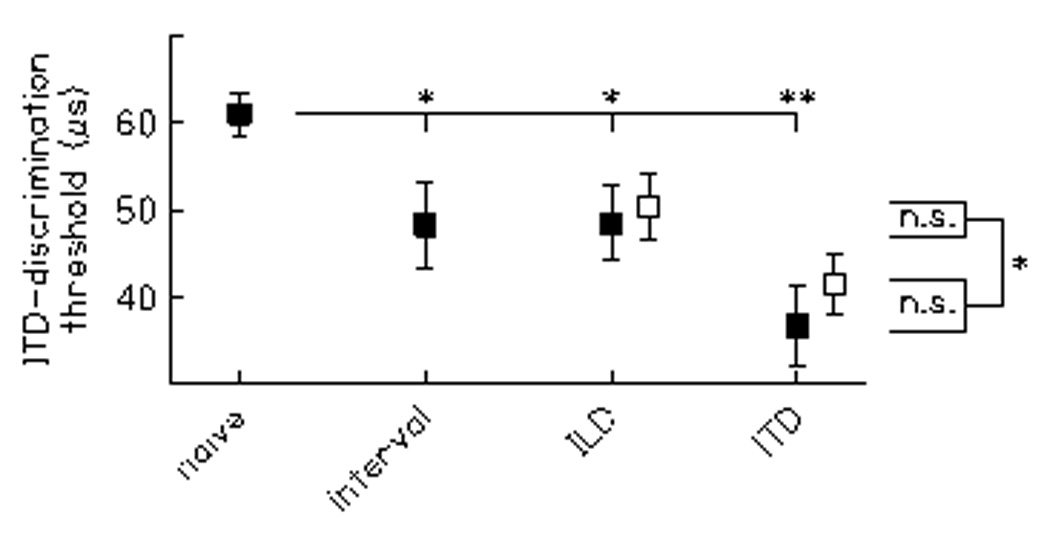
Group means for average thresholds on interaural-time-difference (ITD) discrimination. Filled squares represent data from naïve listeners (n = 94), as well as from three groups of listeners trained for approximately two hours on either temporal-interval (n = 17), interaural-level-difference (ILD, n = 18) or ITD (n = 14) discrimination. Open squares represent supplementary data from listeners trained for approximately 20 minutes on ILD (n = 28) or ITD (n = 17) discrimination. Error bars, s.e.m. Results are based on four to five threshold estimates per listener. *p ≤ 0.05; **p ≤ 0.01.
Nevertheless, there is some indication that stimulus learning may also have contributed to these improvements because the effect size relative to naïve listeners was twice as large for ITD-trained (d = 1.2) than for interval- (d = 0.6) and ILD-trained (d = 0.6) listeners (large versus medium effect size, Cohen, 1988). Further support for this idea arose when the current data were combined with supplementary data from additional ILD- (n = 28, M = 50.29, SD = 20.16) and ITD- (n = 17, M = 41.49, SD = 14.22) trained listeners from whom we obtained only five, rather than 20 to 25, threshold estimates during training (Fig. 1, open squares). The different amounts of training did not affect the amount of threshold improvement on the target ITD condition: a 2 group (ILD- vs. ITD-trained)×2 training amount (five vs. ~25 threshold estimates) ANOVA showed no main effect of training amount (F[1,73] = 0.61, p = 0.44) and no interaction between group and training amount (F[1,73] = 0.12, p = 0.73). However, the ITD thresholds of the ITD-trained listeners were significantly lower than those of the ILD-trained listeners, as revealed by a main effect of group (F[1,73] = 5.87, p = 0.02). The lower thresholds of the ITD-trained listeners in these combined data suggest that some of the threshold improvement in ITD discrimination may be attributed to stimulus learning because the training of ILD- and ITD-trained listeners differed, by our definition, only in the stimulus. Thus, overall, it appears that both procedure and stimulus learning contributed to improvements on ITD-discrimination threshold.
Discussion
The current results suggest that procedure and stimulus learning, but not task learning, contribute to early rapid improvements in ITD discrimination measured the day after a single training session. Evidence for procedure learning comes from the observation that listeners trained on a condition that did not share the task and stimulus in common with the target ITD condition (interval-trained listeners) had significantly lower average thresholds on ITD discrimination than naive listeners. Stimulus learning is revealed by the demonstration that practice on the target ITD stimulus (ITD-trained listeners) yielded significantly lower ITD thresholds than did practice on a condition that differed from the target ITD condition in only the stimulus (ILD-trained listeners). However, task learning did not appear to contribute to improvements on ITD discrimination, as practice on both the target task and procedure (ILD-trained listeners) yielded ITD thresholds that did not differ significantly from those obtained by practicing on the target procedure alone (interval-trained listeners). These data are consistent with numerous previous demonstrations of stimulus learning during early stages of training (see Introduction), and apparently provide the first clear evidence of procedure learning, separated from task and stimulus learning, following brief training. Thus, the present results support the idea that at least two types of learning can contribute to improvements observed early in training.
Although practice on the procedure aided performance, it is not exactly clear what this learning encompassed. Procedure learning may prove to be further divisible, with subtypes potentially including learning of the broad class to which the trained task belongs (e.g., discrimination of sensory stimuli versus memorization of words), the characteristics of the testing environment (e.g., sound-attenuated room versus fMRI scanner) and the testing method (e.g., two-interval forced choice versus identification). However, of these possible factors, there is some indication that learning of the testing method may play a minimal role in procedure learning because in a previous experiment, similar amounts of improvement were seen on a target frequency-discrimination condition employing a two-interval forced choice method, regardless of whether listeners were trained with that method or with a different, three-interval forced choice, method (Hawkey et al., 2004). It is worth noting that exposure to the 30-trial tone detection test at the beginning of the experiment gave all listeners extra practice on the procedure used in the target ITD condition. However, if this additional practice had any influence on ITD thresholds, it would have primarily affected those of naive listeners (who had no intervening training prior to being tested in the target ITD condition), thereby leading to an underestimation of the effect of training for the three trained groups. In any event, the 30-trial exposure was not sufficient for asymptotic procedure learning, because listeners who received two additional hours of training on the target procedure (interval-trained listeners) had ITD thresholds significantly lower than those of naïve listeners.
The observed stimulus learning more clearly reflects improvements in the processing of particular features of the trained stimulus. In the current experiment, ITD-trained listeners appear to have learned something that was unique to the stimulus in the target ITD condition. This stimulus learning likely resulted from modifications in neural circuitry that encodes low-frequency ITDs but not high-frequency ILDs. Similar specificity to the trained cue and frequency is also evident even after multiple hours of training (Wright & Fitzgerald, 2001).
Finally, as with any negative result, there are two potential classes of explanation for the present lack of evidence for task learning. Here we defined task learning as learning of the particular perceptual judgment to be made, in this case, sound lateralization. One possible explanation for the absence of task learning here is that exposure to the lateralization task simply did not benefit performance on the target ITD condition. Another possibility is that practice on lateralization (ILD training) did affect ITD discrimination, but that a different experimental paradigm, such as a different combination of training amount and testing time, is required to reveal this influence. Previous reports of task learning (see Introduction) provide indirect support for the latter explanation.
Taken together, these data are compatible with a framework in which observers select for modification neurons most suited for accomplishing the desired goal (Ahissar & Hochstein, 2004). In the present case, it appears that in a single training session, at least two different neural circuits were selected and modified, and that these circuits differed in the selectivity of their responses to the trained condition, one being broadly-tuned and the other stimulus-specific. Consistent with this conclusion, there is physiological evidence that distinct neural mechanisms that presumably respond, at least in part, to unique aspects of the trained condition are engaged at different time points in the learning process (Atienza, Cantero, & Dominguez-Marin, 2002; Gottselig, Brandeis, Hofer-Tinguely, Borbely, & Achermann, 2004; Karni et al., 1998; Kassubek, Schmidtke, Kimmig, Lucking, & Greenlee, 2001; Pavlides, Miyashita, & Asanuma, 1993; Petersen, van Mier, Fiez, & Raichle, 1998; Toni, Krams, Turner, & Passingham, 1998; Tracy et al., 2001).
Acknowledgments
This work was supported by the National Institutes of Health/National Institute for Deafness and Other Communication Disorders.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/xhp
Contributor Information
Jeanette A. Ortiz, Northwestern University
Beverly A. Wright, Northwestern University
References
- Ahissar M, Hochstein S. Attentional control of early perceptual learning. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(12):5718–5722. doi: 10.1073/pnas.90.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. How early is early vision? Evidence from perceptual learning. In: Papathomas TV, Chubb C, Gorea A, Kowler E, editors. Early Vision and Beyond. Cambridge, Mass: MIT Press; 1995. pp. 199–206. [Google Scholar]
- Ahissar M, Hochstein S. Learning pop-out detection: specificities to stimulus characteristics. Vision Research. 1996;36(21):3487–3500. doi: 10.1016/0042-6989(96)00036-3. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387(6631):401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. The reverse hierarchy theory of visual perceptual learning. Trends in Cognitive Sciences. 2004;8(10):457–464. doi: 10.1016/j.tics.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Amitay S, Irwin A, Moore DR. Discrimination learning induced by training with identical stimuli. Nature Neuroscience. 2006;9(11):1446–1448. doi: 10.1038/nn1787. [DOI] [PubMed] [Google Scholar]
- Atienza M, Cantero JL, Dominguez-Marin E. The time course of neural changes underlying auditory perceptual learning. Learning & Memory. 2002;9(3):138–150. doi: 10.1101/lm.46502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Sekuler R. A Specific and enduring improvement in visual-motion discrimination. Science. 1982;218(4573):697–698. doi: 10.1126/science.7134968. [DOI] [PubMed] [Google Scholar]
- Beard BL, Levi DM, Reich LN. Perceptual-learning in parafoveal vision. Vision Research. 1995;35(12):1679–1690. doi: 10.1016/0042-6989(94)00267-p. [DOI] [PubMed] [Google Scholar]
- Casco C, Campana G, Gidiuli O. Stimulus-specific dynamics of learning in conjunction search tasks. Visual Cognition. 2001;8(2):145–162. [Google Scholar]
- Chou I, Vaina LM. 2-Dimensional symmetrical form discrimination - fast learning, but not that fast. Synthese. 1995;104(1):33–41. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, N.J: L. Erlbaum Associates; 1988. [Google Scholar]
- Delhommeau K, Micheyl C, Jouvent R. Generalization of frequency discrimination learning across frequencies and ears: Implications for underlying neural mechanisms in humans. Jaro-Journal of the Association for Research in Otolaryngology. 2005;6(2):171–179. doi: 10.1007/s10162-005-5055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhommeau K, Micheyl C, Jouvent R, Collet L. Transfer of learning across durations and ears in auditory frequency discrimination. Perception & Psychophysics. 2002;64(3):426–436. doi: 10.3758/bf03194715. [DOI] [PubMed] [Google Scholar]
- Demany L. Perceptual learning in frequency discrimination. Journal of the Acoustical Society of America. 1985;78(3):1118–1120. doi: 10.1121/1.393034. [DOI] [PubMed] [Google Scholar]
- Demany L, Semal C. Learning to perceive pitch differences. Journal of the Acoustical Society of America. 2002;111(3):1377–1388. doi: 10.1121/1.1445791. [DOI] [PubMed] [Google Scholar]
- Doane SM, Alderton DL, Sohn YW, Pellegrino JW. Acquisition and transfer of skilled performance: Are visual discrimination skills stimulus specific? Journal of Experimental Psychology-Human Perception and Performance. 1996;22(5):1218–1248. [Google Scholar]
- Fahle M. Human pattern recognition: Parallel processing and perceptual learning. Perception. 1994;23(4):411–427. doi: 10.1068/p230411. [DOI] [PubMed] [Google Scholar]
- Fahle M, Edelman S. Long-Term learning in vernier acuity - effects of stimulus orientation, range and of feedback. Vision Research. 1993;33(3):397–412. doi: 10.1016/0042-6989(93)90094-d. [DOI] [PubMed] [Google Scholar]
- Fahle M, Edelman S, Poggio T. Fast perceptual learning in hyperacuity. Vision Research. 1995;35(21):3003–3013. doi: 10.1016/0042-6989(95)00044-z. [DOI] [PubMed] [Google Scholar]
- Feddersen WE, Sandel TT, Teas DC, Jeffress LA. Localization of high-frequency tones. Journal of the Acoustical Society of America. 1957;29(9):988–991. [Google Scholar]
- Fiorentini A, Berardi N. Perceptual learning specific for orientation and spatial frequency. Nature. 1980;287(5777):43–44. doi: 10.1038/287043a0. [DOI] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(18):11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nature Neuroscience. 2000;3(12):1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- Gottselig JM, Brandeis D, Hofer-Tinguely G, Borbely AA, Achermann P. Human central auditory plasticity associated with tone sequence learning. Learning & Memory. 2004;11(2):162–171. doi: 10.1101/lm.63304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Harris IM, Diamond ME. The topography of tactile learning in humans. Journal of Neuroscience. 2001;21(3):1056–1061. doi: 10.1523/JNEUROSCI.21-03-01056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey DJ, Amitay S, Moore DR. Early and rapid perceptual learning. Nature Neuroscience. 2004;7(10):1055–1056. doi: 10.1038/nn1315. [DOI] [PubMed] [Google Scholar]
- Irvine DRF, Martin RL, Klimkeit E, Smith R. Specificity of perceptual learning in a frequency discrimination task. Journal of the Acoustical Society of America. 2000;108(6):2964–2968. doi: 10.1121/1.1323465. [DOI] [PubMed] [Google Scholar]
- Karni A, Bertini G. Learning perceptual skills: Behavioral probes into adult cortical plasticity. Current Opinion in Neurobiology. 1997;7(4):530–535. doi: 10.1016/s0959-4388(97)80033-5. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(11):4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365(6443):250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstien BS, Askenasy JJM, et al. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265(5172):679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- Kassubek J, Schmidtke K, Kimmig H, Lucking CH, Greenlee MW. Changes in cortical activation during mirror reading before and after training: an fMRI study of procedural learning. Cognitive Brain Research. 2001;10(3):207–217. doi: 10.1016/s0926-6410(00)00037-9. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. Journal of the Acoustical Society of America. 1971;49(2, Pt. 2):467–477. [PubMed] [Google Scholar]
- Liu ZL, Weinshall D. Mechanisms of generalization in perceptual learning. Vision Research. 2000;40(1):97–109. doi: 10.1016/s0042-6989(99)00140-6. [DOI] [PubMed] [Google Scholar]
- Macpherson EA, Middlebrooks JC. Listener weighting of cues for lateral angle: The duplex theory of sound localization revisited. Journal of the Acoustical Society of America. 2002;111(5):2219–2236. doi: 10.1121/1.1471898. [DOI] [PubMed] [Google Scholar]
- Matthews N, Liu ZL, Geesaman BJ, Qian N. Perceptual learning on orientation and direction discrimination. Vision Research. 1999;39(22):3692–3701. doi: 10.1016/s0042-6989(99)00069-3. [DOI] [PubMed] [Google Scholar]
- Meinhardt G. Learning to discriminate simple sinusoidal gratings is task specific. Psychological Research-Psychologische Forschung. 2002;66(2):143–156. doi: 10.1007/s00426-001-0080-3. [DOI] [PubMed] [Google Scholar]
- Meinhardt G, Grabbe Y. Attentional control in learning to discriminate bars and gratings. Experimental Brain Research. 2002;142(4):539–550. doi: 10.1007/s00221-001-0945-0. [DOI] [PubMed] [Google Scholar]
- Mossbridge JA, Fitzgerald MB, O'Connor ES, Wright BA. Perceptual-learning evidence for separate processing of asynchrony and order tasks. Journal of Neuroscience. 2006;26(49):12708–12716. doi: 10.1523/JNEUROSCI.2254-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan SS, Blake DT, Wright BA, Byl N, Merzenich MM. Practice-related improvements in somatosensory interval discrimination are temporally specific but generalize across skin location, hemisphere, and modality. Journal of Neuroscience. 1998;18(4):1559–1570. doi: 10.1523/JNEUROSCI.18-04-01559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Miyashita E, Asanuma H. Projection from the sensory to the motor cortex is important in learning motor-skills in the monkey. Journal of Neurophysiology. 1993;70(2):733–741. doi: 10.1152/jn.1993.70.2.733. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggio T, Fahle M, Edelman S. Fast perceptual learning in visual hyperacuity. Science. 1992;256(5059):1018–1021. doi: 10.1126/science.1589770. [DOI] [PubMed] [Google Scholar]
- Rayleigh L. On our perception of sound direction. Philosophical Magazine. 1907;13:214–232. [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. Journal of Neuroscience. 1993;13(1):87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K, Summerfield AQ. Adult auditory learning and training. Ear and Hearing. 1996;17(3):S51–S65. doi: 10.1097/00003446-199617031-00006. [DOI] [PubMed] [Google Scholar]
- Rubin N, Nakayama K, Shapley R. Abrupt learning and retinal size specificity in illusory-contour perception. Current Biology. 1997;7(7):461–467. doi: 10.1016/s0960-9822(06)00217-x. [DOI] [PubMed] [Google Scholar]
- Saffell T, Matthews N. Task-specific perceptual learning on speed and direction discrimination. Vision Research. 2003;43(12):1365–1374. doi: 10.1016/s0042-6989(03)00137-8. [DOI] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A. Tactile learning is task specific but transfers between fingers. Perception & Psychophysics. 1997;59(1):119–128. doi: 10.3758/bf03206854. [DOI] [PubMed] [Google Scholar]
- Schoups AA, Vogels R, Orban GA. Human perceptual-learning in identifying the oblique orientation - retinotopy, orientation specificity and monocularity. Journal of Physiology-London. 1995;483(3):797–810. doi: 10.1113/jphysiol.1995.sp020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu LP, Pashler H. Improvement in line orientation discrimination is retinally local but dependent on cognitive set. Perception & Psychophysics. 1992;52(5):582–588. doi: 10.3758/bf03206720. [DOI] [PubMed] [Google Scholar]
- Sireteanu R, Rettenbach R. Perceptual learning in visual search generalizes over tasks, locations, and eyes. Vision Research. 2000;40(21):2925–2949. doi: 10.1016/s0042-6989(00)00145-0. [DOI] [PubMed] [Google Scholar]
- Sowden P, Davies I, Rose D, Kaye M. Perceptual learning of stereoacuity. Perception. 1996;25(9):1043–1052. doi: 10.1068/p251043. [DOI] [PubMed] [Google Scholar]
- Sowden PT, Rose D, Davies IR. Perceptual learning of luminance contrast detection: specific for spatial frequency and retinal location but not orientation. Vision Research. 2002;42(10):1249–1258. doi: 10.1016/s0042-6989(02)00019-6. [DOI] [PubMed] [Google Scholar]
- Toni I, Krams M, Turner R, Passingham RE. The time course of changes during motor sequence learning: A whole-brain fMRI study. Neuroimage. 1998;8(1):50–61. doi: 10.1006/nimg.1998.0349. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Faro SS, Mohammed F, Pinus A, Christensen H, Burkland D. A comparison of 'Early' and 'Late' stage brain activation during brief practice of a simple motor task. Cognitive Brain Research. 2001;10(3):303–316. doi: 10.1016/s0926-6410(00)00045-8. [DOI] [PubMed] [Google Scholar]
- Vogels R, Orban GA. The effect of practice on the oblique effect in line orientation judgments. Vision Research. 1985;25(11):1679–1687. doi: 10.1016/0042-6989(85)90140-3. [DOI] [PubMed] [Google Scholar]
- Wightman FL, Kistler DJ. The dominant role of low-frequency interaural time differences in sound localization. Journal of the Acoustical Society of America. 1992;91(3):1648–1661. doi: 10.1121/1.402445. [DOI] [PubMed] [Google Scholar]
- Wright BA. Why and how we study human learning on basic auditory tasks. Audiology and Neuro-Otology. 2001;6(4):207–210. doi: 10.1159/000046834. [DOI] [PubMed] [Google Scholar]
- Wright BA, Buonomano DV, Mahncke HW, Merzenich MM. Learning and generalization of auditory temporal-interval discrimination in humans. Journal of Neuroscience. 1997;17(10):3956–3963. doi: 10.1523/JNEUROSCI.17-10-03956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BA, Fitzgerald MB. Different patterns of human discrimination learning for two interaural cues to sound-source location. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(21):12307–12312. doi: 10.1073/pnas.211220498. [DOI] [PMC free article] [PubMed] [Google Scholar]


