Abstract
DNA interstrand crosslinks (ICLs) can arise from reactions with endogenous chemicals, such as the lipid peroxidation product malondialdehyde, or from exposure to a variety of clinical anti-cancer drugs, most notably bifunctional alkylators and platinum compounds. Because they covalently link the two strands of DNA, ICLs are absolute blocks to transcription and replication, and as a result, lethal to the cell. It is well established that proteins of nucleotide excision repair and homologous recombination are involved in ICL resolution. Recent work, coupled with a much earlier report, suggest an emerging link between proteins of the base excision repair pathway and crosslink processing.
Overview
DNA interstrand crosslinks (ICLs) consist of a covalent linkage that physically connects the two strands of DNA. As ICLs prevent strand separation, they are absolute blocks to processes such as DNA replication or RNA transcription. Consequently, ICLs are potent toxic DNA lesions that have the ability to promote either genetic instability or cell death, and as a result, contribute to disease and aging1. Whereas prior studies have clearly revealed the contributions of components of nucleotide excision repair (NER) and homologous recombination in the resolution of ICLs, we discuss herein emerging evidence that implicates proteins of base excision repair (BER) in the response to crosslinking agents. Specifically, the nei endonuclease VIII-like 1 (NEIL1) DNA glycosylase and the N-methylpurine DNA glycosylase (MPG; we note that MPG, the approved HUGO nomenclature, is often referred to as AAG in the literature), as well as the major apurinic/apyrimidinic (AP) endonuclease (APE1), contribute to cellular resistance to crosslinking agents. Furthermore, biochemical studies suggest that NEIL1 processes intermediates in the crosslink repair pathway.
Formation of ICLs
ICLs can be formed by both endogenous and exogenous chemical agents (Figure 1). For example, in the former case, malondialdehyde, which is a product of lipid peroxidation, can create etheno adducts in DNA that can undergo rearrangement to create an ICL2. In the latter case, aldehydes, such as acrolein and crotonaldehyde found in tobacco smoke or automotive exhaust, can react with DNA bases in a way that generates ICLs3,4. In addition, psoralens, which are found as natural products in plants, can be activated by long-wave ultraviolet light (UVA) to form covalent monoadducts with pyrimidines or an ICL between the thymines of TA sequences in opposite strands5. Psoralen + UVA is widely used in the treatment of psoriasis, eczema, vitiligo, and cutaneous T-cell lymphoma. Finally, many of the chemotherapeutic agents used today to eradicate cancer involve the formation of DNA ICLs to induce cell killing, including bifunctional alkylating agents, such as 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU, Carmustine) and nitrogen mustard, and the platinum compounds, such as cisplatin (cis-diamminedichloroplatinum(II)) and its derivative forms6,7.
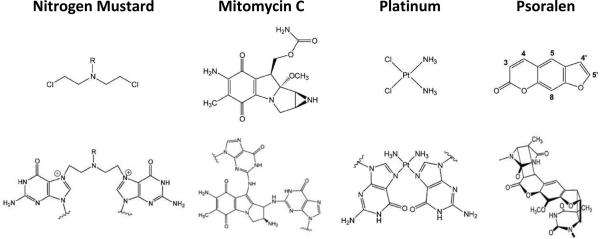
Figure 1.
Examples of DNA Crosslinking Agents. The chemical structure of the indicated agent is shown in the top row. The bottom row depicts the nature of the ICL. (i) Nitrogen mustards form a 1,3 ICL between the N7-positions of two G residues on opposite strands within a 5'-d(GNC) sequence. Mitomycin C (MMC; ii) and cisplatin (iii) generate ICLs between juxtapose Gs of 5'-d(GC). (iv) [SC1]Psoralen can produce ICLs between the T residues of 5'-d(TA) complementary sequences upon exposure to UVA. The frequency of ICLs for cisplatin is around 5–10% of the total adducts, about 5% for nitrogen mustards, less than 10% for MMC, and 20–30% for psoralen. See50 for further details.
Major Repair Mechanisms for ICLs
As ICLs involve covalent linkage of the two strands of DNA, the crosslink must be “unhooked” to allow for strand separation required for either transcription of a coding region or replication of the genome. In the absence of crosslink resolution, the damage will likely result in cell death, or minimally, in chromosomal instability to permit survival. There are multiple pathways for ICL repair, variable as to the cell cycle phase and mechanism of ICL recognition, each requiring two stages to complete removal of the adduct (see8 for review).
In G1 phase (Figure 2a), ICL repair is dependent on the xeroderma pigmentosum group C (XP-C)–human RAD23 homolog (HR23B) protein complex, which binds helical distortions, and directs adducts into the NER pathway, a process defective in the UV sensitive, cancer-prone syndrome XP9. Alternatively, ICLs can be encountered by the transcriptional apparatus and enter a transcription-coupled repair pathway, again requiring NER functions10. Excision repair cross-complementing 1 (ERCC1)–XP-F-dependent incision of one strand on either side of the crosslinked base unhooks the duplex and generates an intermediate that is often depicted as a gapped structure, but likely requires further processing11,12. In particular, it is believed that the incised oligonucleotide is digested by nucleases (SNM1A is a candidate13) to form a gapped intermediate, with the adducted base in the non-incised strand linked to at least one base from the excised oligonucleotide. This crosslink “remnant” will be present during gap-filling synthesis by lesion bypass polymerases. Recent cellular and biochemical work implicates polymerases κ, η and ζ, and the deoxycytidyl transferase REV1, in this process14–16. Moreover, the bypass step can be the source of mutations, which appear to be dependent on polymerase ζ17,18. Closure of the gap restores the duplex (completion of stage I) and forces out the remnant monoadduct, which then enters a second repair cycle involving classic NER processing (stage II).
Figure 2.
Conventional Mechanisms for ICL Repair. Cells cope with ICLs differently depending on the stage of cell cycle and mode of ICL recognition. a) G1 phase repair. ICL recognition is mediated by XPC–HR23B dependent functions, or perhaps by a block to transcription (not shown). The ERCC1–XPF endonuclease is required for crosslink “unhooking”, in which incisions are placed on either side of the linked base on one strand of the duplex. Further processing, perhaps by the SNM1A nuclease, converts the incision product to a gapped structure that can be filled-in by a translesion DNA synthesis (TLS) polymerase. This step completes the first stage of ICL repair and produces an intact duplex with a crosslink remnant that is a substrate for classic NER51, which carries out the second and final stage of ICL repair. b) S phase repair. Recognition as a consequence of collision of the ICL with a progressing replication fork is followed by cleavages by MUS81–EME1 and ERCC1–XPF which unhook the crosslink. Additional processing as in G1 phase results in lesion bypass [with the aid of Fanconi anemia (FA) proteins] to generate a crosslink remnant. Classic NER excises and replaces the segment of DNA harboring the remnant, before the replication fork is reestablished by homologous recombination.
In S phase cells (Figure 2b), the encounter of a replication fork with a crosslink triggers a complex response in which the crosslink is removed and the fork reconstructed. The current view is that the stalled fork is recognized by a number of proteins (including FANCM and FAAP24, which are proteins defective in the rare inherited cancer-prone, blood disorder Fanconi anemia (FA)) followed by incision by the MUS81–EME1 nuclease on one side of the crosslink; ERCC1–XP-F makes a second incision on the other side (reviewed in19,20). This series of events unhooks the crosslink, setting the stage for processing of the incised crosslinked oligonucleotide and gap repair. The FA proteins participate in lesion bypass synthesis21, and are required for mutagenesis22. Classic NER functions, other than the nuclease activity of the ERCC1–XP-F complex, do not play a role in the first stage of repair. Following gap filling, the crosslink remnant is excised in a second stage of repair by the classic NER pathway. Once the ICL has been completely removed, the fork can be reconstructed by homologous recombination (involving additional FA proteins, including the breast cancer-associated protein, FANCD1; also called BRCA2, among others), and replication renewed.
BER Pathway
BER is an evolutionarily conserved pathway that copes primarily with spontaneous decay products and oxidative damage that arises in DNA. In addition, the pathway corrects non-bulky base modifications, abasic (or AP) sites and single-strand breaks that are generated by environmental agents such as alkylators or ionizing radiation. In its most simplistic form, BER involves five major steps (Figure 3): (i) release of the damaged substrate base from the intact sugar-phosphate backbone by a DNA glycosylase, (ii) cleavage of the phosphodiester linkage immediately adjacent to the abasic site product by an AP endonuclease, (iii) clean-up of the 3' and/or 5' termini by a collection of enzymes to create a strand break that harbors a 3'-hydroxyl priming group and a 5'-phosphate, (iv) replacement of the missing nucleotide by a DNA polymerase, and (v) sealing of the final nick by a DNA ligase (see23–26 for reviews). Defects in this pathway have been linked either directly or indirectly to cancer predisposition and neurodegenerative disease27.
Figure 3.

BER Pathway. The five enzymatic steps of BER are indicated to the left, and the major proteins involved in executing these steps are shown to the right. In brief, a DNA glycosylase (e.g. MPG or NEIL1) cleaves the bond that links the substrate base (filled diamond) to the sugar phosphate backbone, creating an AP site intermediate (filled circle). The AP site is typically incised by AP endonuclease 1 (APE1) 5' to the lesion to create a strand break with a 3'-hydroxyl group and a 5'-abasic residue (deoxyribose phosphate, dRP). Polymerase β (POLβ) then replaces the missing nucleotide (thicker line) and excises the 5'-dRP group. The remaining nick is sealed by a complex comprising X-ray repair cross-complementing 1 (XRCC1) and DNA ligase 3 (LIG3α). Shown is short-patch (or single nucleotide) BER, but there are alternative BER-related responses, such as long-patch BER, which involves the incorporation of multiple nucleotides and the formation of a 5'-flap DNA intermediate that requires processing by flap-endonuclease 1 (FEN1) (see26,52 for further details).
Participation of BER in the Response to ICLs
Typically we think of DNA glycosylases as processing simple, non-bulky base adducts. However, early findings that suggested a role for BER factors in the removal of complex DNA lesions were the observation of binding of MPG to cisplatin intrastrand diadducts28, and the mild hypersensitivities of homozygous Mpg-deficient mouse cells to BCNU and mitomycin C (MMC)29,30. At the time, it was unknown whether or not this increased sensitivity resulted from impaired removal of ICLs or one of the many precursor monoadducts. Moreover, yeast cells lacking the equivalent DNA glycosylase (MAG1) were shown to be hypersensitive to nitrogen mustard treatment31. More recently, Samson and colleagues reported that Mpg−/− mouse embryonic stem cells are more sensitive than their wild-type counterparts to the interstrand crosslinking agent 4,5',8-trimethylpsoralen, but behave normally when challenged with angelicin, a psoralen derivative which forms mainly monoadducts and no crosslinks32,33. In addition, Mpg−/− cells displayed an altered double-strand break response and increased apoptosis when exposed to 4,5',8-trimethylpsoralen and UVA. The lack of increased sensitivity to angelicin and the appearance of the double-strand break marker γ-H2AX (albeit with abnormal kinetics) in Mpg-deficient cells implies that the protein does not function in monoadduct repair or prevent the formation of ICLs. In vitro studies of the Samson group show that MPG has no obvious glycosylase activity on DNA substrates harboring a MMC ICL, an N2-guanine monoadduct, or a monoadduct produced by 4,5',8-trimethylpsoralen or 8-methoxypsoralen30,32, suggesting a non-enzymatic, structural role for MPG in the early steps of ICL resolution (Figure 4). Moreover, MPG, at least under conditions optimal for binding cognate base damage, does not form a stable protein–DNA complex with ICL-containing DNA in electrophoretic mobility shift assays, suggesting that the protein does not play a direct role in ICL recognition.
Figure 4.
Potential Involvement of BER Proteins in ICL Resolution. NEIL1 might initiate a classic BER response by excising (i) a psoralen monoadduct (MA) prior to the commencement of Stage I of ICL repair or (ii) the crosslink remnant (CR) formed at the completion of the first stage of ICL repair (see also Figure 2). This excision (glycosylase) activity would generate an abasic site (filled circle) that would be processed by APE1 and the remaining BER proteins (Figure 3). The precise involvement of MPG is less clear, but evidence suggests participation after ICL formation in a structural, non-enzymatic capacity.
Prompted in part by the observation that cells depleted for APE1 exhibit hypersensitivity to MMC, Saparbaev and colleagues initiated an effort to evaluate the contribution of BER to psoralen–DNA photoadducts34. Strikingly, they found that siRNA depletion of APE1 resulted in a hypersensitivity of HeLa cells to 8-methoxypsoralen + UVA similar to that seen with cells depleted for FANCD2. Subsequent biochemical studies revealed that NEIL1, but not the human glycosylases MPG, NEIL2, nth endonuclease III-like 1 (NTH1) or 8-oxoguanine DNA-glycosylase 1 (OGG1), was able to act in concert with APE1 to initiate repair of photoactivated psoralen-induced DNA monoadducts, but not ICLs, presumably via a classic BER-type response (Figure 4). Consistently, Neil1-deficient cells exhibited increased sensitivity to 8-methoxypsoralen + UVA. Unfortunately, it was not determined whether Neil1 or Ape1 mutant cells are hypersensitive to angelicin. In a more recent report35, Saparbaev and colleagues found that NEIL1 efficiently excises an unhooked ICL fragment within a three-stranded DNA structure, suggesting that the glycosylase can also operate at the later stages of ICL removal (Figure 4).
Concluding Remarks and Future Perspectives
Participation of DNA Glycosylases in ICL Resolution
Current evidence suggests that a traditional BER response involving excision of the modified base from the sugar-phosphate backbone could operate as an alternative mechanism in crosslinking agent resistance to the major protective pathways, namely NER and homologous recombination (Figure 2). Specifically, biochemical data indicate a direct role for NEIL1 glycosylase function in the removal of the initial precursor monoadducted base (i.e. before ICL formation) or the unhooked three-stranded base remnant product (i.e. prior to stage II repair) (Figure 4). As NEIL1 typically processes base modifications such as formamidopyrimidine and 5-hydroxyuracil, which involve relatively subtle chemical changes to DNA, how NEIL1 recognizes and promotes enzymatic release of such sizeable DNA lesions needs to be determined.
How might NEIL1 recognize a psoralen monoadduct? Glycosylases are generally thought to bind specifically to modified bases that exhibit increased residence in an extrahelical configuration36,37. However, the suggestion that NEIL1 is active on a psoralen–T monoadduct requires additional consideration. The reaction of psoralen with thymidine destroys the aromaticity and planarity of the thymine ring, and as a result, the stacking interactions of the thymine are suppressed. NMR analysis of psoralen monoadducts, however, revealed intercalation of the psoralen attached to the thymidine, accompanied by stacking of the psoralen with the adjacent adenines38. This feature actually reduced the frequency of flipping of the adducted thymidine out of the helix. Nevertheless, as pointed out by the Spielmann group39,40, the structural interactions of other bases in the vicinity of the monadduct were perturbed. They proposed that these perturbations could be recognized by DNA damage recognition proteins. Thus, although the frequency of extrahelical presentation of a thymidine would be reduced by the psoralen monoadduct, the affinity of a repair protein, such as NEIL1, for the immediate vicinity might be increased. Consequently, the glycosylase would spend relatively more time near the monoadducted thymidine, increasing the probability of capturing the base when it does become extrahelical. Of course all of this conjecture is predicated on the ability of the glycosylase active site to accommodate the rather large base modification, a fact that will need to be verified by further studies. Alternatively, it remains a possibility that the NEIL1 glycosylase actually excises an unstable neighboring base, as opposed to the monoadduct itself, facilitating a subsequent nearby incision event.
As for an ICL, the linked bases have no opportunity to become extrahelical, and so would not be substrates for a DNA glycosylase. If, however, the ICL enters a classic repair response, then a crosslink remnant will appear as a product of the first repair cycle (Figure 2). The restoration of the duplex following repair synthesis across the crosslinked base of the intact strand might force the base, and any joining bases remaining from the incised fragment, out of the helix. If that occurred, then the extrahelical structure could be a substrate for a glycosylase such as NEIL1 or possibly MPG. Obviously, this scenario similarly requires that the thymine (in the case of psoralen) with its bulky additions be accommodated in the active site of the glycosylase41,42. It would therefore be of interest to compare the kinetics of cleavage by the glycosylases on duplexes containing a “classic” base substrate (e.g. formamidopyrimidine), a psoralen monoadduct, or a psoralen-T crosslink remnant. It should be emphasized that the work on MPG suggests that this protein does not function in the excision of monoadducts (e.g. angelicin), is not involved in crosslink recognition, and contributes at some step after initial ICL formation in a non-enzymatic/structural capacity (Figure 4). Whether the DNA glycosylase activity of MPG is required for crosslinking agent resistance should be further evaluated using a catalytically-inactive MPG protein in complementation assays.
BER in the Cellular Context
When considering the recruitment and activity of glycosylases on the various crosslink substrates, it is important to note that crosslinks are the targets of other DNA repair pathways, including NER8. Thus, entry of the different DNA structures into NER can obscure a role for glycosylases in the repair of these adducts in vivo. Consequently, experiments designed to examine a role for DNA glycosylases at various stages in crosslink repair would probably be best performed in NER-deficient cells, in which a major competing repair pathway has been removed. Furthermore, when assessing the influence of a glycosylase deficiency on the sensitivity of cells to crosslinking agents, it would again be best examined in an NER-deficient background. Additionally, the nature of the crosslinking agent is an important consideration, as different agents will elicit different repair responses. For example, MMC forms crosslinks that are relatively non-distorting and thus not engaged effectively by NER43. Conversely, psoralen forms more distorting crosslinks44, which can be processed by the NER pathway45,46. As crosslinks can be “recognized” by replication forks, it will also be necessary to consider a contribution for DNA glycosylases in ICL repair in the context of a replication fork, in addition to a non-replicative setting.
Strengthening the Link to BER
Recently, using a photo-crosslinking technique, Lippard and colleagues found that XRCC1, a nuclear protein that contributes to single-strand break repair and BER, binds to cisplatin-DNA ICLs47. This observation expands the list of BER-related factors that might participate in ICL recognition and resolution, as well as the spectrum of ICL chemical forms that could be processed by a BER-type response. How broadly BER might function in crosslinking agent resistance is presently unclear, and it would be valuable to explore the sensitivity of additional BER-deficient cell lines to various crosslinking compounds (for instance those in Figure 1). However, it is important to keep in mind that treatment with most exogenous agents, including MMC and cisplatin, induces not only a range of DNA crosslinks and monoaddcuts, but also oxidative stress and an assortment of associated oxidative DNA base lesions, which are known to be substrates for DNA glycosylases such as MPG (hypoxanthine and 1,N(6)-ethenoadenine) and NEIL1. As an additional strategy to evaluate the contribution of BER to ICL repair, the use of modified versions of ICLs that permit easy visualization would allow one to monitor the excision kinetics of such DNA lesions in a particular repair-deficient background48. It will also be interesting, using technologies that employ site-specific damage-introduction and protein re-localization detection, to define with greater accuracy the molecular stages at which BER factors arrive in response to ICLs49. Obviously, defining the molecular mechanisms of ICL repair; the BER proteins that participate in this process, either directly, as auxiliary factors, or in a backup capacity; and the extent to which BER operates in crosslinking agent resistance are important steps towards the design of more effective treatment strategies that involve clinical DNA crosslinking drugs.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. We thank Drs. Robert Brosh (NIA), Parameswary Muniandy (NIA), and James Stivers (Johns Hopkins Medical Institute) for valuable input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Grillari J, et al. Contributions of DNA interstrand cross-links to aging of cells and organisms. Nucleic Acids Res. 2007;35:7566–7576. doi: 10.1093/nar/gkm1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Summerfield FW, Tappel AL. Detection and measurement by high-performance liquid chromatography of malondialdehyde crosslinks in DNA. Anal. Biochem. 1984;143:265–271. doi: 10.1016/0003-2697(84)90662-6. [DOI] [PubMed] [Google Scholar]
- 3.Kozekov ID, et al. Interchain cross-linking of DNA mediated by the principal adduct of acrolein. Chem. Res. Toxicol. 2001;14:1482–1485. doi: 10.1021/tx010127h. [DOI] [PubMed] [Google Scholar]
- 4.Cho YJ, et al. Stereospecific formation of interstrand carbinolamine DNA cross-links by crotonaldehyde- and acetaldehyde-derived alpha-CH3-gamma-OH-1,N2-propano-2'-deoxyguanosine adducts in the 5'-CpG-3' sequence. Chem. Res. Toxicol. 2006;19:195–208. doi: 10.1021/tx050239z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole RS. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim. Biophys. Acta. 1970;217:30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- 6.Drablos F, et al. Alkylation damage in DNA and RNA--repair mechanisms and medical significance. DNA Repair (Amst) 2004;3:1389–1407. doi: 10.1016/j.dnarep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muniandy PA, et al. DNA interstrand crosslink repair in mammalian cells: step by step. Crit Rev. Biochem. Mol. Biol. 2009 doi: 10.3109/10409230903501819. PMID: 20039786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkar S, et al. DNA interstrand crosslink repair during G1 involves nucleotide excision repair and DNA polymerase zeta. EMBO J. 2006;25:1285–1294. doi: 10.1038/sj.emboj.7600993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vos JM, Hanawalt PC. Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell. 1987;50:789–799. doi: 10.1016/0092-8674(87)90337-0. [DOI] [PubMed] [Google Scholar]
- 11.Li L, et al. Interstrand cross-links induce DNA synthesis in damaged and undamaged plasmids in mammalian cell extracts. Mol. Cell Biol. 1999;19:5619–5630. doi: 10.1128/mcb.19.8.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuraoka I, et al. Repair of an interstrand DNA cross-link initiated by ERCC1-XPF repair/recombination nuclease. J. Biol. Chem. 2000;275:26632–26636. doi: 10.1074/jbc.C000337200. [DOI] [PubMed] [Google Scholar]
- 13.Hazrati A, et al. Human SNM1A suppresses the DNA repair defects of yeast pso2 mutants. DNA Repair (Amst) 2008;7:230–238. doi: 10.1016/j.dnarep.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Minko IG, et al. Role for DNA polymerase kappa in the processing of N2-N2-guanine interstrand cross-links. J. Biol. Chem. 2008;283:17075–17082. doi: 10.1074/jbc.M801238200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicks JK, et al. Differential roles for DNA Polymerases Eta, Zeta and REV1 in lesion bypass of intrastrand versus interstrand DNA cross-links. Mol. Cell Biol. 2009 doi: 10.1128/MCB.00993-09. PMID: 20028736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zietlow L, et al. Evidence for the involvement of human DNA polymerase N in the repair of DNA interstrand cross-links. Biochemistry. 2009;48:11817–11824. doi: 10.1021/bi9015346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards S, et al. Triplex targeted genomic crosslinks enter separable deletion and base substitution pathways. Nucleic Acids Res. 2005;33:5382–5393. doi: 10.1093/nar/gki851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen X, et al. REV3 and REV1 play major roles in recombination-independent repair of DNA interstrand cross-links mediated by monoubiquitinated proliferating cell nuclear antigen (PCNA) J. Biol. Chem. 2006;281:13869–13872. doi: 10.1074/jbc.C600071200. [DOI] [PubMed] [Google Scholar]
- 19.Moldovan GL, D'Andrea AD. How the fanconi anemia pathway guards the genome. Annu. Rev. Genet. 2009;43:223–249. doi: 10.1146/annurev-genet-102108-134222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson LH, Hinz JM. Cellular and molecular consequences of defective Fanconi anemia proteins in replication-coupled DNA repair: mechanistic insights. Mutat. Res. 2009;668:54–72. doi: 10.1016/j.mrfmmm.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knipscheer P, et al. The Fanconi anemia pathway promotes replication-dependent DNA interstrand cross-link repair. Science. 2009;326:1698–1701. doi: 10.1126/science.1182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirchandani KD, et al. The Fanconi anemia core complex is required for efficient point mutagenesis and Rev1 foci assembly. DNA Repair (Amst) 2008;7:902–911. doi: 10.1016/j.dnarep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida KH, Sobol RW. A unified view of base excision repair: lesion-dependent protein complexes regulated by post-translational modification. DNA Repair (Amst) 2007;6:695–711. doi: 10.1016/j.dnarep.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegde ML, et al. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 2008;18:27–47. doi: 10.1038/cr.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maynard S, et al. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson AB, et al. DNA repair in mammalian cells: Base excision repair: the long and short of it. Cell Mol. Life Sci. 2009;66:981–993. doi: 10.1007/s00018-009-8736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson DM, III, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair (Amst) 2007;6:544–559. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Kartalou M, et al. Cisplatin adducts inhibit 1,N(6)-ethenoadenine repair by interacting with the human 3-methyladenine DNA glycosylase. Biochemistry. 2000;39:8032–8038. doi: 10.1021/bi000417h. [DOI] [PubMed] [Google Scholar]
- 29.Engelward BP, et al. Repair-deficient 3-methyladenine DNA glycosylase homozygous mutant mouse cells have increased sensitivity to alkylation-induced chromosome damage and cell killing. EMBO J. 1996;15:945–952. [PMC free article] [PubMed] [Google Scholar]
- 30.Allan JM, et al. Mammalian 3-methyladenine DNA glycosylase protects against the toxicity and clastogenicity of certain chemotherapeutic DNA cross-linking agents. Cancer Res. 1998;58:3965–3973. [PubMed] [Google Scholar]
- 31.McHugh PJ, et al. Excision repair of nitrogen mustard-DNA adducts in Saccharomyces cerevisiae. Nucleic Acids Res. 1999;27:3259–3266. doi: 10.1093/nar/27.16.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maor-Shoshani A, et al. 3-Methyladenine DNA glycosylase is important for cellular resistance to psoralen interstrand cross-links. DNA Repair (Amst) 2008;7:1399–1406. doi: 10.1016/j.dnarep.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant EL, et al. Mutagenicity of cross-links and monoadducts of furocoumarins (psoralen and angelicin) induced by 360-nm radiation in excision-repair-defective and radiation-insensitive strains of Saccharomyces cerevisiae. Environ. Mutagen. 1979;1:55–63. doi: 10.1002/em.2860010112. [DOI] [PubMed] [Google Scholar]
- 34.Couve-Privat S, et al. Psoralen-induced DNA adducts are substrates for the base excision repair pathway in human cells. Nucleic Acids Res. 2007;35:5672–5682. doi: 10.1093/nar/gkm592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couve S, et al. The human oxidative DNA glycosylase NEIL1 excises psoralen-induced interstrand DNA cross-links in a three-stranded DNA structure. J. Biol. Chem. 2009;284:11963–11970. doi: 10.1074/jbc.M900746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang W. Structure and mechanism for DNA lesion recognition. Cell Res. 2008;18:184–197. doi: 10.1038/cr.2007.116. [DOI] [PubMed] [Google Scholar]
- 37.Stivers JT. Extrahelical damaged base recognition by DNA glycosylase enzymes. Chemistry. 2008;14:786–793. doi: 10.1002/chem.200701501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spielmann HP, et al. DNA structural reorganization upon conversion of a psoralen furan-side monoadduct to an interstrand cross-link: implications for DNA repair. Proc. Natl. Acad. Sci. U. S. A. 1995;92:2345–2349. doi: 10.1073/pnas.92.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spielmann HP. Dynamics in psoralen-damaged DNA by 1H-detected natural abundance 13C NMR spectroscopy. Biochemistry. 1998;37:5426–5438. doi: 10.1021/bi972536b. [DOI] [PubMed] [Google Scholar]
- 40.Isaacs RJ, Spielmann HP. A model for initial DNA lesion recognition by NER and MMR based on local conformational flexibility. DNA Repair (Amst) 2004;3:455–464. doi: 10.1016/j.dnarep.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Imamura K, et al. Structural characterization of a viral NEIL1 ortholog unliganded and bound to abasic site-containing DNA. J. Biol. Chem. 2009;284:26174–26183. doi: 10.1074/jbc.M109.021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lau AY, et al. Molecular basis for discriminating between normal and damaged bases by the human alkyladenine glycosylase, AAG. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13573–13578. doi: 10.1073/pnas.97.25.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomasz M. Mitomycin C: small, fast and deadly (but very selective) Chem. Biol. 1995;2:575–579. doi: 10.1016/1074-5521(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 44.Eichman BF, et al. The crystal structures of psoralen cross-linked DNAs: drug-dependent formation of Holliday junctions. J. Mol. Biol. 2001;308:15–26. doi: 10.1006/jmbi.2001.4567. [DOI] [PubMed] [Google Scholar]
- 45.Cleaver JE, et al. Formation and repair of psoralen-DNA adducts and pyrimidine dimers in human DNA and chromatin. Environ. Health Perspect. 1985;62:127–134. doi: 10.1289/ehp.8562127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wauthier EL, et al. Differential repair and replication of damaged DNA in ribosomal RNA genes in different CHO cell lines. J. Cell Biochem. 1990;43:173–183. doi: 10.1002/jcb.240430208. [DOI] [PubMed] [Google Scholar]
- 47.Zhu G, Lippard SJ. Photoaffinity labeling reveals nuclear proteins that uniquely recognize cisplatin-DNA interstrand cross-links. Biochemistry. 2009;48:4916–4925. doi: 10.1021/bi900389b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thazhathveetil AK, et al. Psoralen conjugates for visualization of genomic interstrand cross-links localized by laser photoactivation. Bioconjug. Chem. 2007;18:431–437. doi: 10.1021/bc060309t. [DOI] [PubMed] [Google Scholar]
- 49.Muniandy PA, et al. Repair of laser-localized DNA interstrand cross-links in G1 phase mammalian cells. J. Biol. Chem. 2009;284:27908–27917. doi: 10.1074/jbc.M109.029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajski SR, Williams RM. DNA Cross-Linking Agents as Antitumor Drugs. Chem. Rev. 1998;98:2723–2796. doi: 10.1021/cr9800199. [DOI] [PubMed] [Google Scholar]
- 51.Cleaver JE, et al. Disorders of nucleotide excision repair: the genetic and molecular basis of heterogeneity. Nat. Rev. Genet. 2009;10:756–768. doi: 10.1038/nrg2663. [DOI] [PubMed] [Google Scholar]
- 52.Fortini P, Dogliotti E. Base damage and single-strand break repair: mechanisms and functional significance of short- and long-patch repair subpathways. DNA Repair (Amst) 2007;6:398–409. doi: 10.1016/j.dnarep.2006.10.008. [DOI] [PubMed] [Google Scholar]