Abstract
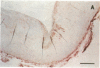
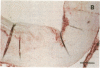
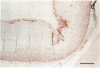
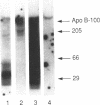
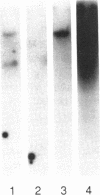
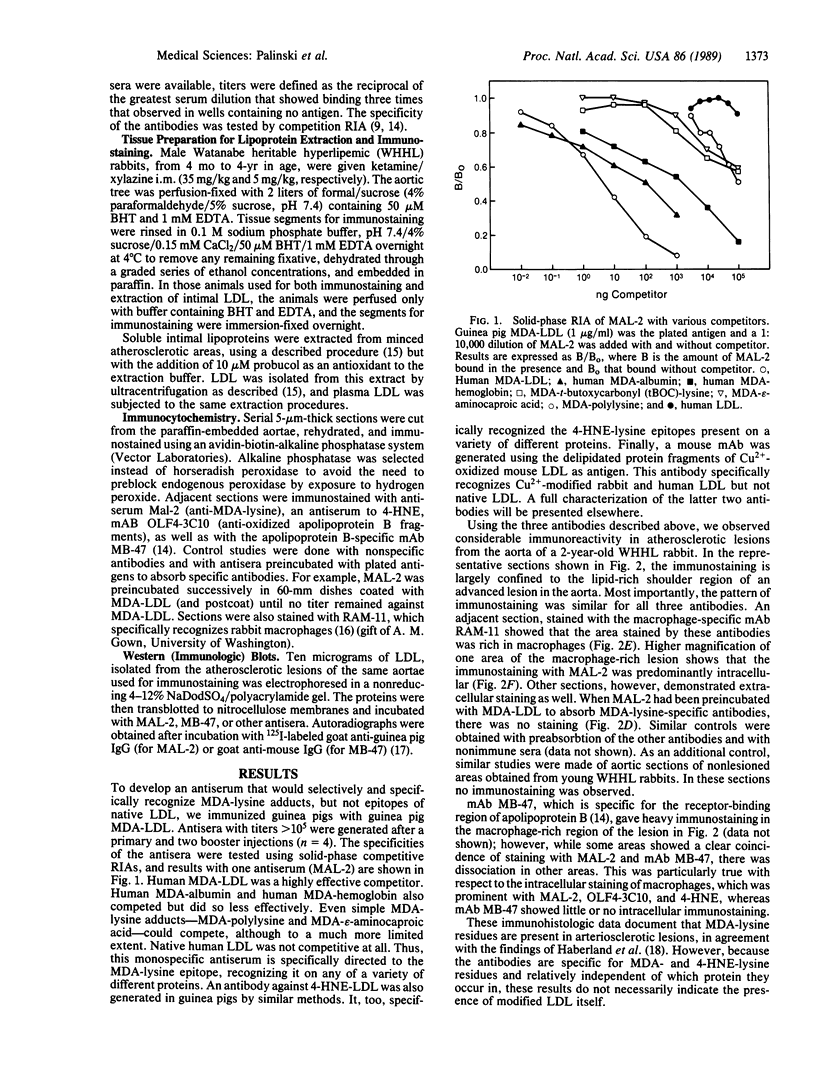
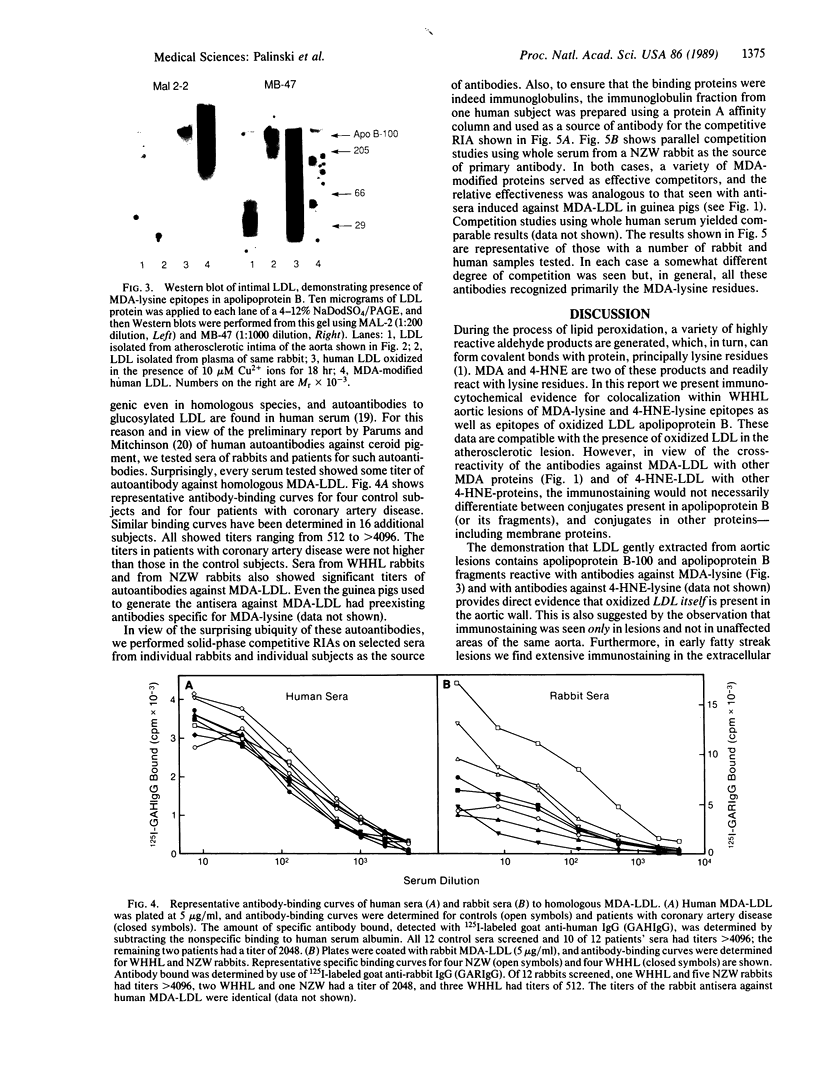
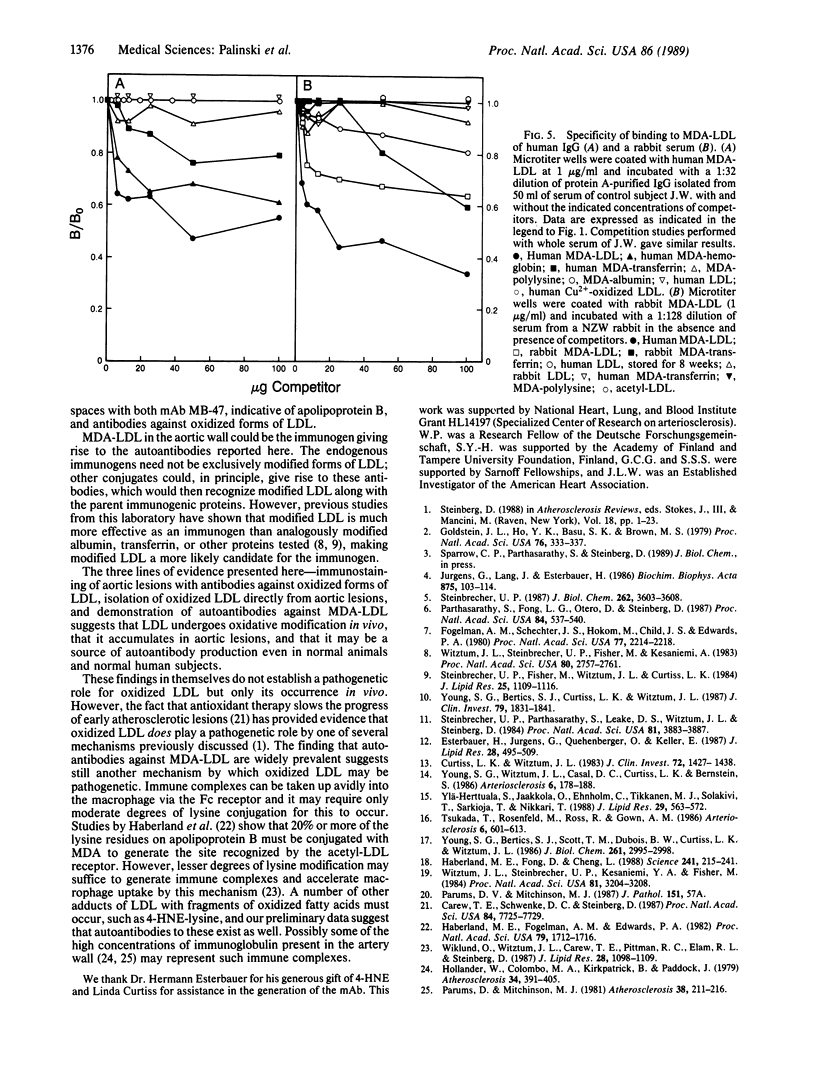
It has been proposed that low density lipoprotein (LDL) must undergo oxidative modification before it can give rise to foam cells, the key component of the fatty streak lesion of atherosclerosis. Oxidation of LDL probably generates a broad spectrum of conjugates between fragments of oxidized fatty acids and apolipoprotein B. We now present three mutually supportive lines of evidence for oxidation of LDL in vivo: (i) Antibodies against oxidized LDL, malondialdehyde-lysine, or 4-hydroxynonenal-lysine recognize materials in the atherosclerotic lesions of LDL receptor-deficient rabbits; (ii) LDL gently extracted from lesions of these rabbits is recognized by an antiserum against malondialdehyde-conjugated LDL; (iii) autoantibodies against malondialdehyde-LDL (titers from 512 to greater than 4096) can be demonstrated in rabbit and human sera.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss L. K., Witztum J. L. A novel method for generating region-specific monoclonal antibodies to modified proteins. Application to the identification of human glucosylated low density lipoproteins. J Clin Invest. 1983 Oct;72(4):1427–1438. doi: 10.1172/JCI111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Jürgens G., Quehenberger O., Koller E. Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res. 1987 May;28(5):495–509. [PubMed] [Google Scholar]
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fogelman A. M., Edwards P. A. Specificity of receptor-mediated recognition of malondialdehyde-modified low density lipoproteins. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1712–1716. doi: 10.1073/pnas.79.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Hollander W., Colombo M. A., Kirkpatrick B., Paddock J. Soluble proteins in the human atherosclerotic plaque. With spectral reference to immunoglobulins, C3-complement component, alpha 1-antitrypsin and alpha 2-macroglobulin. Atherosclerosis. 1979 Dec;34(4):391–405. doi: 10.1016/0021-9150(79)90064-9. [DOI] [PubMed] [Google Scholar]
- Jürgens G., Lang J., Esterbauer H. Modification of human low-density lipoprotein by the lipid peroxidation product 4-hydroxynonenal. Biochim Biophys Acta. 1986 Jan 3;875(1):103–114. doi: 10.1016/0005-2760(86)90016-0. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Fong L. G., Otero D., Steinberg D. Recognition of solubilized apoproteins from delipidated, oxidized low density lipoprotein (LDL) by the acetyl-LDL receptor. Proc Natl Acad Sci U S A. 1987 Jan;84(2):537–540. doi: 10.1073/pnas.84.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parums D., Mitchinson M. J. Demonstration of immunoglobulin in the neighbourhood of advanced atherosclerotic plaques. Atherosclerosis. 1981 Jan-Feb;38(1-2):211–216. doi: 10.1016/0021-9150(81)90118-0. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Fisher M., Witztum J. L., Curtiss L. K. Immunogenicity of homologous low density lipoprotein after methylation, ethylation, acetylation, or carbamylation: generation of antibodies specific for derivatized lysine. J Lipid Res. 1984 Oct;25(10):1109–1116. [PubMed] [Google Scholar]
- Steinbrecher U. P. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J Biol Chem. 1987 Mar 15;262(8):3603–3608. [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Rosenfeld M., Ross R., Gown A. M. Immunocytochemical analysis of cellular components in atherosclerotic lesions. Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis. 1986 Nov-Dec;6(6):601–613. doi: 10.1161/01.atv.6.6.601. [DOI] [PubMed] [Google Scholar]
- Wiklund O., Witztum J. L., Carew T. E., Pittman R. C., Elam R. L., Steinberg D. Turnover and tissue sites of degradation of glucosylated low density lipoprotein in normal and immunized rabbits. J Lipid Res. 1987 Sep;28(9):1098–1109. [PubMed] [Google Scholar]
- Witztum J. L., Steinbrecher U. P., Fisher M., Kesaniemi A. Nonenzymatic glucosylation of homologous low density lipoprotein and albumin renders them immunogenic in the guinea pig. Proc Natl Acad Sci U S A. 1983 May;80(9):2757–2761. doi: 10.1073/pnas.80.9.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum J. L., Steinbrecher U. P., Kesaniemi Y. A., Fisher M. Autoantibodies to glucosylated proteins in the plasma of patients with diabetes mellitus. Proc Natl Acad Sci U S A. 1984 May;81(10):3204–3208. doi: 10.1073/pnas.81.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Jaakkola O., Ehnholm C., Tikkanen M. J., Solakivi T., Särkioja T., Nikkari T. Characterization of two lipoproteins containing apolipoproteins B and E from lesion-free human aortic intima. J Lipid Res. 1988 May;29(5):563–572. [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Curtiss L. K., Witztum J. L. Characterization of an abnormal species of apolipoprotein B, apolipoprotein B-37, associated with familial hypobetalipoproteinemia. J Clin Invest. 1987 Jun;79(6):1831–1841. doi: 10.1172/JCI113025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Scott T. M., Dubois B. W., Curtiss L. K., Witztum J. L. Parallel expression of the MB19 genetic polymorphism in apoprotein B-100 and apoprotein B-48. Evidence that both apoproteins are products of the same gene. J Biol Chem. 1986 Mar 5;261(7):2995–2998. [PubMed] [Google Scholar]
- Young S. G., Witztum J. L., Casal D. C., Curtiss L. K., Bernstein S. Conservation of the low density lipoprotein receptor-binding domain of apoprotein B. Demonstration by a new monoclonal antibody, MB47. Arteriosclerosis. 1986 Mar-Apr;6(2):178–188. doi: 10.1161/01.atv.6.2.178. [DOI] [PubMed] [Google Scholar]