The most commonly reported adverse events with donepezil are gastrointestinal (nausea, diarrhoea, and constipation).1 Donepezil has been licensed in the United Kingdom since April 1997, and from April to December 1997 only seven adverse reactions affecting the skin were reported through the yellow card adverse drug reaction reporting scheme (Committee on Safety of Medicines, personal communication). To our knowledge, this is the first report of purpuric rash associated with donepezil treatment.
An 82 year old woman was seen with a two year history of memory problems. She was also hypertensive and receiving long term treatment with atenolol and doxazosin. On examination she was normotensive (140/80 mm Hg) and had moderate cognitive impairment (score in mini-mental state examination 15/30 and on the cognitive subscale of the Alzheimer’s disease assessment scale 46/75). Routine haematological and biochemical tests gave normal results (platelet count 146× 109/l), and a computed tomogram of the brain was normal. Probable Alzheimer’s disease was diagnosed according to published criteria,2 and treatment with donepezil 5 mg daily was started. After 4 days she developed diarrhoea and vomiting. On review she had a purpuric rash on her trunk and her arms and legs (figure). Donepezil treatment was stopped, with resolution of the gastrointestinal symptoms, which were thought to be a result of gastroenteritis as another family member was affected. The rash began to fade.
After discussion with the patient and her carer we cautiously restarted donepezil treatment. On review 16 days later an obvious recurrence of the purpuric rash was noted on her trunk and legs, although she had not had a recurrence of the gastrointestinal symptoms. Donepezil treatment was stopped. Successive platelet counts were 119×109/l and 157×109/l, and the rash had almost resolved when she was reviewed six weeks after rechallenge with donepezil.
Donepezil was thought to be the cause of this rash because of the temporal association with treatment and its recurrence on rechallenge.
Figure.
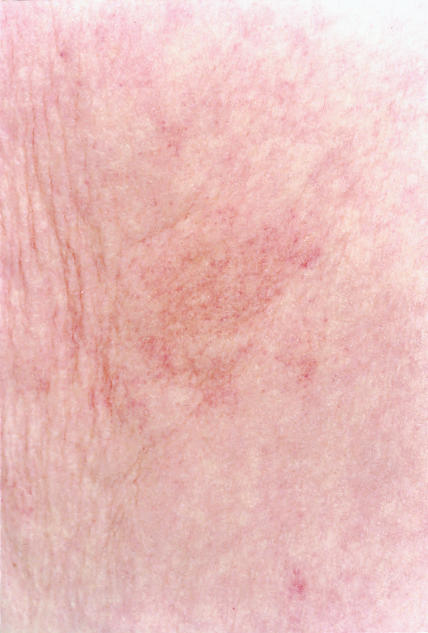
Purpuric rash with donepezil treatment
References
- 1.Rogers SL, Friedhoff LT. The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicentre, randomized, double-blind, placebo-controlled trial. The Donepezil Study Group. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- 2.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]


