Abstract
The Hedgehog (Hh) signal transduction pathway is essential for the development and patterning of numerous organ systems, and has important roles in a variety of human cancers. Genetic screens for mouse embryonic patterning mutants first showed a connection between mammalian Hh signaling and intraflagellar transport (IFT), a process required for construction of the primary cilium, a small cellular projection found on most vertebrate cells. Additional genetic and cell biological studies have provided very strong evidence that mammalian Hh signaling depends on the primary cilium. Here, we review the evidence that defines the integral roles that IFT proteins and cilia play in the regulation the Hh signal transduction pathway in vertebrates. We discuss the mechanisms that control localization of Hh pathway proteins to the cilium, focusing on the transmembrane protein Smoothened, which moves into the cilium in response to Hh ligand. The phenotypes caused by loss of cilia-associated proteins are complex, which suggests that cilia and IFT play active roles in mediating Hh signaling rather than serving simply as a compartment in which pathway components are concentrated. Hedgehog signaling in Drosophila does not depend on cilia, but there appear to be ancient links between cilia and components of the Hh pathway that may reveal how this fundamental difference between the Drosophila and mammalian Hh pathways arose in evolution.
INTRODUCTION
The primary cilium is a single, small, hair-shaped projection that emanates from the surface of nearly all non-dividing mammalian cells. Genetic studies of mouse mutants that affect the formation of primary cilia have shown that these structures are essential for developmental signaling through the Hedgehog (Hh) pathway (Huangfu and Anderson, 2005; Huangfu et al., 2003; May et al., 2005). This discovery was surprising, as the Hh-responsive cells in Drosophila, where the pathway was first characterized, are not ciliated. Nevertheless, the connection between cilia and mammalian Hh signaling is now clear, as all the key components of the Hh signal transduction pathway are enriched in the cilium (Corbit et al., 2005; Haycraft et al., 2005; Ocbina and Anderson, 2008; Rohatgi et al., 2007). As the Hh pathway is required for normal development of every organ system in mammals (McMahon et al., 2003) and Hh signaling depends on cilia in all tissues tested (Wong and Reiter, 2008), the primary cilium plays a central role in mammalian development. In addition, inappropriate Hh signaling causes tumors such as basal cell carcinoma and medulloblastoma (Jiang and Hui, 2008) and Hh appears to support the growth of a variety of other tumors (Ruiz i Altaba et al., 2002; Yauch et al., 2008).
As we describe here, mutations in different proteins required for cilia formation have distinct effects on Hh signaling: some block responses to Hh ligands, others cause ligand-independent pathway activation and yet others change the spatial organization of pathway activation. Paralleling the complexity in the mouse embryo, mutations in a number of human genes encoding proteins that localize to primary cilia or basal bodies, which nucleate cilia, are associated with a set of pleiotropic human syndromes, including Bardet-Biedl (Tobin and Beales, 2007), Meckel-Gruber, Joubert, Oral-Facial Digital, Kartagener and Alstrom syndromes (Badano et al., 2006). These syndromes associated with seemingly unrelated abnormalities as renal cysts, retinal degeneration, brain malformations, situs reversal, obesity and polydactyly (Badano et al., 2006); it is not yet known how many of these abnormalities are due to the disruption of Hh signaling.
Because the primary cilium projects into the extracellular space, it has been proposed that the cilium acts as an antenna to facilitate the reception of Hh signals. This is an interesting perspective, however it is now clear that the transport machinery within the cilium provides an apparatus that is essential for mammalian Hh signal transduction. Here, we focus on findings that define the cellular and molecular mechanisms by which IFT, a trafficking process required for the construction and maintenance of cilia, regulates Hh signaling. Based on the analysis of mutations that affect IFT and other cilia-associated components, we propose that, in addition to providing a compartment where signaling pathway components are enriched, ciliary proteins participate actively in the regulation of the Hh pathway.
Genetic Screens Reveal a Requirement for Cilia in Mammalian Hh Signaling
The connection between Hh signaling and primary cilia was first uncovered in forward genetic screens undertaken to identify genes required for patterning in the mid-gestation mouse embryo (Caspary and Anderson, 2006; Huangfu et al., 2003). Because forward genetic screens are based on phenotype, this approach can define unexpected functions of any gene, or group of genes, in the genome. As the central nervous system is prominent at mid-gestation, many mutations were identified that affect patterning of the neural tube (Garca-Garcia et al., 2005).
The specification of distinct types of neural progenitors along the dorsal-ventral axis of the neural tube requires highly regulated signaling events of several different pathways, including Wnt (Patapoutian and Reichardt, 2000), BMP (Liem et al., 1997), retinioic acid (Appel and Eisen, 2003; Pierani et al., 1999), as well as Shh (Briscoe and Ericson, 1999; Dessaud et al., 2008; Ho and Scott, 2002; Wilson and Maden, 2005). The initial source of the Shh signal in neural patterning is the notochord that lies below the ventral midline of the neural plate, and specification of cell types within the ventral neural tube depends on levels Shh (Fig. 1A). The ventral-most cell fates, floorplate and V3 interneuron progenitors, depend on high levels of Shh for their identities, motor neuron progenitors require intermediate levels of Shh, and V2, V1 and V0 interneurons require progressively lower levels of Hh signals for their specification. In the absence of any Hh signaling, as in Smo mutant embryos, all these ventral cell fates are missing and the neural tube is entirely dorsalized (Wijgerde et al., 2002). Because the identity of cells within the ventral neural tube is highly sensitive to the levels and duration of Shh signal (Dessaud et al., 2008), neural patterning provides a readout of Hh pathway activity.
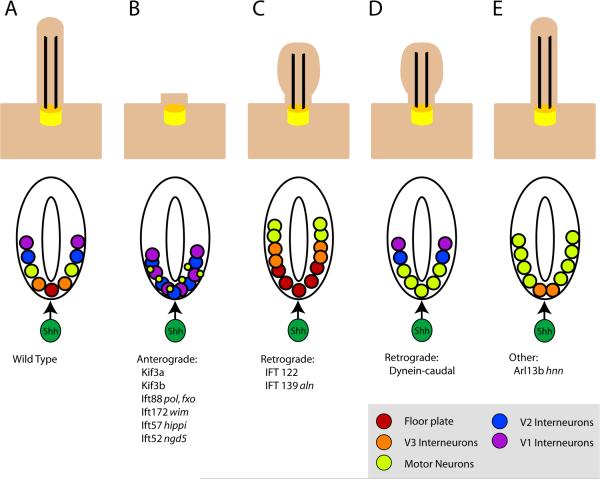
Figure 1. Summary of IFT mutants and their associated neural patterning defects.
Upper row: schematic of cilia morphology; lower row, schematic of neural tube patterning in the same mutants. (A) In wild-type, cilia are required for cells to respond to Shh, which is essential for the specification of a set of cell types in the ventral neural tube. The notochord (dark green) located ventral to the neural tube acts as the initial source of the Shh signal (arrow). In the absence of Shh, none of the indicated ventral neural cell types are specified. (B) In mutants that lack the anterograde IFT motor Kinesin-II or components of the IFT-B complex, cilia are severely shortened or absent, the most Shh-dependent ventral neural cell fates, floor plate and V3 interneurons are absent, and the number of motor neurons is severely reduced (Huangfu et al., 2003; Houde et al., 2006; Liu, 2005). (C) IFT-A complex mutants have abnormal cilia with bulges and tend to be short. The neural tubes in these embryos show expansion of Hh-dependent ventral cell fates (Cortellino et al., 2009; Tran et al., 2008). (D) Dynein mutants, which are deficient in retrograde trafficking, have bulged cilia similar to those of IFT-A mutants, but their neural patterning phenotype is consistent with a loss of Hh signaling. Rostrally, the phenotype resembles that depicted in (B), while caudally motor neurons are specified (Huangfu and Anderson, 2005; May et al., 2005). (E) Arl13bhnn cilia have structural defects in the axoneme. Neural patterning in these mutants is unusual, with a loss of ventral-most cell fates, but an expansion of motor neurons, which depend of intermediate levels of Shh for their specification (Caspary et al., 2007). Cilia in Kif7 mutants appear normal, but motor neurons are expanded dorsally (not shown).
A number of the mutations identified in the genetic screen based on altered neural patterning were found to disrupt components of the evolutionarily conserved Hh pathway (Caspary et al., 2002). In addition, two mutants, wimple (wim) and flexo (fxo), exhibited exencephaly (the failure to close the anterior neural tube) as well as loss of Shh-dependent cell types in the neural tube, including floorplate, V3 interneurons and motor neurons (Huangfu et al., 2003) (Fig 1B). The wim and fxo mutations were shown to affect two IFT proteins: Ift172 (wim) and Ift88 (fxo). In addition to a loss of Hh signaling, null mutants of these genes lack cilia (Huangfu et al., 2003; Murcia et al., 2000), consistent with phenotypes observed when homologues of these genes are mutated in Chlamydomonas reinhardtii (Pazour et al., 2000; Pedersen et al., 2005), the single celled alga in which IFT was first described (Rosenbaum and Witman, 2002). A null mutation in Kif3a, which encodes a subunit of the Kinesin-II motor required for anterograde trafficking within the cilium, was also shown to disrupt Shh signaling and block the specification of Shh-dependent neural fates (Huangfu et al., 2003), supporting a requirement for IFT for mammalian Hh signaling (Fig 1B).
IFT proteins are required at the heart of the Hh cytoplasmic signal transduction pathway
The Hh signaling pathway plays critical roles in regulating embryonic development in metazoan animals from cnidarians (Matus et al., 2008) to mammals (Jiang and Hui, 2008). Hh signaling was discovered and first characterized in Drosophila, where it is required for embryonic segment polarity (Nüsslein-Volhard and Wieschaus, 1980; Mohler and Vani, 1992) as well as patterning of the imaginal discs that give rise to external structures of the adult (Ma et al., 1993; Tabata and Kornberg, 1994).
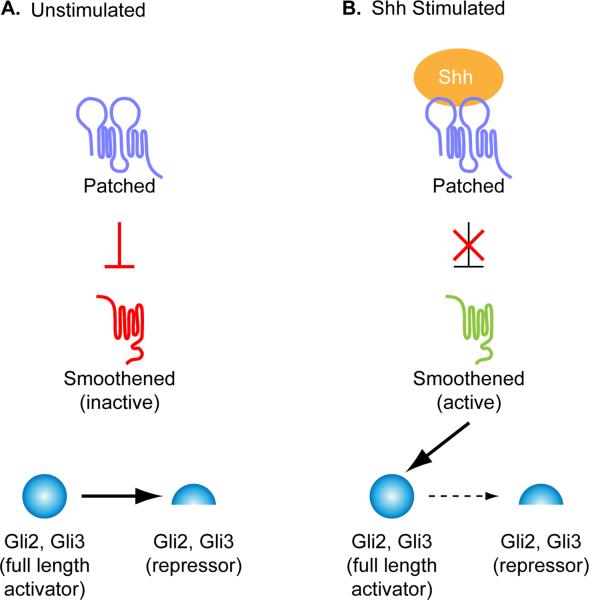
The core components and regulatory relationships are conserved between Drosophila and vertebrates (Fig. 2). In both flies and mammals, signaling is initiated upon binding of the secreted Hh ligand to the transmembrane receptor, Patched (Ptch) (Chen and Struhl, 1996; Marigo et al., 1996a; Marigo and Tabin, 1996; Stone et al., 1996). There is a single Hh ligand in Drosophila, whereas mammals have three Hh ligands - Sonic hedgehog (Shh), Indian hedgehog (Ihh) and Desert hedgehog (Dhh). Unlike traditional receptors, the Ptch receptor is a negative regulator of the pathway: in the absence of ligand, Ptch blocks the activation of another transmembrane protein Smoothened (Smo) (Marigo and Tabin, 1996). In this state, Cubitus interruptus (Ci), the transcription factor that acts as the effector of the Drosophila pathway, is proteolytically processed into a repressor form and blocks transcription of target genes. Upon Hh binding to Ptch, the repression of Smo is relieved, processing of the Ci repressor form is blocked, the Ci transcriptional activator is produced and target genes are expressed (Aza-Blanc et al., 1997). There are three vertebrate homologues of Ci: Gli1, Gli2 and Gli3. Like Ci, Gli2 and Gli3 can both act as a proteolytically processed repressor or a full-length activator (Marigo et al., 1996b; Pan et al., 2006; Wang et al., 2000). Gli3 functions primarily as a repressor, Gli2 as a transcriptional activator, and Gli1 cannot be processed into a repressor and is a transcriptional target of the Hh pathway (Matise and Joyner, 1999) (Fig. 2).
Figure 2. The core Hh signaling pathway.
The vertebrate Hedgehog (Hh) pathway in the absence (A) or presence (B) of Sonic hedgehog (Shh) ligand. (A) In the absence of ligand, the Shh receptor Ptch1 prevents the activation of another transmembrane domain protein, Smo. In this state, full length Gli2 and Gli3 are proteolytically processed into a smaller repressor form. (B) Upon Shh ligand binding to Ptch1, the inhibition on Smo is relieved and activates full-length Gli proteins and blocks production of Gli repressors.
Although mutations in Drosophila IFT genes do not affect Hh signaling, genetic and biochemical experiments showed that mouse IFT proteins are required for transmission of information from Shh, Ptch and Smo to the Gli transcription factors that mediate activity of the Hh pathway. For example, double mutant embryos that lack both an IFT-B protein (IFT172 or IFT88) and an upstream signaling component (Shh, Ptch or Smo) have phenotypes very similar to those seen in the IFT mutants, both with respect to overall morphology as well as specification of ventral neural cell fates (Huangfu et al., 2003). IFT172, IFT88 and Kif3a are required for processing of Gli3 repressor (Huangfu and Anderson, 2005; Liu et al., 2005) and IFT172 has been shown to be required for Gli activator function (Ocbina et al., 2008). Thus intraflagellar transport is required for transduction of Hh signals from the membrane proteins Ptch and Smo to the downstream Gli transcription factors in the mouse, but not in Drosophila.
Hh Pathway Proteins are Localized to Cilia
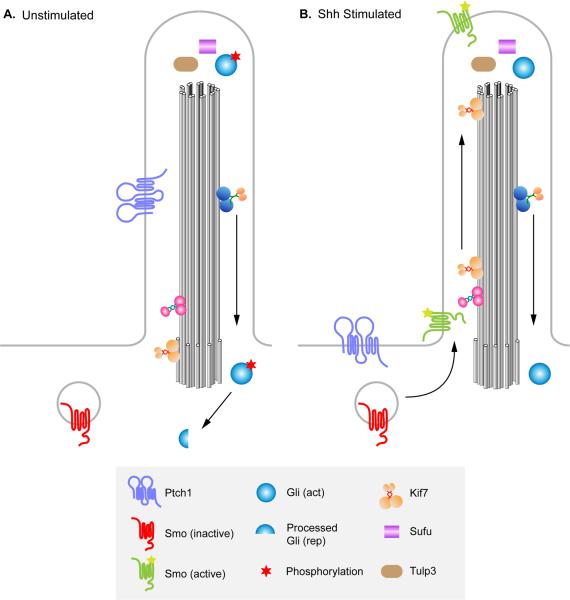
The phenotypes of mouse IFT mutants demonstrate a tight correlation between the process of IFT and the ability to properly transduce Hh signals. Further evidence that Hh signaling in mammals requires the primary cilium as an organelle came from studies showing that core components of the Hh signaling pathway are localized to cilia (Fig. 3). Ptch1, the mouse Hh receptor, is enriched within the primary cilium in the absence of ligand and moves out of the axonemal shaft to surround the base of the cilium after exposure to ligand (Rohatgi et al., 2007). In a parallel time course, Smo protein becomes enriched in primary cilia in response to ligand (Corbit et al., 2005; Rohatgi et al., 2007). The Gli transcription factors are found at the tip of the cilium both in the presence and absence of ligand. This localization to the ciliary tip is thought to be required for the activation and/or processing of the Gli transcription factors (Haycraft et al., 2005), although the mechanism by which localization to the cilium is required for activation of the Gli proteins in response to Hh signaling is not understood. Sufu, a negative regulator of the pathway, also localizes to the tip of the cilium in both the presence and absence of ligand (Haycraft et al., 2005). However, analysis of Sufu; Ift88 double mutants suggests that Sufu is able to act as a repressor of Hh pathway even in the absence of cilia (Jia et al., 2009).
Figure 3. Localization of Hh pathway components to the primary cilium in the presence and absence of Shh.
(A) In the absence of Shh ligand, Ptch1 is enriched in primary cilia and Smo is not. Sufu and Gli are present at the cilia tip, and events take place that promote proteolytic processing of Gli3 to the transcriptional repressor form (red star), causing repression of Hh target genes. KIf7, a kinesin homologous to Drosophila Cos2, is present at the base of the cilium and helps prevent activation of the pathway. (B) In the presence of Shh ligand, Shh binds to Ptch1, and Ptch1 moves out of the ciliary axoneme. In parallel, Smo is enriched in primary cilia and promotes activation rather than proteolytic processing of Gli proteins. Kif7 moves from the base to the tips of cilia and contributes to the activation of Gli proteins. Activated Gli proteins are transported out of the cilium to turn on Shh target gene expression in the nucleus. Sufu and Gli proteins are localized to the tip of the cilium in both the presence and absence of ligand, as is Tulp3.
Mutations in different IFT proteins cause a variety of defects on Hh signaling
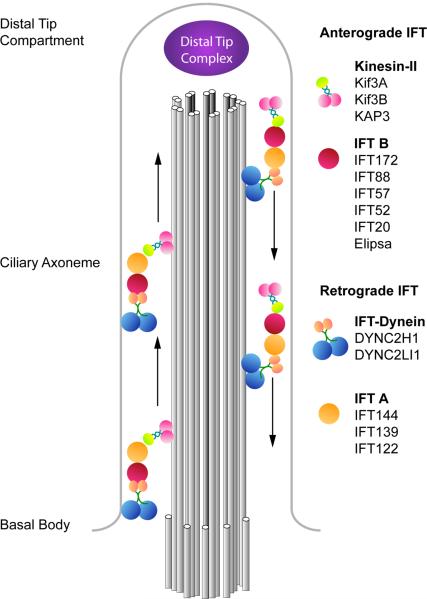
Each primary cilium is formed of a ring of nine doublet microtubules that extends from a basal body, a modified form of one of the two centrioles of the cell, the mother centriole (Dutcher, 2003). Construction and maintenance of the ciliary axoneme of the cilium depends the bidirectional transport system of IFT (Fig. 4). The process of IFT, as well as its protein components, appears to be conserved among cilia and flagella across eukaryotes. Two protein complexes, IFT-A and IFT-B, form large protein platforms that deliver cargo to the tip of the cilium or flagellum (Cole, 2003). Genetic analysis has identified more than a dozen other cilia-associated proteins that are required for normal Hh signaling, and the roles of those proteins in cilia structure and Hh signaling are varied (Table 1; Fig. 1).
Figure 4. The components of anterograde and retrograde IFT complexes.
Anterograde transport from the basal body to the distal tip is driven by the heterotrimeric Kinesin-II complex, composed of Kif3a, Kif3b (pink) and KAP3 (green), and the multiprotein IFT-B complex (red). At the tip of the cilium, IFT turnaround and remodeling of the IFT complexes occur in a poorly-understood process (Pedersen et al., 2006). Recycling of ciliary components to the base of the cilium is mediated by cytoplasmic dynein 2 (blue), together with the IFT-A complex (orange).
Table 1.
Cilia-associated proteins and their effects on cilia morphology and mouse Hh signaling.
| Role in cilia | Vertebrate Gene | Mutant Cilia Phenotype | Neural Tube Patterning Phenotype | Reference | |
|---|---|---|---|---|---|
| Kinesin-II | kinesin-II subunit | Kif3a | Absent | Loss of ventral cell types | Huangfu et al., 2003 |
| kinesin-II subunit | Kif3b | Absent | Loss of ventral cell types (inferred) | Nonaka et al., 1998 | |
| Kinesin-like | kinesin-like | Kif7 | Normal | Expansion of lateral cell types | Cheung et al., 2009; Endoh-Yamagami et al., 2009, Liem et al., 2009 |
| Dynein | heavy chain | Dync2h1 | Abnormal bulges | Loss of ventral cell types | Huangfu and Anderson, 2005; May et al., 2005 |
| light intermediate chain | Dync2li1 | n.d. | Loss of ventral cell types | Rana et al., 2004 | |
| IFT complex B | anterograde IFT | Ift172 | Absent | Loss of ventral cell types | Huangfu et al., 2003 |
| Ift88 | Absent | Loss of ventral cell types | Huangfu et al., 2003; Liu et al., 2005 | ||
| Ift52 | n.d. | Loss of ventral cell types | Liu et al., 2005 | ||
| Ift57 | Absent | Loss of ventral cell types | Houde et al., 2006 | ||
| Ift20 | Short/absent | n.d | Follit et al., 2006 | ||
| Elipsa/Ift54 | Short | n.d | Omori et al., 2008 | ||
| IFT complex A | retrograde IFT | Ift139 | Short, with abnormal bulges | Expansion of ventral cell types | Tran et al., 2008 |
| Ift122 | Bulged | Expansion of ventral and lateral cell types | Cortellino et al., 2009 | ||
| Basal body | Basal body protein | Ofd1 | Absent | Loss of ventral cell types | Ferrante et al., 2006; Romio et al., 2004; |
| Basal body protein | Rpgrip1l/Ftm | Sparse and short | Loss of ventral cell types | Vierkotten et al., 2007 | |
| Basal body protein | Evc | Normal | n.d. | Ruiz-Perez et al., 2000; Ruiz-Perez et al., 2003 | |
| Basal body protein | C2cd3 (hearty) | Short/absent | Loss of ventral cell types | Hoover et al., 2008 | |
| Arl13b | localizes to axoneme | Arl13b | Short, abnormal structure | Expansion of lateral cell types | Caspary et al., 2007 |
| Tulp3 | localizes to cilia tip | Tulp3 | Normal | Expansion of ventral cell types | Norman et al., 2009; Patterson et al., 2009 |
n.d: not determined
As described above, the requirement for cilia in Hh signaling was initially discovered based upon the phenotypes of mutations in Ift172 and Ift88 (Huangfu et al., 2003). In addition, mutations in Ift52 and Ift57 show the same loss of Shh-dependent ventral neural cell types (Houde et al., 2006; Liu, 2005) (Fig. 1B). IFT172, IFT88, IFT52 and IFT57 are components of a single protein complex, IFT-B, and null alleles of these mouse genes lack cilia completely. The same phenotype, the failure to form cilia, is observed in Chlamydomonas mutants for components of the IFT-B complex (Pazour et al., 2000; Scholey, 2008). The IFT-B complex is thought to mediate anterograde trafficking within the cilium, nevertheless, the detailed mechanisms by which IFT-B regulates anterograde trafficking are still the subject of investigation (Fig 4). The anterograde motor, Kinesin-II, is composed of two motor subunits, Kif3a and Kif3b, and an accessory subunit, kinesin-associated protein-3 (KAP-3) (Scholey, 1996; Yamazaki et al., 1996). In both Chlamydomonas and in mammals, loss of either Kinesin-II motor subunit blocks formation of the cilium/flagellum (Kozminski et al., 1995; Marszalek et al., 1999; Nonaka et al., 1998), and mouse Kif3a mutants show the same loss of ventral neural cell types seen in IFT-B mutants (Huangfu et al., 2003).
Once IFT complexes and motors have reached the tip of the cilium, they dissociate from the microtubules that make up the axoneme and release their cargo. In a process called tip turnaround, the IFT complexes are remodeled, dynein is activated, Kinesin-II is inactivated, and the complex takes on cargo to be recycled to the base of the cilium (Iomini et al., 2001; Pedersen et al., 2005).
Retrograde transport from the tip of the ciliary axoneme back to the base is mediated by cytoplasmic dynein 2 (Fig. 4). Mouse mutations in the dynein heavy chain gene, Dync2h1, produce cilia that are shorter than wild type and have characteristic bulges along the axoneme (Huangfu and Anderson, 2005; May et al., 2005; Ocbina and Anderson, 2008), similar to those seen in Chlamydomonas mutants lack the dynein heavy chain gene (cDhc1b) (Piperno et al., 1998). Like Kif3a and IFT-B mutants, mouse Dync2h1 mutants fail to specify Shh-dependent cell types in the ventral neural tube (Huangfu and Anderson, 2005; May et al., 2005). As in IFT-B mutants, Gli3 is not properly proteolytically processed in Dync2h1 mutants (Huangfu and Anderson, 2005; May et al., 2005) and Gli activator function is also blocked (Ocbina and Anderson, 2008). Nevertheless, the Dync2h1 mutants are not identical to the IFT-B complex mutants: the effect on neural patterning is stronger in rostral than caudal regions of the Dync2h1 mutant neural tube (Huanagfu and Anderson, 2005) (Fig. 1D), in contrast to the uniformly strong effect of IFT-B complex mutants. Mouse embryos lacking the light intermediate chain component of IFT-dynein, Dync2li1 (also known as mD2LIC) show the same loss floor plate markers seen in Dync2h1 mutants, although neural patterning was not further characterized (Rana et al., 2004). Thus loss of either loss of anterograde or retrograde IFT blocks normal Hh signal transduction.
The IFT-A complex appears to cooperate with dynein to mediate retrograde transport (Pedersen et al., 2008) and may play a role in tip turnaround (Iomini et al., 2001), as IFT-A complex mutants in both Chlamydomonas or mammals caused bulged cilia/flagella, similar to those seen in the dynein mutants (Cortellino et al., 2009; Pedersen et al., 2005; Piperno et al., 1998; Tran et al., 2008) (Fig 1C).
In contrast to the loss of Hh signaling seen in IFT-B and dynein mutants, mouse mutations identified in two different genes that encode IFT Complex A proteins have the opposite effect on Hh signaling: they cause ectopic activation of the Hh pathway in the dorsal neural tube (Tran et al., 2008; Cortellino et al., 2009) (Fig. 1C). The alien (aln) mutation disrupts the function of Tetratricopeptide repeat-containing Hedgehog modulator-1 (THM-1) (Tran et al., 2008), one of two mouse homologs of Chlamydomonas IFT139 (http://www.ncbi.nlm.nih.gov/protein/156766595). In aln mutant embryos, Shh-dependent cell fates including floor plate, V3 interneurons and motor neurons are dorsally expanded (Tran et al., 2008). A similar expansion of ventral cell fates is seen in mutants that lack IFT122, another component of the IFT complex A (Cortellino et al., 2009).
The opposing effects of IFT-A and IFT-B mutants on Hedgehog signaling are surprising and difficult to explain based on what is known about the two classes of proteins. IFT-A and IFT-B were identified as subcomplexes of a single large complex (Cole et al., 1998). In both Chlamydomonas and C. elegans the two subcomplexes move coordinately in the same particle (Ou et al., 2005; Qin et al., 2005). Studies in Chlamydomonas and C. elegans suggest that the IFT complex B is required for anterograde IFT and transports complex A proteins to the ciliary tip where IFT-A proteins are required for retrograde IFT (Pedersen et al., 2006; Pedersen et al., 2005). However, studies on the phenotype of C. elegans dyf-2 mutants suggest that the distinction between the functions of the two IFT subcomplexes may be an over-simplification. C. elegans dyf-2 encodes the homologue of Chlamydomonas IFT144 (called Wdr19 in the mouse), an IFT A complex protein (Cole, 2003). dyf-2 mutants have very short sensory cilia, similar to those of complex B mutants, but they also accumulate IFT complex B proteins in cilia, like complex A mutants (Efimenko et al., 2006). Thus at least one IFT-A protein, DYF-2/IFT144, appears to play roles in both anterograde and retrograde IFT.
It is particularly surprising that mutations in the IFT dynein and IFT-A proteins, which are both thought to mediate retrograde IFT, cause similar defects in cilia morphology but exert opposite effects on the Hh pathway. Both the IFT-A mutants analyzed (THM-1/aln and Ift122) have cilia defects similar to the phenotypes seen in Dync2h1 mutants (Cortellino et al., 2009; Huangfu and Anderson, 2005; May et al., 2005; Tran et al., 2008). The opposing phenotypes of IFT-dynein and IFT- A mutants suggest that these two classes of protein have distinct effects on the trafficking of Hh components.
Other Cilia-associated proteins and Hedgehog signaling
Another mouse mutation, hennin (hnn), has a novel effect on neural patterning: cell types within the neural tube that depend on high levels of Hh signaling, such as floor plate, are lost, while cell types that depend on lower levels of Hh, such as motor neurons, are expanded dorsally (Caspary et al., 2007) (Fig. 1E). Positional cloning showed that hnn inactivates Arl13b, a member of the Arl subfamily of Ras GTPases that is highly enriched in cilia. In addition to the defect in Hh signaling, loss of Arl13b causes a specific disruption in the microtubules of the ciliary axoneme, the failure to close the B tubule of the outer doublet microtubules of the ciliary axoneme. Loss of Arl13b does not affect Gli3 processing and instead causes Hh-independent, low-level activation of Gli proteins (Caspary et al., 2007). Thus perturbation of the structure of the ciliary axoneme can have specific, unexpected effects on the activity of the Hh pathway.
Another cilia-associated protein that plays an important role in Hh signaling is Tubby-like protein 3 (Tulp3). Tulp3 is one of four TULP members related to Tubby (Tub), a regulator of late-onset obesity (Coleman and Eicher, 1990). All TULPs, including Tub, are expressed at highest levels in the retina (Ikeda et al., 2002). Tulp3 is more widely expressed throughout the nervous system and Tulp3 mutant embryos fail to close the anterior neural tube (Ikeda et al., 2001), but its role in neural tube patterning was not investigated until recently. Like the IFT-A mutants, Tulp3 mutants show an expansion of Shh-dependent ventral neural cell types, indicating that Tulp3 is a negative regulator of the pathway (Cameron et al., 2009; Norman et al., 2009; Patterson et al., 2009). Tulp3 is required at the same step in the genetic hierarchy as the IFT proteins: downstream of Smo and upstream of the Gli proteins. Tulp3 protein localizes to the tips of primary cilia in the neural tube, as well as to both the plasma membrane and the nucleus (Norman et al., 2009; Patterson et al., 2009). Tulp3 mutant cells do not show obvious defects in ciliogenesis (Norman et al., 2009; Patterson et al., 2009), however the localization of Tulp3 to the tip of the cilium suggests that it could regulate the Gli transcription factors in that compartment (Fig. 3).
Hedgehog pathway proteins that affect the cilia structure and trafficking
Thus far, we have focused on how mutations that disrupt cilia structure and trafficking also alter Hh signaling. Several recent studies suggest that there is a deep and ancient connection between Hh signaling and cilia: two proteins, Costal2 (Cos2) and Fused, first identified for their roles in Drosophila Hh signaling (Lum et al., 2003; Robbins et al., 1997; Sisson et al., 1997), have roles in cilia function in vertebrates.
Drosophila Fused is a serine/threonine kinase required for phosphorylation of the atypical kinesin Cos2, Sufu and perhaps other components of the Hh pathway to promote signal transduction (Aikin et al., 2008). Although Fused is important for Hh signaling in zebrafish (Wolff et al., 2003), the single mouse Fused homologue apparently does not participate in mammalian Shh signaling (Chen et al., 2005; Merchant et al., 2005). Instead, mouse Fused is required for the formation of the central pair of microtubules in motile cilia in both zebrafish and mouse (Wilson et al., 2009b).
Cos2 is a kinesin-related protein that serves as an essential scaffold for Drosophila Hh signaling protein complexes. Recent work has shown that the mouse kinesin Kif7, a homologue of Cos2, acts as both a positive and negative regulator of the Shh pathway (Liem et al., 2009; Cheung et al., 2009; Endoh-Yamagami et al., 2009). Kif7 moves to the tip of the cilium in response to activation of the pathway (Liem et al., 2009; Endoh-Yamagami et al., 2009) (Fig. 3). Movement of Kif7 to the cilia tip depends on its motor domain, suggesting that it acts as an anterograde motor within the cilium, in parallel with Kinesin-II (Liem et al, 2009). Perhaps, like the C. elegans homodimeric kinesin Osm-3/Kif17 (Ou et al., 2005), Kif7 cooperates with Kinesin-II to control the cargo and rate of trafficking within the ciliary axoneme.
Certain basal body proteins are required for cilia formation and Hh signaling
The ciliary basal body is a microtubule-based structure that forms from the mother centriole. A number of proteins that localize to the centriole/basal body have been shown to be important for both the assembly of primary cilia and for Hh signaling. The chicken talpid3 mutation was first described over 40 years ago based on limb polydactyly (Ede and Kelly, 1964a; Ede and Kelly, 1964b) and causes a set of developmental defects that are consistent with disrupted Hh signaling (Lewis et al., 1999). The talpid3 is caused by a mutation in KIAA0586, a centrosomal protein. talpid3 mutant embryos lack cilia, apparently due to a failure of the basal body to dock at the apical membrane (Yin et al., 2009).
Several basal body proteins associated with human disorders have also been implicated in primary cilia formation and Hh signaling. Mutations in Ofd1 cause the human disorder oral-facial-digital type 1 syndrome (Ferrante et al., 2001), and mutations in Ftm/Rpgrip1l cause Joubert syndrome type B and Meckel syndrome (Delous et al., 2007). Null mutations in mouse Ftm and Ofd1 cause embryonic lethality and phenotypes characteristic of abnormal Hh signaling, including loss of ventral neural cell fates, polydactyly and reduced expression of Hh target genes (Ferrante et al., 2006; Vierkotten et al., 2007). Both Ftm and Ofd1 proteins localize to the basal body (Romio et al., 2004; Vierkotten et al., 2007). Cilia in Ftm null embryos are reduced in number and the cilia that do form on fibroblasts derived from Ftm mutant embryos are short (Vierkotten et al., 2007). In male mouse embryos hemizygous for a deletion of X-linked Ofd1, no cilia can be detected (Ferrante et al., 2006). Thus, in addition to proteins associated with IFT, some proteins associated with the basal body are also required for primary cilia formation and therefore for Hh signaling.
Another basal body associated protein, EVC, is required for Hh signaling in a tissue-restricted manner (Ruiz-Perez et al., 2007). The Evc gene is mutated in the human disorder Ellis-van Creveld syndrome, a chondroectodermal dysplasia (Ruiz-Perez et al., 2000; Ruiz-Perez et al., 2003). Evc is expressed specifically within the developing orofacial region and the cartilaginous components of the axial skeleton. In chondrocytes, EVC protein is present at the distal end of the mother centriole, which becomes the basal body. However, in contrast with Ofd1 hemizygous and Ftm null embryos, Evc null mouse mutants can survive to adult stages, but show skeletal and craniofacial abnormalities. Cilia are present, even in chondrocytes, but the skeletal phenotypes appear to be due to reduced Indian hedgehog (Ihh) signaling: the mutants show premature differentiation in growth plates of the long bones, and reduced expression of Ptch and Gli1 in these tissues (Ruiz-Perez et al., 2007). Thus this basal body protein apparently does not affect ciliogenesis, but is required for Hh signaling in specific cell types.
Vesicle trafficking, cilia formation and Hh signaling
Vesicle trafficking from the Golgi to the cilium appears to be important in the initial stages of cilia formation from the mother centriole, as well as the subsequent growth and the maintenance of the cilium (Sorokin, 1962; Pedersen et al., 2008). IFT20, an IFT B protein, is localized both to cilia and to the Golgi complex (Follit et al., 2006) and may define one direct link between vesicle trafficking and cilia formation.
Further evidence for a role for vesicle trafficking in cilia formation comes from a study linking a proteins associated with Bardet-Biedl syndrome (BBS) to the small GTPase Rab8, which is known to be important in vesicle trafficking and membrane fusion (Nachury et al., 2007). The majority of the BBS proteins appear to form a stable complex that localizes to the base of the cilium and recruits factors important for membrane and vesicle trafficking, including Rab8 (Nachury et al., 2007). Rab8 traffics within the cilium and can promote the fusion of membrane vesicles to the growing cilium. Overexpression of Rab8 or expression of a constitutively activated form of Rab8 in cells results in extension of the ciliary membrane uncoupled from extension of the axoneme. Conversely, a dominant negative Rab8 blocks cilia formation in cells (Nachury et al., 2007); however mouse Rab8 mutant mice are viable until about 5 weeks of age (Sato et al., 2007), which is not consistent with an essential role in ciliogenesis.
A study in zebrafish identified a cilia-localized protein, Elipsa, that also appears to link IFT with vesicle trafficking via Rab8 (Omori et al., 2008). Elipsa is the homologue of IFT54 (Follit et al., 2009) and interacts with IFT20 and the Rab effector Rabaptin5. Rabaptin5 was shown to interact biochemically and genetically with Rab8, thus providing further evidence that the IFT B complex is linked with a pathway important for vesicle trafficking (Omori et al., 2008).
There are also indications that regulation of trafficking of vesicles from the Golgi to the cilium can affect Hh signaling. The mouse ENU-induced mutation hearty (hty), which disrupts C2cd3, a C2 domain-containing protein (Hoover et al., 2008), exhibits a number of defects consistent with a loss of Shh signaling, including loss of ventral neural cell fates, and polydactyly. These Shh pathway defects are apparently the result of cilia defects, as embryos homozygous for hty, which is a hypomorphic allele of C2cd3, show a reduced number of cilia. Embryos homozygous for a gene trap allele of C2cd3, which causes a more severe disruption of the protein, fail to form any detectable cilia (Hoover et al., 2008). As C2 domain-containing proteins are involved with calcium-dependent lipid binding (Nalefski and Falke, 1996), it was suggested that C2cd3 may be involved with vesicle trafficking. GFP-tagged C2cd3 localizes to the basal body (Hoover et al., 2008), but a direct role in vesicle trafficking has not yet been established.
Furthermore, Rab23, a small GTPase disrupted in mouse open brain (opb) mutants, is a critical negative regulator of the Shh pathway in the mouse (Eggenschwiler et al., 2001). Rab23 acts downstream of Smo and upstream of the Gli receptors (Eggenschwiler et al., 2006), the same step in the pathway as that affected by IFT. However, it is not known whether Rab23 regulates trafficking of vesicles to the cilium or affects a different kind of vesicle trafficking.
Trafficking of Smo to and within the Cilium
Trafficking both to and within the cilium also appears to play a crucial role in the regulation of the seven-pass transmembrane protein Smo. A key step in activation of the Hh pathway is the movement of Smo into cilia when cells are exposed to Shh (Fig. 3). Localization of Smo to the primary cilium is tightly correlated with Hh pathway activation. For example, mutations in Smo that cause ligand-independent activation of the pathway (e.g. SmoM2; Taipale et al., 2000) also cause constitutive localization of the mutant Smo to primary cilia (Corbit et al., 2005; Han et al., 2008), and SmoM2 mutant protein cannot activate the pathway in the absence of cilia (Han et al., 2008; Ocbina and Anderson, 2008). The transmembrane receptor Ptch1 is a negative regulator of pathway activity, and Smo is constitutively localized to cilia in Ptch1 mutant cells (Rohatgi et al., 2007; Ocbina and Anderson, 2008), however the mechanism by which Ptch1 regulates Smo localization and activation is not known. Ptch is similar in structure to bacterial transporters, and it has been suggested that Ptch may modulate the concentration of a small molecule that controls Smo activation (Taipale et al., 2002). Treatment of NIH3T3 fibroblasts with 20α-hydroxycholesterol (20α-OHC), an oxysterol derivative of cholesterol, is sufficient to promote Smo translocation to cilia and activate the pathway, which suggests that an oxysterol-like molecule might regulate Smo movement to the cilium (Rohatgi et al., 2007), although the identity of that molecule is unknown.
The localization of Smo to cilia is necessary for its activation. For example, amino acid substitutions in Smo that prevent its localization to the cilium also block activation of the pathway (Corbit et al., 2005; Aanstad et al., 2009). However, a variety of experiments have shown that Smo localization in cilia is not sufficient for pathway activity. Over-expression of wild-type Smo in NIH3T3 cells leads to Smo enrichment in cilia without fully activating the pathway (Rohatgi et al., 2007), and loss of the dynein motor that directs retrograde IFT leads to constitutive ciliary Smo localization without pathway activation (Ocbina and Anderson, 2008). Furthermore, cyclopamine is a small molecule that inhibits activity of the pathway, but it promotes Smo localization to cilia (Rohatgi et al., 2009; Wang et al., 2009; Wilson et al., 2009a). These observations have suggested the hypothesis that Smo is activated in a two-step process: the first step being localization to the cilium and a second step a conformational change of Smo within the cilium that triggers downstream signaling events (Rohatgi et al., 2009) (Fig. 5).
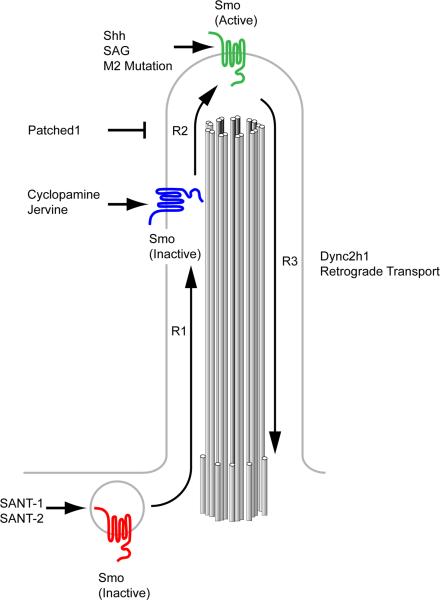
Figure 5. Smo activation requires localization to the primary cilium.
Modified from Rohatgi et al. 2009. Smo is activated through a series of steps and conformations that can be stabilized by small molecule regulators of Hh signaling. Full activation of Smo requires the transport of inactive cytoplasmic Smo (red) from the cytoplasm into the cilium controlled by step R1. The Smo antagonists SANT-1 and -2 stabilize Smo in this inactive state. Smo within the cilium can exist in an inactive state (blue) that is trafficked out of the cilium when no further activation is achieved (R3). This inactive form is stabilized by the antagonist cyclopamine. Smo can be activated (green) through a second step, R2, to promote activation of downstream pathway components such as Gli proteins. This state is promoted by Shh signal, and can be stabilized by the Smo agonist SAG as well as activating mutations such as SmoM2. IFT-Dynein mediates retrograde transport of Smo (R3) in either an active or inactive state, as Dync2h1 mutant cilia constitutively localize Smo without Shh pathway activation. The mechanisms that deliver Smo to the cilium are not known.
The trafficking events that target Smo to the cilium are not known. The accumulation of Smo in the cilia of IFT dynein mutants, in which retrograde IFT is disrupted, suggests that Smo is trafficked through the cilium at a low basal rate in the absence of ligand (Ocbina and Anderson, 2008). Several G-protein coupled receptors that localize to the cilia of sensory neurons require the basal body associated proteins BBS2 and 4 for their localization (Berbari et al., 2008), but Hh signaling and cilia formation are normal in Bbs2 and Bbs4 mouse mutants (Kulaga et al., 2004; Mykytyn et al., 2004; Nishimura et al., 2004), and there is no evidence of a role for these proteins in trafficking Smo. A recent study has suggested that ß-arrestins mediate the interaction of Smo with Kif3a in NIH3T3 cells (Kovacs et al., 2008). However, the exact function of ß-arrestins within the cilium and their relationship to Smo are controversial (Molla-Herman et al., 2008), as mouse knockouts for either ß-arrestin-1 or -2 do not affect early mouse development (Bohn et al., 1999; Conner et al., 1997). Thus additional genetic and cell biological experiments will be needed to define how Smo moves into the cilium.
Summary
Given the vital role of Shh signaling in regulating the patterning of the early embryo and the development of nearly every organ and roles of the Shh pathway in cancer, it is critical to understand mammalian-specific regulation of Hh signaling by primary cilia. Studies conducted over the past several years have revealed the complexity of the mechanisms active in primary cilia to regulate the Hh pathway. If the cilium were simply a compartment where components of the Hh pathway are enriched, mutations that disrupt cilia formation would be predicted to have similar effects on pathway activity. However, as we have described, mutations in different cilia-associated proteins can increase, decrease or change the spatial distribution of the activity of the pathway. A striking example of the unexplained complexity of the pathway is that different mutations in two kinds of components that affect retrograde IFT, dynein and IFT-A proteins, have opposite effects on Shh signaling.
Proteins associated with the basal body also differ in terms of their effects on the Hh pathway. Some basal body proteins are clearly required for Hh signaling, while others, most notably BBS proteins, do not appear to be individually required to regulate Hh signaling. Of the basal body proteins that are required for Hh signaling, some are clearly required for cilia formation. Loss of function of other basal body proteins however, including EVC, does not result in overt defects in cilia formation. Thus, the specific functions of proteins associated with the basal body with respect to cilia formation need to be defined.
Although understanding of the mechanisms that control Smo localization to cilia in response to Hh ligands is essential for understanding why and how the mammalian Hh pathway is tied to the primary cilium, much remains to be learned. In vivo, Shh acts as a morphogen: different concentrations of Shh direct different cell fates (Jiang and Hui, 2008; Ruiz i Altaba et al., 2003). The question of whether the gradient of Shh activity depends on regulation of Smo localization to the cilium or activation within the cilium has yet to be tackled.
It remains a mystery why vertebrate, but not Drosophila, Hh signaling is coupled to cilia. The Hh pathway is ancient and must have been present in the common ancestor of both protostomes and deuterostomes (Matus et al., 2008), and may have co-opted from more primitive eukaryotes before the advent of multicellular animals (Hausmann et al., 2009). Cilia and IFT are also conserved across evolution from the single celled alga Chlamydomonas to mammals. It will be important to determine whether Hh signaling in other invertebrate metazoans depends on cilia. It is interesting to note that Chlamydomonas IFT proteins play an integral role in a signaling cascade important during fertilization (Wang et al., 2006), suggesting that role of cilia in cell signaling goes far back in evolutionary history.
Acknowledgments
Work in our lab in this area was supported by NIH grant NS044385. S. C. G. is an American Cancer Society postdoctoral fellow.
References
- Aanstad P, et al. The Extracellular Domain of Smoothened Regulates Ciliary Localization and Is Required for High-Level Hh Signaling. Curr Biol. 2009 doi: 10.1016/j.cub.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikin RA, et al. The role of kinases in the Hedgehog signalling pathway. EMBO Rep. 2008;9:330–6. doi: 10.1038/embor.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel B, Eisen JS. Retinoids run rampant: multiple roles during spinal cord and motor neuron development. Neuron. 2003;40:461–4. doi: 10.1016/s0896-6273(03)00688-3. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, et al. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–53. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- Badano JL, et al. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–48. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Berbari NF, et al. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA. 2008;105:4242–6. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, et al. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–8. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Ericson J. The specification of neuronal identity by graded Sonic Hedgehog signalling. Semin Cell Dev Biol. 1999;10:353–62. doi: 10.1006/scdb.1999.0295. [DOI] [PubMed] [Google Scholar]
- Cameron DA, et al. Tulp3 is a critical repressor of mouse hedgehog signaling. Dev Dyn. 2009;238:1140–9. doi: 10.1002/dvdy.21926. [DOI] [PubMed] [Google Scholar]
- Caspary T, Anderson KV. Uncovering the uncharacterized and unexpected: unbiased phenotype-driven screens in the mouse. Dev Dyn. 2006;235:2412–23. doi: 10.1002/dvdy.20853. [DOI] [PubMed] [Google Scholar]
- Caspary T, et al. Mouse Dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Curr Biol. 2002;12:1628–32. doi: 10.1016/s0960-9822(02)01147-8. [DOI] [PubMed] [Google Scholar]
- Caspary T, et al. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–78. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Chen MH, et al. Mice deficient in the fused homolog do not exhibit phenotypes indicative of perturbed hedgehog signaling during embryonic development. Mol Cell Biol. 2005;25:7042–53. doi: 10.1128/MCB.25.16.7042-7053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–63. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- Cheung HO, et al. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Sci Signal. 2009;2:ra29. doi: 10.1126/scisignal.2000405. [DOI] [PubMed] [Google Scholar]
- Cole DG. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic. 2003;4:435–42. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- Cole DG, et al. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DL, Eicher EM. Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered. 1990;81:424–7. doi: 10.1093/oxfordjournals.jhered.a111019. [DOI] [PubMed] [Google Scholar]
- Conner DA, et al. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res. 1997;81:1021–6. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- Corbit KC, et al. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–21. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Cortellino S, et al. Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Developmental Biology. 2009;325:225–37. doi: 10.1016/j.ydbio.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–81. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- Dessaud E, et al. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–2503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- Dutcher SK. Elucidation of basal body and centriole functions in Chlamydomonas reinhardtii. Traffic. 2003;4:443–51. doi: 10.1034/j.1600-0854.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- Ede DA, Kelly WA. Developmental Abnormalities in the Head Region of the Talpid Mutant of the Fowl. J Embryol Exp Morphol. 1964a;12:161–82. [PubMed] [Google Scholar]
- Ede DA, Kelly WA. Developmental Abnormalities in the Trunk and Limbs of the Talpid3 Mutant of the Fowl. J Embryol Exp Morphol. 1964b;12:339–56. [PubMed] [Google Scholar]
- Efimenko E, et al. Caenorhabditis elegans DYF-2, an orthologue of human WDR19, is a component of the intraflagellar transport machinery in sensory cilia. Mol Biol Cell. 2006;17:4801–11. doi: 10.1091/mbc.E06-04-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler J, et al. Mouse Rab23 regulates Hedgehog signaling from Smoothened to Gli proteins. Developmental Biology. 2006;290:1–12. doi: 10.1016/j.ydbio.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, et al. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194–8. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- Endoh-Yamagami S, et al. The Mammalian Cos2 Homolog Kif7 Plays an Essential Role in Modulating Hh Signal Transduction during Development. Curr Biol. 2009 doi: 10.1016/j.cub.2009.06.046. [DOI] [PubMed] [Google Scholar]
- Ferrante M, et al. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet. 2006;38:112–117. doi: 10.1038/ng1684. [DOI] [PubMed] [Google Scholar]
- Ferrante MI, et al. Identification of the gene for oral-facial-digital type I syndrome. Am J Hum Genet. 2001;68:569–76. doi: 10.1086/318802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, et al. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17:3781–92. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, et al. Characterization of mouse IFT complex B. Cell Motil. Cytoskeleton. 2009 doi: 10.1002/cm.20346. NA-NA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia MJ, et al. Analysis of mouse embryonic patterning and morphogenesis by forward genetics. Proc Natl Acad Sci U S A. 2005;102:5913–9. doi: 10.1073/pnas.0501071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Hausmann G, et al. The hedgehog signaling pathway: where did it come from? PLoS Biol. 2009;7:e1000146. doi: 10.1371/journal.pbio.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft C, et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KS, Scott MP. Sonic hedgehog in the nervous system: functions, modifications and mechanisms. Curr Opin Neurobiol. 2002;12:57–63. doi: 10.1016/s0959-4388(02)00290-8. [DOI] [PubMed] [Google Scholar]
- Hoover AN, et al. C2cd3 is required for cilia formation and Hedgehog signaling in mouse. Development. 2008;135:4049–58. doi: 10.1242/dev.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde C, et al. Hippi is essential for node cilia assembly and Sonic hedgehog signaling. Dev Biol. 2006;300:523–33. doi: 10.1016/j.ydbio.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA. 2005;102:11325–30. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–7. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Ikeda A, et al. Neural tube defects and neuroepithelial cell death in Tulp3 knockout mice. Hum Mol Genet. 2001;10:1325–34. doi: 10.1093/hmg/10.12.1325. [DOI] [PubMed] [Google Scholar]
- Ikeda A, et al. The tubby-like proteins, a family with roles in neuronal development and function. J Cell Sci. 2002;115:9–14. doi: 10.1242/jcs.115.1.9. [DOI] [PubMed] [Google Scholar]
- Iomini C, et al. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J Cell Biol. 2001;153:13–24. doi: 10.1083/jcb.153.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, et al. Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia. Developmental Biology. 2009:1–31. doi: 10.1016/j.ydbio.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–12. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, et al. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–81. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, et al. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–27. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaga H, et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36:994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- Lewis KE, et al. Expression of ptc and gli genes in talpid3 suggests bifurcation in Shh pathway. Development. 1999;126:2397–407. doi: 10.1242/dev.126.11.2397. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr., et al. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–38. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Liu A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Lum L, et al. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol Cell. 2003;12:1261–74. doi: 10.1016/s1097-2765(03)00426-x. [DOI] [PubMed] [Google Scholar]
- Ma C, et al. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell. 1993;75:927–38. doi: 10.1016/0092-8674(93)90536-y. [DOI] [PubMed] [Google Scholar]
- Marigo V, et al. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996a;384:176–9. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- Marigo V, et al. Sonic hedgehog differentially regulates expression of GLI and GLI3 during limb development. Dev Biol. 1996b;180:273–83. doi: 10.1006/dbio.1996.0300. [DOI] [PubMed] [Google Scholar]
- Marigo V, Tabin CJ. Regulation of patched by sonic hedgehog in the developing neural tube. Proc Natl Acad Sci U S A. 1996;93:9346–51. doi: 10.1073/pnas.93.18.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, et al. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A. 1999;96:5043–8. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matise MP, Joyner AL. Gli genes in development and cancer. Oncogene. 1999;18:7852–9. doi: 10.1038/sj.onc.1203243. [DOI] [PubMed] [Google Scholar]
- Matus DQ, et al. The Hedgehog gene family of the cnidarian, Nematostella vectensis, and implications for understanding metazoan Hedgehog pathway evolution. Developmental Biology. 2008;313:501–18. doi: 10.1016/j.ydbio.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May SR, et al. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Developmental Biology. 2005;287:378–89. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- McMahon AP, et al. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- Merchant M, et al. Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus. Mol Cell Biol. 2005;25:7054–68. doi: 10.1128/MCB.25.16.7054-7068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler J, Vani K. Molecular organization and embryonic expression of the hedgehog gene involved in cell-cell communication in segmental patterning of Drosophila. Development. 1992;115:957–71. doi: 10.1242/dev.115.4.957. [DOI] [PubMed] [Google Scholar]
- Molla-Herman A, et al. Targeting of beta-arrestin2 to the centrosome and primary cilium: role in cell proliferation control. PLoS One. 2008;3:e3728. doi: 10.1371/journal.pone.0003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia NS, et al. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–55. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- Mykytyn K, et al. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci U S A. 2004;101:8664–9. doi: 10.1073/pnas.0402354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury M, et al. A Core Complex of BBS Proteins Cooperates with the GTPase Rab8 to Promote Ciliary Membrane Biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci. 1996;5:2375–90. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura DY, et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci U S A. 2004;101:16588–93. doi: 10.1073/pnas.0405496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–37. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Norman RX, et al. Tubby-like protein 3 (TULP3) regulates patterning in the mouse embryo through inhibition of Hedgehog signaling. Hum Mol Genet. 2009;18:1740–54. doi: 10.1093/hmg/ddp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Ocbina PJ, Anderson KV. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev Dyn. 2008;237:2030–8. doi: 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, et al. elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- Ou G, et al. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–7. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- Pan Y, et al. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365–77. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr Opin Neurobiol. 2000;10:392–9. doi: 10.1016/s0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson VL, et al. Mouse hitchhiker mutants have spina bifida, dorso-ventral patterning defects and polydactyly: identification of Tulp3 as a novel negative regulator of the Sonic hedgehog pathway. Hum Mol Genet. 2009;18:1719–39. doi: 10.1093/hmg/ddp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–18. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, et al. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–81. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, et al. Dissecting the molecular mechanisms of intraflagellar transport in chlamydomonas. Curr Biol. 2006;16:450–9. doi: 10.1016/j.cub.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Pedersen LB, et al. Chlamydomonas IFT172 is encoded by FLA11, interacts with CrEB1, and regulates IFT at the flagellar tip. Curr Biol. 2005;15:262–6. doi: 10.1016/j.cub.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Pedersen LB, et al. Assembly of primary cilia. Dev Dyn. 2008;237:1993–2006. doi: 10.1002/dvdy.21521. [DOI] [PubMed] [Google Scholar]
- Pierani A, et al. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell. 1999;97:903–15. doi: 10.1016/s0092-8674(00)80802-8. [DOI] [PubMed] [Google Scholar]
- Piperno G, et al. Distinct mutants of retrograde intraflagellar transport (IFT) share similar morphological and molecular defects. J Cell Biol. 1998;143:1591–601. doi: 10.1083/jcb.143.6.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, et al. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr Biol. 2005;15:1695–9. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Rana AA, et al. Targeted deletion of the novel cytoplasmic dynein mD2LIC disrupts the embryonic organiser, formation of the body axes and specification of ventral cell fates. Development. 2004;131:4999–5007. doi: 10.1242/dev.01389. [DOI] [PubMed] [Google Scholar]
- Robbins DJ, et al. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90:225–34. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, et al. Hedgehog signal transduction by Smoothened: Pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0813373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, et al. Patched1 Regulates Hedgehog Signaling at the Primary Cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Romio L, et al. OFD1 is a centrosomal/basal body protein expressed during mesenchymal-epithelial transition in human nephrogenesis. J Am Soc Nephrol. 2004;15:2556–68. doi: 10.1097/01.ASN.0000140220.46477.5C. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–25. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, et al. The emergent design of the neural tube: prepattern, SHH morphogen and GLI code. Current Opinion in Genetics & Development. 2003;13:513–21. doi: 10.1016/j.gde.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, et al. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–72. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, et al. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134:2903–12. doi: 10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, et al. Mutations in a new gene in Ellis-van Creveld syndrome and Weyers acrodental dysostosis. Nat Genet. 2000;24:283–6. doi: 10.1038/73508. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, et al. Mutations in two nonhomologous genes in a head-to-head configuration cause Ellis-van Creveld syndrome. Am J Hum Genet. 2003;72:728–32. doi: 10.1086/368063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature. 2007;448:366–9. doi: 10.1038/nature05929. [DOI] [PubMed] [Google Scholar]
- Scholey JM. Kinesin-II, a membrane traffic motor in axons, axonemes, and spindles. J Cell Biol. 1996;133:1–4. doi: 10.1083/jcb.133.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey JM. Intraflagellar transport motors in cilia: moving along the cell's antenna. The Journal of Cell Biology. 2008;180:23–9. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signor D, et al. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J Cell Biol. 1999;147:519–30. doi: 10.1083/jcb.147.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JC, et al. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell. 1997;90:235–45. doi: 10.1016/s0092-8674(00)80332-3. [DOI] [PubMed] [Google Scholar]
- Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–77. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DM, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–34. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- Tabata T, Kornberg TB. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell. 1994;76:89–102. doi: 10.1016/0092-8674(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Taipale J, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–9. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- Taipale J, et al. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–7. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- Tobin JL, Beales PL. Bardet-Biedl syndrome: beyond the cilium. Pediatr Nephrol. 2007;22:926–36. doi: 10.1007/s00467-007-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P, et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierkotten J, et al. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–77. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- Wang B, et al. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–34. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Wang Q, et al. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell. 2006;125:549–62. doi: 10.1016/j.cell.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. Selective translocation of intracellular Smoothened to the primary cilium in response to Hedgehog pathway modulation. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0812110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijgerde M, et al. A direct requirement for Hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord. Genes Dev. 2002;16:2849–64. doi: 10.1101/gad.1025702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CW, et al. Smoothened adopts multiple active and inactive conformations capable of trafficking to the primary cilium. PLoS One. 2009a;4:e5182. doi: 10.1371/journal.pone.0005182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CW, et al. Fused has evolved divergent roles in vertebrate Hedgehog signalling and motile ciliogenesis. Nature. 2009b;459:98–102. doi: 10.1038/nature07883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L, Maden M. The mechanisms of dorsoventral patterning in the vertebrate neural tube. Dev Biol. 2005;282:1–13. doi: 10.1016/j.ydbio.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Wolff C, et al. Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr Biol. 2003;13:1169–81. doi: 10.1016/s0960-9822(03)00461-5. [DOI] [PubMed] [Google Scholar]
- Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, et al. Cloning and characterization of KAP3: a novel kinesin superfamily-associated protein of KIF3A/3B. Proc Natl Acad Sci U S A. 1996;93:8443–8. doi: 10.1073/pnas.93.16.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- Yin Y, et al. The Talpid3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development. 2009;136:655–64. doi: 10.1242/dev.028464. [DOI] [PMC free article] [PubMed] [Google Scholar]