Abstract
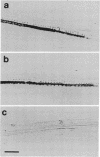
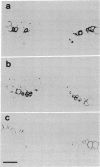
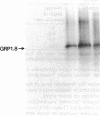
An antibody against glycine-rich protein 1.8 of bean (Phaseolus vulgaris L.) was used for immunogold/silver localization of the protein in different organs of the plant. In hypocotyls, ovaries, and seed coats, the protein was found specifically in xylem cells of the vascular tissue. In hypocotyls, only protoxylem cells were labeled with the antibody, which indicates a remarkable cell-type specificity for accumulation of this cell wall protein. In mature hypocotyls, the protein was restricted to the same subset of xylem cells but was no longer detected on tissue prints, where a positive antibody reaction depends on the transfer of soluble material from plant tissue to the nitrocellulose filter. This indicates that the glycine-rich protein is insolubilized in the cell wall during development. In longitudinal sections of tracheary elements of young hypocotyls and seed coats, the antibody stained a pattern very similar to that of the lignified secondary thickenings of the cell wall, which suggests a close functional relationship between glycine-rich protein and lignin deposition during cell wall biogenesis in protoxylem cells.
Keywords: immunocytolocalization, lignin, Phaseolus vulgaris
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bürglin T. R., De Robertis E. M. The nuclear migration signal of Xenopus laevis nucleoplasmin. EMBO J. 1987 Sep;6(9):2617–2625. doi: 10.1002/j.1460-2075.1987.tb02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassab G. I., Varner J. E. Immunocytolocalization of extensin in developing soybean seed coats by immunogold-silver staining and by tissue printing on nitrocellulose paper. J Cell Biol. 1987 Dec;105(6 Pt 1):2581–2588. doi: 10.1083/jcb.105.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin D. R., Sauer N., Lamb C. J. Differential regulation of a hydroxyproline-rich glycoprotein gene family in wounded and infected plants. Mol Cell Biol. 1987 Dec;7(12):4337–4344. doi: 10.1128/mcb.7.12.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blas A. L., Cherwinski H. M. Detection of antigens on nitrocellulose paper immunoblots with monoclonal antibodies. Anal Biochem. 1983 Aug;133(1):214–219. doi: 10.1016/0003-2697(83)90245-2. [DOI] [PubMed] [Google Scholar]
- Keller B., Sauer N., Lamb C. J. Glycine-rich cell wall proteins in bean: gene structure and association of the protein with the vascular system. EMBO J. 1988 Dec 1;7(12):3625–3633. doi: 10.1002/j.1460-2075.1988.tb03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Showalter A. M., Bell J. N., Cramer C. L., Bailey J. A., Varner J. E., Lamb C. J. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6551–6555. doi: 10.1073/pnas.82.19.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springall D. R., Hacker G. W., Grimelius L., Polak J. M. The potential of the immunogold-silver staining method for paraffin sections. Histochemistry. 1984;81(6):603–608. doi: 10.1007/BF00489542. [DOI] [PubMed] [Google Scholar]
- Thomas E. D. Bone marrow grafts and tolerance. Nature. 1986 Sep 11;323(6084):110–111. doi: 10.1038/323110b0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]