Abstract
Podocytes are highly specialized cells in the vertebrate kidney. They participate in the formation of the size-exclusion barrier of the glomerulus/glomus and recruit mesangial and endothelial cells to form a mature glomerulus. At least six transcription factors (wt1, foxc2, hey1, tcf21, lmx1b and mafb) are known to be involved in podocyte specification, but how they interact to drive the differentiation program is unknown. The Xenopus pronephros was used as a paradigm to address this question. All six podocyte transcription factors were systematically eliminated by antisense morpholino oligomers. Changes in the expression of the podocyte transcription factors and of four selected markers of terminal differentiation (nphs1, kirrel, ptpru and nphs2) were analyzed by in situ hybridization. The data were assembled into a transcriptional regulatory network for podocyte development. Although eliminating the six transcription factors individually interfered with aspects of podocyte development, no single gene regulated the entire differentiation program. Only the combined knockdown of wt1 and foxc2 resulted in a loss of all podocyte marker gene expression. Gain-of-function studies showed that wt1 and foxc2 were sufficient to increase podocyte gene expression within the glomus proper. However, the combination of wt1, foxc2 and Notch signaling was required for ectopic expression in ventral marginal zone explants. Together, this approach demonstrates how complex interactions are required for the correct spatiotemporal execution of the podocyte gene expression program.
Keywords: Amphibian, Glomus, Glomerulus, Mesangial cells, Notch, Slit diaphragm, Xenopus
INTRODUCTION
The kidney is required for water homeostasis and waste excretion (Saxén, 1987; Vize et al., 2003). In vertebrates, three successively more complex kidney structures – the pronephros, mesonephros and metanephros – have evolved. The metanephros is the adult kidney in higher vertebrates, such as humans and mice, whereas the mesonephros is the adult kidney of fish and amphibians. The pronephros is the simplest and earliest kidney form. It is rudimentary in mammals, but is required in aquatic animals for water homeostasis at larval stages (Howland, 1916). Moreover, the development of the pronephros is a prerequisite for the subsequent formation of the mesonephric and metanephric kidney (Torrey, 1965; Bouchard et al., 2002). Despite the differing complexity of the three kidney types, their functional unit, the nephron, is organized very similarly. Many transcription factors, structural proteins and signaling pathways pattern the pronephros and metanephros in an evolutionarily conserved manner (Carroll et al., 1999; McLaughlin et al., 2000; Cheng et al., 2003; Wang et al., 2003; Zhou and Vize, 2004; Cheng et al., 2007; Wingert et al., 2007; Raciti et al., 2008).
Among the different cell types present in the nephron, podocytes are unique in that they link the vasculature to the urinary system (Johnstone and Holzman, 2006; Quaggin and Kreidberg, 2008). They are highly specialized epithelial cells required for kidney filtration. Podocytes form the slit diaphragm, a sieve-like structure with a 4-nm pore size that restricts the transport of molecules from the blood to the ultrafiltrate (Rodewald and Karnovsky, 1974; Edwards et al., 1999; Takahashi-Iwanaga, 2002). The slit diaphragm contains proteins that are commonly found in adherens junctions (e.g. α-, β-, γ-Catenin, Vinculin and α-Actinin-4) and tight junctions [e.g. Tjp1 (ZO1)], as well as a set of slit diaphragm-specific proteins [e.g. Nphs1 (Nephrin), Kirrel (Neph1) and Nphs2 (Podocin)] (Pavenstadt et al., 2003; Johnstone and Holzman, 2006; Quaggin and Kreidberg, 2008). Although the importance of the slit diaphragm complex is well established, the details of its formation during podocyte differentiation are still unknown.
Several transcription factors and signaling pathways have been implicated in this process (Quaggin and Kreidberg, 2008). Among these, Wilms tumor 1 (Wt1) is regarded as the key regulator of podocyte development. Although Wt1 mutant mice do not form kidneys (Kreidberg et al., 1993), mice lacking the transcriptionally active Wt1 splice variant Wt1-KTS develop kidneys with very few immature glomeruli (Hammes et al., 2001). The role of Wt1 is evolutionarily conserved. Studies in zebrafish and Xenopus show that Wt1 is also important for glomerulogenesis in the pronephros (Majumdar et al., 2000; Taelman et al., 2006; Perner et al., 2007). Other podocyte transcription factors develop weaker podocyte phenotypes in mouse loss-of-function studies. Foxc2 homozygous mouse mutants have hypoplastic kidneys with few glomeruli, exhibiting dilated capillary loops and expressing decreased levels of Nphs2 and Mafb (Takemoto et al., 2006). Mafb, Tcf21 and Lmx1b mouse mutants develop glomeruli, but the podocytes show defects at the ultrastructural level (Quaggin et al., 1999; Miner et al., 2002; Quaggin, 2002; Rohr et al., 2002; Sadl et al., 2002; Cui et al., 2003; Moriguchi et al., 2006). Notch is one of the best-documented signaling pathways in the kidney. It is involved in the initial patterning of the nephron, determining proximal (i.e. glomerulus and proximal tubules) versus distal cell fates in both mouse and Xenopus (McLaughlin et al., 2000; Cheng et al., 2003; Wang et al., 2003; Taelman et al., 2006; Cheng et al., 2007; Naylor and Jones, 2009).
Although glomerular transcription factors have been studied extensively, it is still not known how they cooperate to form a functional podocyte and regulate podocyte-specific transcription. To address this question, we have performed a systematic knockdown of six transcription factors (wt1, foxc2, hey1, mafb, tcf21 and lmx1b) using the Xenopus pronephros as a model for podocyte development. This analysis identified a specific role for each transcription factor in podocyte specification. More importantly, the data were assembled into a gene regulatory network to visualize interactions between the transcription factors. This revealed that wt1 and foxc2 together are required, but not sufficient, for podocyte development. A third input, activated Notch signaling, was necessary to induce ectopic podocyte gene expression. This suggested that the correct spatiotemporal execution of the podocyte program relies on the concerted action of at least three independent inputs: wt1, foxc2 and Notch signaling.
MATERIALS AND METHODS
Embryo manipulations and RT-PCR analyses
Xenopus embryos obtained by in vitro fertilization were maintained in 0.1× modified Barth medium (Sive et al., 2000) and staged following Nieuwkoop and Faber (Nieuwkoop and Faber, 1994). For antisense morpholino oligomer (MO) injections, a total of 3.2 pmol of MO was injected radially into Xenopus embryos at the 2- to 4-cell stage. MOs were dissolved to 1 mM stock solutions and were used at a final concentration of 200 μM. The following MOs were obtained from Gene Tools: 5′-ATTCATATCCCGCACATCAGATCCC-3′ (wt1-MO1), 5′-CATATCCCGGACATCAGACCCCATC-3′ (wt1-MO2) (Taelman et al., 2006), 5′-ACGCGCCTGCATCATTAGTGCTGAA-3′ (foxc2-MO), 5′-TAGTCGTGTCCCCGCTTCATGGCTG-3′ (hey1-MO) (Taelman et al., 2006), 5′-CATCACTGAGAGAACCGGTGGACAT-3′ (tcf21-MO), 5′-CGGGACCTGTTGCAATATCCATGCC-3′ (lmx1b-MO), 5′-GCCACTCTCCAAAACTCACTTCAGT-3′ (lmx1b-MO2) (Haldin et al., 2008), 5′-AATGGGCAACTCTCCAGCCATACTG-3′ (mafb-MO) and a standard control MO (Std-MO).
For synthetic mRNA, plasmids were linearized as indicated and transcribed with SP6 RNA polymerase using the mMessage mMachine (Applied Biosystems) as follows: pCS2-foxc2* (ApaI), pCS2-GFP-foxc2 (NotI), pCS2-lmx1b* (NotI), pCS2-GFP-lmx1b (NotI), pCS2-mafb* (NotI), pCS2-GFP-mafb (NotI), pCS2-NICD (NotI) (Coffman et al., 1990), pCS2-GFP-tcf21 (NotI), pCS2-wt1* (KspI), pCS2-wt1-GFP (NotI), pCS2-wt1(mut)-GFP (NotI); pXEX-β-Gal was linearized with Asp718 and transcribed with T7 RNA polymerase (detailed information about the individual constructs is available upon request).
Ectodermal explants were excised at stage 9; marginal zone explants were excised at stage 10. Explants were cultured in 0.5× MMR (Sive et al., 2000) until sibling embryos reached stage 35. For Activin A treatment, explants were incubated for 3 hours with 10 ng/ml Activin A (Cell Sciences) in 0.1% BSA low Ca2+/low Mg2+ Ringer's Solution (Sive et al., 2000). Explants and whole control embryos were processed for RT-PCR analysis as described (Sasai et al., 1995). The sequences of the primers are listed in Table S1 in the supplementary material. RT-PCR products were analyzed by polyacrylamide gel electrophoresis. In some cases results were quantified using a GelDoc-It Imaging System (UVP). The percentage change in gene expression in injected embryos compared with controls was calculated and averaged between three experiments. The F-statistic was used to verify equal variance between the experimental and control groups so that statistical significance could be calculated using a one-tailed Student's t-test.
GFP reporter assays
For the GFP reporter assays, 250 pg of synthetic mRNA was injected into the animal pole of each blastomere at the 4-cell stage, alone or preceding animal injection of 3.2 pmol of the corresponding MO at the 2-cell stage. Once sibling uninjected embryos reached stage 10, embryos or dissected animal poles were analyzed for fluorescence on a Zeiss Axio Imager A1 with an Axiocam digital camera. Fluorescence intensity was quantified using ImageJ (NIH). MO-injected embryos and transcription factor-GFP + MO co-injected embryos were compared using Student's t-test.
Whole-mount in situ hybridization
Whole-mount in situ hybridization and in situ hybridization on Paraplast sections were performed as described (Tran et al., 2007; Agrawal et al., 2009). The following plasmids were linearized and transcribed for antisense probes: pSK-aplnr, NotI/T7 (Devic et al., 1996); pSK-foxc2, EcoRI/T7 (GenBank accession AJ249225); pCS2-hey1, EcoRI/T7 (DC130331); pCS2p-kirrel, ClaI/T7 (BC057728); pSK-lmx1b, BamHI/T7 (AF414086); pCS2p-mafb, EcoRI/T7 (BC077255); pCMV-SPORT6-nphs1, EcoRI/T7 (Tran et al., 2007); pGEM-T-Easy-nphs2, ApaI/SP6 (GQ370808); pCMV-SPORT6-ptpru, EcoRI/T7 (CD300952); pCMV-SPORT6-tcf21, EcoRI/T7 (BC073597); pSK-wt1, BamHI/T7 (Carroll and Vize, 1996).
All experiments were performed in triplicate and analyzed by comparing the expression patterns of the podocyte genes at stage 35 between morphants and sibling control embryos of the same experiment. Embryos were embedded in Paraplast and sectioned transversely at 25 μm. For each gene at least six control and six morphant embryos were evaluated by examining every section of the embryo. The effects of knockdowns on the expression of podocyte genes were recorded as increased, decreased, absent or unchanged. Sectioning of whole-mount embryos was essential to accurately judge the individual expression patterns as the analysis of whole-mount in situ hybridizations tended to underestimate the effects. Moreover, the podocyte expression of foxc2 and mafb was obscured by other expression domains and could not be evaluated in whole-mounts.
Histology and immunohistochemistry
For histological staining, Xenopus embryos were fixed in Bouin's Fixative, dehydrated, embedded in Paraplast, sectioned at 7 μm, dewaxed, and stained with Hematoxylin and Eosin. For immunohistochemistry, embryos were fixed in Dent's Fixative. For whole-mount immunostaining, embryos were incubated overnight with Vimentin antiserum [14h7 (Dent et al., 1989)] followed by incubation with a horseradish peroxidase-coupled anti-mouse IgG and developed using the ImmPACT DAB Kit (Vector Laboratories). Embryos were subsequently embedded in Paraplast and sectioned coronally at 25 μm to visualize the glomus. Immunohistochemistry for β1-Integrin [8C8 (Gawantka et al., 1992)] was performed on Paraplast sections using an Alexa Fluor 488-conjugated goat anti-mouse secondary antibody (Invitrogen).
RESULTS
Temporal expression pattern of podocyte genes
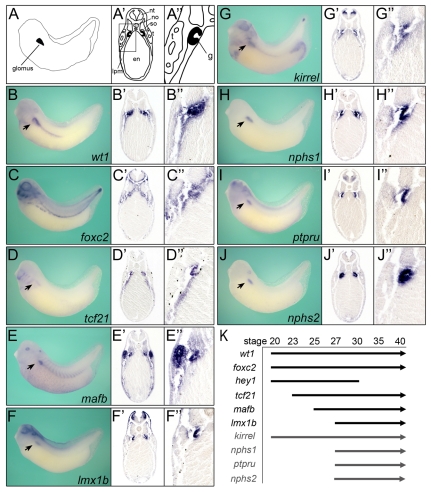
As a first step to understand podocyte development in Xenopus, the expression patterns of six podocyte transcription factors [wt1, foxc2, hey1 (xhrt1), tcf21, mafb and lmx1b] were compared with the expression of four markers of terminal differentiation [nphs1 (Nephrin), Kirrel (neph1) and nphs2 (Podocin)] that are expressed in the slit diaphragm (Ruotsalainen et al., 1999; Schwarz et al., 2001; Barletta et al., 2003) and with ptpru (glepp1) as a non-slit diaphragm protein (Thomas et al., 1994). wt1, hey1, mafb, lmx1b, tcf21 and nphs1 have been studied previously in Xenopus whole-mounts (Carroll and Vize, 1996; Ishibashi and Yasuda, 2001; Haldin et al., 2003; Coolen et al., 2005; Gerth et al., 2005; Simrick et al., 2005; Taelman et al., 2006; Haldin et al., 2008), but to precisely compare the glomus expression domains it was important to perform a side-by-side analysis of all ten genes. We initially analyzed embryos at stage 35 because Xenopus podocytes are functional at stage 38 and vascularization of the glomus occurs around stage 32 (Nieuwkoop and Faber, 1994; Vize et al., 2003; Doherty et al., 2007). Both whole-mount in situ hybridizations and Paraplast-embedded sections thereof revealed distinct expression patterns. wt1 mRNA was detected in the glomus, the intermediate mesoderm surrounding the pronephric duct and the heart (Fig. 1B-B′ and data not shown). foxc2 was expressed in the glomus, the eyes, the branchial arches, hypaxial muscles and the tailbud, as well as in the mesenchyme surrounding the notochord and neural tube (Fig. 1C-C′). tcf21 was expressed in the glomus, lateral plate mesoderm and branchial arches (Fig. 1D-D′). mafb mRNA was detected in the glomus, proximal tubules, somites, ventral blood islands, eyes and hindbrain (Fig. 1E-E′). lmx1b was detected in the glomus, eyes, otic placodes, dorsal ectoderm and the dopaminergic neurons of the neural tube (Fig. 1F-F′). kirrel was expressed in the glomus, eyes, brain, branchial arches, heart, tailbud and the ventral pancreatic bud (Fig. 1G-G′). nphs1 was exclusively expressed in the glomus (Fig. 1H-H′). ptpru was detected in the glomus, eyes, brain and neural tube (Fig. 1I-I′). nphs2 was detected in the glomus and the inner lining of the neural tube (Fig. 1J-J′). As previously reported (Taelman et al., 2006), hey1 was no longer expressed in the glomus region at stage 35 (data not shown). Examining in situ hybridizations at progressively earlier stages also established a temporal profile of glomus gene expression (Fig. 1K and data not shown). Based on this analysis, wt1, foxc2 and hey1 are the earliest expressed transcription factors at stage 20, followed by tcf21 at stage 23, mafb at stage 25 and lmx1b at stage 27. Interestingly, of the terminal differentiation genes, kirrel was already detected at stage 20, whereas the other three were not detected until stage 27.
Fig. 1.
Spatiotemporal expression of podocyte genes. (A-A′) Schematics depicting the glomus (black) in whole Xenopus embryos (A) and in transverse section at low (A′) and high (A′) magnification at stage 35. (B-J′) In situ hybridization of podocyte transcription factors wt1 (B-B′), foxc2 (C-C′), tcf21 (D-D′), mafb (E-E′) and lmx1b (F-F′) and of markers of podocyte differentiation kirrel (G-G′), nphs1 (H-H′), ptpru (I-I′) and nphs2 (J-J′) at stage 35 in whole-mounts (B-J) and transverse sections (B′-J′). (K) Temporal profile of podocyte gene expression in the glomus. en, endoderm; g, pronephric glomus; lpm, lateral plate mesoderm; no, notochord; nt, neural tube; so, somites; t, pronephric tubules.
Together, these data showed that the podocyte genes analyzed here are present in the glomus by stage 27, even though the pronephros is not functional until stage 38 (Nieuwkoop and Faber, 1994). Interestingly, none of the podocyte transcription factors and only one terminal differentiation marker is glomus specific. Thus, combinatorial inputs most likely play an important role in restricting podocyte development to the glomus anlage.
Knockdown of wt1
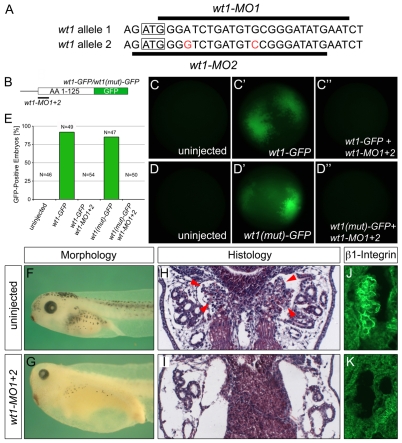
The expression analysis suggested that wt1 is among the earliest transcription factors expressed in the future glomus. Work by others has suggested that wt1 is a key regulator in podocyte development (Hammes et al., 2001; Taelman et al., 2006; Perner et al., 2007). Thus, to better understand the role of wt1 in podocyte specification, the protein was eliminated using two antisense morpholino oligomers (wt1-MO1, wt1-MO2) that target the ATG of the two pseudo-alleles of wt1 mRNA (Fig. 2A). In the absence of an antibody recognizing Xenopus wt1 protein, a reporter assay was developed to test the efficacy of the wt1-MO. Part of the 5′UTR and the first 125 amino acids of wt1 were fused in-frame with eGFP (wt1-GFP, Fig. 2B). A second construct mimicking the second allele of wt1 was generated by site-directed mutagenesis [wt1(mut)-GFP, Fig. 2B]. Synthetic wt1-GFP or wt1(mut)-GFP mRNA (1 ng) was injected into the animal pole of Xenopus embryos at the 4-cell stage in the presence or absence of a mixture of the two wt1 MOs (3.2 pmol each, wt1-MO1+2). Embryos were cultured until gastrula stage and imaged by fluorescence microscopy (Fig. 2C-E). Whereas fluorescence could be detected in the wt1-GFP-injected and wt1(mut)-GFP-injected embryos, it was completely abolished upon co-injection of wt1-MO1+2.
Fig. 2.
Knockdown of wt1 results in a glomus phenotype. (A) Sequences of the two pseudo-alleles of Xenopus wt1 and the location of wt1-MO1 and wt1-MO2. Mismatches between wt1-MO and the second allele of wt1 are indicated in red. The translational start site is boxed. (B) Schematic of the wt1-GFP reporter constructs. (C-E) Fluorescence of uninjected control embryos (C,D), embryos injected with the wt1-GFP reporter mRNA (C′), with wt1-MO1+2 and the wt1-GFP reporter (C′), wt1(mut)-GFP (D′) or with wt1-MO1+2 and the wt1(mut)-GFP (D′) at stage 10. The results of multiple experiments were quantified (E); the number of embryos analyzed is indicated above the bars. (F-K) Phenotype, histology and β1-Integrin immunofluorescence of wt1-MO1+2-injected embryos (G,I,K) and sibling controls (F,H,J) at stage 40 (F,G) and stage 42 (H-K). Red arrowheads indicate glomus in H.
Next, Xenopus embryos were injected radially with wt1-MO1+2 at the 2- to 4-cell stage, cultured until sibling embryos reached stage 40/42 and analyzed by morphology and histology. wt1-MO1+2-injected embryos were characterized by the formation of fluid-filled edema (Fig. 2F-I). This phenotype was most likely caused by a defective pronephros. Histological sections and immunofluorescence for β1-Integrin, which marks the basolateral side of podocytes (Pozzi et al., 2008), indicated a complete absence of the glomus domain in the injected embryos (Fig. 2H-K).
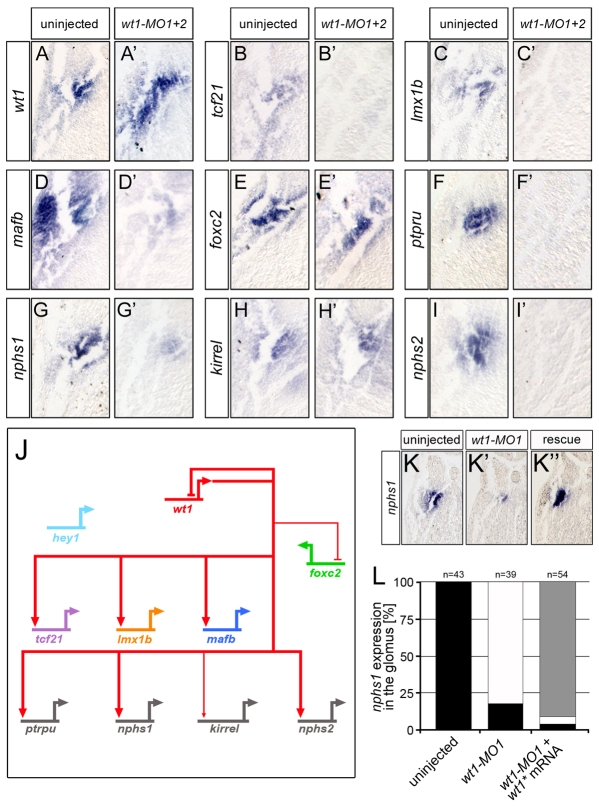
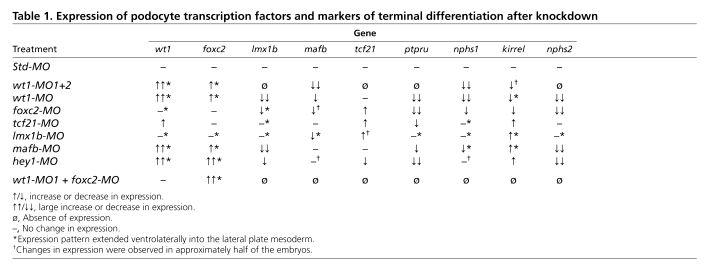
To substantiate this phenotype at the molecular level, whole-mount in situ hybridizations were performed to analyze the expression of the podocyte transcription factors wt1, foxc2, tcf21, lmx1b and mafb and the terminal differentiation genes nphs1, kirrel, ptpru and nphs2. Embryos were analyzed at stage 35 because all the genes, with the exception of hey1, were robustly expressed at this stage (Fig. 1K). It is very difficult to dissect the glomus without contamination from the surrounding tissue, and this might influence the results of quantitative RT-PCR analysis. Instead, whole-mounts and Paraplast sections thereof were used to compare gene expression patterns or levels (see Materials and methods for details on the evaluation process). wt1-MO1+2-injected embryos displayed a wide range of changes in the podocyte expression domains (Fig. 3A-I′, Table 1; see Fig. S1 and Table S2 in the supplementary material). Expression of lmx1b, tcf21, ptpru and nphs2 was completely lost (Fig. 3B-C′,F,F′,I,I′) and mafb and nphs1 were markedly reduced (Fig. 3D,D′,G,G′). By contrast, kirrel was weakly reduced (Fig. 3H,H′) and wt1 and foxc2 were elevated (Fig. 3A,A′,E,E′). In addition to these changes in expression levels, the expression domain of wt1 was enlarged and those of foxc2 and kirrel were expanded ventrolaterally into the lateral plate mesoderm (Fig. 3A,A′,E,E′,H,H′). Injection of 3.2 pmol of wt1-MO1 alone resulted in a qualitatively similar, yet less robust, loss-of-function phenotype (Table 1; see Fig. S2A-I′ in the supplementary material). This was noteworthy because the two pseudo-alleles of wt1 only differ by two nucleotides within the wt1-MO1 binding site (Fig. 2A).
Fig. 3.
wt1 regulates podocyte gene expression. (A-I′) High magnification of representative transverse sections of whole-mount in situ hybridizations comparing the expression of podocyte genes wt1 (A,A′), tcf21 (B,B′), lmx1b (C,C′), mafb (D,D′), foxc2 (E,E′), ptpru (F,F′), nphs1 (G,G′), kirrel (H,H′) and nphs2 (I,I′) between wt1 morphants and sibling control Xenopus embryos at stage 35. (J) Wire diagram summarizing the in situ hybridization data. Arrows, positive regulation; T-bars, negative regulation; thick lines, strong effects; thin lines, weak effects. Note that none of the interactions is necessarily direct and might involve intermediary players. (K-L) In situ hybridization of nphs1 in uninjected controls (K), embryos injected with wt1-MO1 (K′) and with wt1* mRNA and wt1-MO1 (K′). The results of multiple experiments were quantified (L). Black, strong bilateral expression; white, loss of expression; gray, unilateral expression rescued by wt1* mRNA.
Table 1.
Expression of podocyte transcription factors and markers of terminal differentiation after knockdown
To document these changes in gene expression a wire diagram was developed, similar to those initially described for sea urchin mesendoderm formation (Davidson et al., 2003). Genes were connected by arrows or inhibitory bars to indicate how a given gene product regulates the transcription of other genes. Since we did not perform promoter studies, such as chromatin immunoprecipitations, the connections do not necessarily reflect a direct interaction between the transcription factor and its target, but might involve a transcriptional relay. In the case of the wt1-MO1+2 experiment the following connections were made (Fig. 3J): as the expression of mafb, lmx1b, tcf21, ptpru, nphs1 and nphs2 was lost or strongly reduced, wt1 was connected to these genes with a thick arrow to indicate a positive input; kirrel mRNA levels were only weakly reduced and therefore connected by a thin arrow; conversely, wt1 and foxc2 expression was increased and so these genes were connected with an inhibitory bar symbolizing transcriptional repression, again using thin and thick lines to reflect the strength of the effect.
The specificity of the wt1-MO1+2 results was addressed using two assays. First, injections of a standard control MO (Std-MO) did not show any reproducible changes in any of the podocyte genes analyzed (Table 1 and see Fig. S3 in the supplementary material). Secondly, Xenopus embryos were injected with the wt1-MO1 in the presence or absence of a one-sided injection of 500 pg wt1* mRNA, a wt1-KTS construct that was mutated in the wt1-MO1 binding site and was therefore resistant to the wt1-MO1 activity. Whole-mount in situ hybridizations were performed using nphs1 expression as readout. nphs1 was strongly reduced following wt1 knockdown, but was regained on the wt1*-injected side in 90% of cases (Fig. 3K-L and see Fig. S2J-L in the supplementary material). It is important to note that the wt1 construct used in the present study differs from the constructs used by others (Wallingford et al., 1998; Perner et al., 2007) as it contains an optimized Kozak sequence and lacks the entire 3′UTR, and single injections did not result in gross morphological defects (data not shown).
This analysis showed that wt1 is a very important player in podocyte specification, but is not sufficient to block all aspects of podocyte gene expression.
Knockdown of other podocyte transcription factors
Next, we examined the contribution of the other transcription factors to the podocyte program. foxc2, hey1, tcf21, lmx1b and mafb were eliminated using MOs, following the experimental layout described for wt1 knockdown. With the exception of the previously described MO targeting hey1 (Taelman et al., 2006), all MOs were tested for efficacy and specificity. Knockdown phenotypes were characterized by morphology and histology, and podocyte gene expression was examined by in situ hybridization and the data assembled into a wire diagram (results are summarized in Table 1, Figs S4-S12 and Table S2 in the supplementary material). There were only two deviations from this scheme. Despite noticeable changes in the glomus architecture of tcf21 morphants, only weak changes in gene expression were detected (see Figs S6 and S7 in the supplementary material). Since these were not amendable as readouts of the tcf21-MO phenotype, no rescue experiment was attempted. Secondly, the results of the lmx1b knockdown were confirmed using a second previously published MO (Haldin et al., 2008) (see Table S2 in the supplementary material). It is also noteworthy that the effect of the hey1-MO seemed to be stage dependent. Although wt1 and nphs1 were strongly downregulated at stage 28 (data not shown) (Taelman et al., 2006), the expression recovered by stage 35.
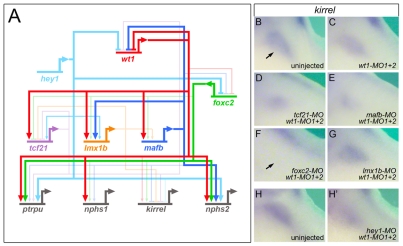
Once all the data were obtained, the individual wire diagrams were combined into a single gene regulatory network (Fig. 4A). It is important to note that the connections might not reflect direct transcriptional effects. The analysis did not exclude relays of consecutive gene activations. Despite its complexity, five messages were readily apparent. First, the network displays a high degree of redundancy. All genes have multiple transcriptional inputs. Second, genes are integrated into feedback loops. For example, lmx1b activates mafb transcription and mafb activates lmx1b. Third, mafb, a downstream transcription factor, negatively regulates its upstream activators, wt1 and foxc2. Fourth, no single transcription factor regulates the entire network. Lastly, wt1 is the most potent podocyte transcription factor.
Fig. 4.
wt1 and foxc2 are key regulators of the podocyte gene regulatory network. (A) Wire diagram combining the data for the individual knockdowns of the six podocyte transcription factors, showing all the connections. Note that none of the interactions is necessarily direct and might involve intermediary players. (B-G) Whole-mount in situ hybridization for kirrel mRNA expression in uninjected control embryos (B) and Xenopus embryos injected radially with wt1-MO1+2 alone (C) and in the presence of tcf21-MO (D), mafb-MO (E), foxc2-MO (F) or lmx1b-MO (G). (H,H′) kirrel expression in a Xenopus embryo injected unilaterally with wt1-MO1+2 and hey1-MO showing the uninjected (H) or injected (H′) side.
wt1 and foxc2 are required together for podocyte gene expression
The analysis so far indicated a high redundancy among the podocyte transcription factors. Since knockdown of wt1 had the most dramatic effect, we next asked whether any of the other five transcription factors cooperates with wt1 and whether this cooperation can explain all of the podocyte gene expression patterns. The initial analysis focused on kirrel, as it was least affected by the knockdown of wt1 (Fig. 3H,H′ and see Fig. S1G,G′ in the supplementary material). Xenopus embryos were injected radially with wt1-MO1+2 in combination with either foxc2-MO, tcf21-MO, mafb-MO or lmx1b-MO at the 2- to 4-cell stage. hey1 radial injections impaired overall development of the embryos (data not shown), and wt1-MO1+2 and hey1-MO were therefore injected unilaterally into one blastomere at the 2-cell stage. Whole-mount in situ hybridization at stage 35 demonstrated that from all the combinations, only co-injection of wt1-MO1+2 and foxc2-MO resulted in a loss of kirrel expression in the glomus region (Fig. 4B-H′).
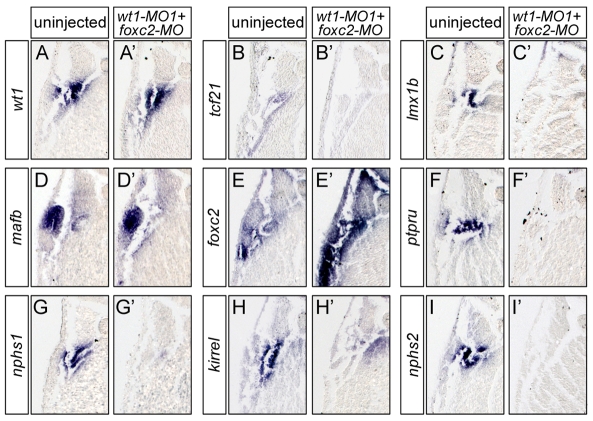
To address whether this effect of wt1 and foxc2 is restricted to kirrel, we extended the analysis to the other genes of the network. Injection of wt1-MO1+2 alone already strongly reduced the expression of many of the podocyte genes and resulted in the absence of a histologically identifiable glomus (Figs 2 and 3 and see Fig. S1 in the supplementary material). Thus, to achieve a more sensitive assay, only one of the two wt1 MOs (wt1-MO1) was used in this part of the study. Knockdown with wt1-MO1 indeed resulted in a less pronounced, but qualitatively similar phenotype (see Fig. S2A-I′ in the supplementary material). Xenopus embryos were co-injected with 3.2 pmol wt1-MO1 and 3.2 pmol foxc2-MO, cultured until the uninjected control embryos reached stage 35 and then processed for in situ hybridization. The glomus expression of the other three transcription factors (tcf21, lmx1b and mafb) and of all four terminal differentiation markers (ptpru, nphs1, kirrel and nphs2) was lost (Fig. 5, Table 1). wt1 expression was unchanged, whereas foxc2 mRNA was greatly increased throughout the embryo. In the case of kirrel, a new expression domain of unknown function was observed in the dorsal endoderm (Fig. 5H′). These data suggested that wt1 and foxc2 cooperate in the expression of podocyte markers.
Fig. 5.
wt1 and foxc2 are required together for expression of podocyte genes. (A-I′) High magnification of transverse sections of whole-mount in situ hybridizations comparing the expression of podocyte genes wt1 (A,A′), tcf21 (B,B′), lmx1b (C,C′), mafb (D,D′), foxc2 (E,E′), ptpru (F,F′), nphs1 (G,G′), kirrel (H,H′) and nphs2 (I,I′) between wt1-MO1 plus foxc2-MO co-injected Xenopus embryos (A′-I′) and sibling controls (A-I) at stage 35.
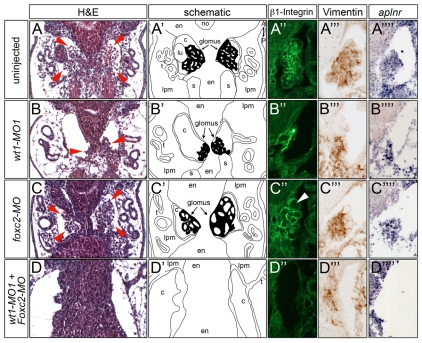
Next, we extended our analysis to the morphological differentiation of the glomus. The pronephric glomus is composed of three cell types: podocytes, endothelial and mesangial-like cells (Ellis and Youson, 1991; Kramer-Zucker et al., 2005). In Xenopus, the detailed organization of the glomus and the existence of mesangial-like cells have not been previously demonstrated. However, all three cell types could be identified in the Xenopus pronephric glomus using a combination of histology, immunohistochemistry and in situ hybridization on coronal sections at stage 40 (Fig. 6A-A′′′): podocytes were marked by β1-Integrin (Pozzi et al., 2008), the mesangial-like cells by the mesenchymal marker Vimentin (Dent et al., 1989; Gonlusen et al., 2001) and the endothelial cells by apelin receptor [aplnr (X-msr)] mRNA (Devic et al., 1996). In contrast to wt1-MO1+2-injected embryos, which do not show any glomerular structures (Fig. 2H-K), injection of wt1-MO1 resulted in a compacted glomus with no capillary loops and a few endothelial and mesangial-like cells present in its center (Fig. 6B-B′′′). foxc2 morphants displayed a slightly smaller glomus with enlarged capillary loops. Both endothelial and mesangial-like cells were grouped in the center of the glomus and did not extend towards its lateral edges (Fig. 6C-C′′′). wt1-MO1 and foxc2-MO co-injected embryos exhibited a more severe phenotype than the wt1-MO1 or foxc2-MO single knockdowns, characterized by the complete absence of the glomus (Fig. 6D-D′′′). Strong staining of aplnr and Vimentin at the midline of the embryos suggested that endothelial and mesangial-like cells were present, but could not assemble into a glomus structure in the absence of podocytes. Together, these experiments suggest that even though wt1 is the most potent podocyte transcription factor, it still cooperates with foxc2 in some aspects of glomus formation.
Fig. 6.
wt1 and foxc2 regulate glomulogenesis.
(A-D′′′) Uninjected control Xenopus embryos (A-A′′′) and embryos injected with wt1-MO1 (B-B′′′), foxc2-MO (C-C′′′) or with wt1-MO1 plus foxc2-MO (D-D′′′) at stage 40 were analyzed by histological staining with Hematoxylin and Eosin (H&E, A-D), immunohistochemistry for β1-Integrin (A′-D′) or Vimentin (A′′D′′), and in situ hybridization for aplnr (A′′′-D′′′). (A′-D′) Schematics of the histology, in which the glomus is highlighted in black. The arrowhead in C′ indicates enlarged capillary loops in foxc2 morphants. c, coelom; en, endoderm; lpm, lateral plate mesoderm; lu, lung bud; no, notochord; s, somites; t, tubule.
wt1, foxc2 and Notch signaling induce ectopic podocyte marker gene expression
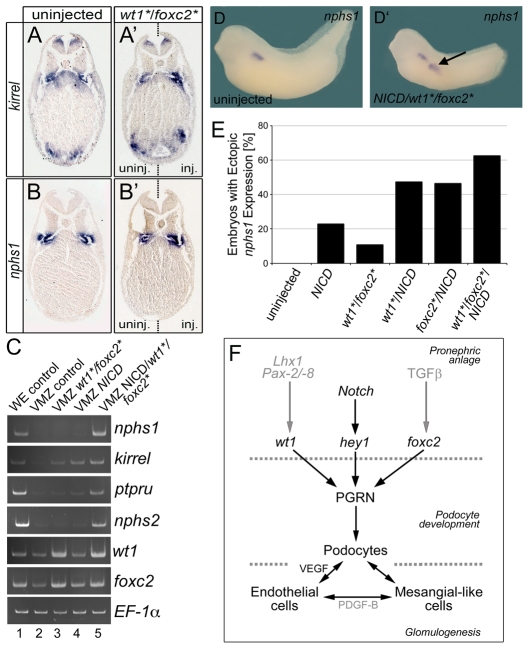
The experiments so far demonstrated that the combined knockdown of wt1 and foxc2 abolishes podocyte formation, but did not address whether the two transcription factors are also sufficient to do so. To address this point, three approaches were used. First, Xenopus embryos were microinjected marginally into a single blastomere at the 4-cell stage with a mixture of wt1* and foxc2* mRNA (1 ng each), as rescue constructs shown to be biologically active (Fig. 3K-L and see Fig. S5K-L in the supplementary material). In situ hybridization at stage 35 showed that the expression domains of the podocyte markers nphs1 and kirrel were increased in size and intensity within the glomus proper in 90% of the embryos (Fig. 7A-B′) (nphs1, n=12; kirrel, n=11). kirrel was detected in the ventral mesoderm in 63% of the embryos, but this domain was most likely an expansion of the heart-specific expression of kirrel and unrelated to its expression in the glomus. These experiments were extended by co-injecting lacZ mRNA with wt1* plus foxc2*. Although lacZ staining could be detected outside of the glomus, these cells did not co-express nphs1 or kirrel (data not shown). In a second approach analogous to experiments spearheaded by Asashima and colleagues (Asashima et al., 2009), ectodermal explants were treated with 10 ng/ml Activin A for 3 hours at stage 9.5. This exposure was sufficient to induce podocyte terminal differentiation genes as assessed by RT-PCR analysis and suggested that ectodermal explants can be transdifferentiated into podocytes (see Fig. S13A, lane 3, in the supplementary material). However, ectodermal explants from embryos injected with 2 ng wt1* and foxc2* mRNA did not show any podocyte gene expression (see Fig. S13A, lane 4, in the supplementary material). A similar result was obtained when ventral marginal zone explants were used; this tissue is already committed to mesoderm and should be more easily converted to podocytes. However, RT-PCR analysis of these explants at stage 35 did not detect significant expression of nphs1, kirrel, ptpru or nphs2 (Fig. 7C, compare lanes 2 and 3, and see Fig. S13B in the supplementary material). Based on these data, we concluded that wt1 and foxc2 are not sufficient to induce podocyte formation by themselves.
Fig. 7.
wt1, foxc2 and NICD are sufficient to activate podocyte gene expression. (A-B′) Transverse sections of whole-mount in situ hybridizations comparing kirrel (A,A′) and nphs1 (B,B′) expression in wt1* plus foxc2* mRNA-injected Xenopus embryos (A′,B′) and uninjected sibling controls (A,B) at stage 35. Injected and uninjected sides are indicated. (C) RT-PCR analysis comparing the expression of podocyte terminal differentiation genes nphs1, kirrel, ptpru and nphs2 and the transcription factors wt1 and foxc2 in uninjected whole embryos (WE control, lane 1), uninjected ventral marginal zone explants (VMZ control, lane 2) or ventral marginal zones injected with wt1* plus foxc2* mRNA (VMZ wt1*/foxc2*, lane 3), with NICD mRNA (VMZ NICD, lane 4), or co-injected with NICD, wt1* and foxc2* mRNA (VMZ NICD/wt1*/foxc2*, lane 5). (D,D′) Whole-mount in situ hybridization of control embryos (D) and embryos co-injected with NICD, wt1* and foxc2* mRNA (D′) for nphs1 expression at stage 35. Arrow indicates an ectopic patch of nphs1 expression. (E) Quantification of ectopic nphs1 patches in embryos injected with wt1*/foxc2*, NICD, wt1*/NICD, foxc2*/NICD and wt1*/foxc2*/NICD mRNA. (F) Schematic of Xenopus glomus development. The three transcription factors, wt1, foxc2 and hey1, activate the PGRN (podocyte gene regulatory network). Subsequently, differentiated podocytes secrete Vegf to recruit endothelial cells and mesangial cells to form the differentiated glomus (see Discussion for further details).
The time course of podocyte gene expression (Fig. 1K) demonstrated that hey1 is co-expressed with wt1 and foxc2 as early as stage 20. Moreover, hey1 is downstream of Notch signaling, a pathway known to be involved in podocyte development (McLaughlin et al., 2000; Cheng et al., 2003; Wang et al., 2003; Taelman et al., 2006; Cheng et al., 2007; Naylor and Jones, 2009). Thus, we next tested whether Notch signaling was the missing input to induce podocyte development outside of the glomus. Xenopus embryos were injected with either 4 ng of wt1* mRNA and 1 ng foxc2* mRNA, or 4 ng of NICD mRNA [a constitutively active Notch receptor (Coffman et al., 1990)], or a mixture of all three mRNAs at the 2- to 4-cell stage. Ventral marginal zone explants were excised at stage 10, cultured until sibling embryos reached stage 35 and processed for RT-PCR analysis. Whereas neither wt1* plus foxc2* nor NICD induced the podocyte terminal differentiation markers, the combination of wt1*, foxc2* and NICD strongly upregulated all four (Fig. 7C, compare lanes 2-4 with lane 5). This effect was specific to the presence of all three mRNAs because neither wt1* plus NICD mRNA nor foxc2* plus NICD mRNA induced nphs1 expression (see Fig. S13C in the supplementary material).
Interestingly, the same combination of mRNAs (1 ng wt1*, 200 pg foxc2*, 1 ng NICD) injected into a single vegetal blastomere at the 8-cell stage was sufficient to induce ectopic patches of nphs1 expression in 65% of the embryos (n=51) (Fig. 7D-E). These ectopic domains were mostly restricted to the posterior intermediate mesoderm, but isolated cases also showed expression in the head region (data not shown). In contrast to the ventral marginal zone explants (Fig. 7C), injection of NICD mRNA alone, wt1* plus NICD or foxc2* plus NICD also resulted in ectopic nphs1 expression, albeit at lower frequency than when all three mRNAs were co-injected (Fig. 7E). It is noteworthy that wt1 and foxc2 are expressed at lower levels throughout the intermediate mesoderm and only become restricted to the podocyte lineage later during development (Fig. 1B,C). Thus, for example, injected wt1 mRNA can already cooperate with endogenous foxc2, resulting in some ectopic patches of nphs1 expression, and co-injection with foxc2 mRNA only augments this.
In summary, these experiments extend reports by others (McLaughlin et al., 2000; Cheng et al., 2003; Wang et al., 2003; Taelman et al., 2006; Cheng et al., 2007; Naylor and Jones, 2009) that Notch signaling is a very important aspect of kidney development. The interplay between the two transcription factors wt1 and foxc2 and Notch signaling specifies the podocyte lineage and highlights the importance of combinatorial signaling.
DISCUSSION
The data presented here provide the first model of a gene regulatory network for cell fate decisions in the kidney. They show that the differentiation of a very specialized cell type, the podocyte, does not rely on a single master gene, but instead requires multiple inputs. These inputs ensure the correct spatiotemporal execution of the program so that podocytes are only found within the glomus and not in other parts of the kidney or elsewhere in the embryo.
The main conclusion of our study is that the two transcription factors wt1 and foxc2 and Notch signaling are not only very important for podocyte development, but also for the assembly of the entire glomus structure (Fig. 7F). Intense midline staining of aplnr and Vimentin in the wt1/foxc2 double morphants (Fig. 6D′,D′′) suggested that endothelial and mesangial-like cells were present, but lacked the interaction with podocytes required to form a glomus. In mouse, it has been shown that podocytes secrete Vegf to attract endothelial cells (Jokelainen, 1963; Eremina et al., 2007) and that endothelial cells secrete Pdgfβ to recruit mesangial cells (Bernstein et al., 1981; Lindahl et al., 1998). If Vegf is not present, neither endothelial nor mesangial cells can be recruited to the glomerulus. Similarly, Xenopus embryos express vegfa mRNA in the developing podocytes (Cleaver et al., 1997) and this expression is lost in embryos injected with wt1-MO (our unpublished data). Importantly, the transcriptional network presented here (Fig. 4A) only provides a snapshot and needs to be extended in the future. It will, for example, be worthwhile investigating how the upper-tier transcription factors are induced and what their function is in non-podocyte tissues.
Interestingly, foxc2, wt1 and Notch signaling seem to reflect three different aspects in determining future podocytes (Fig. 7F). foxc2 is broadly expressed in the mesoderm, is a direct transcriptional target of Activin/Tgfβ signaling (Koster et al., 2000) and probably determines the mesodermal precursor population of the future kidney. Indeed, studies in mouse suggest that Foxc2 and its close relative Foxc1 regulate the decision between paraxial and intermediate mesoderm cell fates (Wilm et al., 2004). Wt1, by contrast, is a more kidney-restricted transcription factor. In the Xenopus early nephric mesenchyme, wt1 is co-expressed with the three kidney-specific transcription factors lhx1 (lim1), pax2 and pax8 (Carroll et al., 1999) and its podocyte expression has been shown in several species to be regulated by cross-talk between Wt1 and Pax2 (Ryan et al., 1995; Dehbi et al., 1996; Majumdar et al., 2000). Finally, Notch signaling and its downstream effector hey1 provide the positional information for podocyte development (McLaughlin et al., 2000; Taelman et al., 2006; Naylor and Jones, 2009). As recently demonstrated by Naylor and Jones (Naylor and Jones, 2009), Notch signaling patterns the pronephros along its mediolateral axis. Notch signaling is required for the expression of wnt4, a secreted factor that has a central role in kidney development in Xenopus and mouse (Stark et al., 1994; Saulnier et al., 2002).
The Xenopus pronephros proved to be a very powerful animal model for our study. In mammals, the molecular analysis of podocyte maturation is complicated by the asynchronous development of podocytes. At any given time during kidney development, the metanephros contains mature as well as developing podocytes, making it difficult to follow individual cell fate decisions. In Xenopus, by contrast, the bilateral pronephros and its glomus develop synchronously and independently of the tubules and duct (Urban et al., 2006). In addition, loss-of-function studies with MOs circumvented the difficulty in obtaining compound mouse mutants of more than two genes at the same time. In our case, two MOs were sufficient, but other studies have successfully eliminated up to four genes at the same time (Khokha et al., 2005; Reversade and De Robertis, 2005; Eisen and Smith, 2008). Finally, insights into pronephric development are easily applicable to the metanephric kidney. The molecular regulation of nephron development in both kidney types follows an evolutionarily conserved path (Carroll et al., 1999; Zhou and Vize, 2004; Reggiani et al., 2007; Raciti et al., 2008). For example, some of the regulations reported in this study (e.g. the autoregulation of wt1) have also been observed in mouse (Rupprecht et al., 1994; Rohr et al., 2002; Wagner et al., 2004; Moriguchi et al., 2006; Takemoto et al., 2006).
Notably, some of our observations are not in complete agreement with all previously published reports. In contrast to Taelman et al. (Taelman et al., 2006), we still detected low-level expression of nphs1 upon injection of wt1-MO1+2, probably owing to longer staining of the whole-mount in situ hybridizations. Similarly, Haldin et al. (Haldin et al., 2008) showed a marked downregulation of wt1 and nphs1 in Xenopus lmx1b knockdowns, whereas we only observed small changes, even when using their published lmx1b MO (see Figs S8, S9 and Table S2 in the supplementary material). These rather mild phenotypes of lmx1b morphants are similar to those observed in mouse. Animal-wide, as well as podocyte-specific, knockouts in mouse did not show strong changes in the transcription of genes such as wt1 and nphs1, and induced rather mild changes in the foot processes of the podocytes (Miner et al., 2002; Rohr et al., 2002; Suleiman et al., 2007).
Our model of a podocyte gene regulatory network is based on six transcription factors and four terminal differentiation genes. This analysis obviously does not provide a global transcription profile of all genes expressed in the podocyte. The six transcription factors studied here (wt1, foxc2, hey1, tcf21, lmx1b and mafb) are the best-characterized regulators of podocyte specification (Rascle et al., 2007; Quaggin and Kreidberg, 2008). Although this is unlikely to be an exhaustive list, only a few other transcription factors, which were not tested here, have been implicated in this process. They include members of the ZHX (zinc-fingers and homeoboxes) family of proteins that regulate podocyte gene expression during disease formation, but have not yet been linked to podocyte development (Liu et al., 2006), and Ldb1 and E47 (Tcf3) heterodimerization partners of Lmx1b. Although E47 is not necessary for podocyte development, mice lacking Ldb1 in podocytes display a phenotype that is similar, but less severe, than that observed in Lmx1b mutants (Suleiman et al., 2007; Haldin et al., 2008).
For the terminal differentiation genes the scenario is even more complex. Hundreds of genes are involved in podocyte function (Takemoto et al., 2006). Of the four genes chosen for this study, the expression of three is localized to the slit diaphragm (nphs1, kirrel and nphs2) and one is expressed outside of this junctional complex (ptpru). Interestingly, all had inputs from at least three transcription factors, and no combination of inputs was identical. This suggests a high degree of redundancy in the regulation of terminal differentiation genes, which probably provides robustness to the podocyte program. However, the type of analysis performed here does not rule out the possibility that some of the observed connections are indirect. To completely understand all the nuances of this regulation, it will be necessary to address in vivo promoter occupancy using high-throughput sequencing of chromatin immunoprecipitation products (Wold and Myers, 2008).
In Xenopus, naïve ectoderm can be transdifferentiated into kidney tissue by the addition of Activin and retinoic acid (Moriya et al., 1993; Uochi and Asashima, 1996; Brennan et al., 1999; Osafune et al., 2002). These in vitro induced embryonic kidneys could even restore kidney function upon transplantation into nephrectomized Xenopus embryos (Chan et al., 1999). Thus, it will be very interesting to see whether this better-defined gene set (wt1, foxc2 and Notch signaling) will allow the in vitro generation of podocytes from pluripotent cells. Since podocyte malfunction is one of the main causes of kidney failure, the regeneration of podocytes might provide a novel therapeutic approach to restore podocyte and kidney function in humans.
Supplementary Material
Acknowledgments
We thank Drs J. Larraín, T. Obara, E. Pera, S. Piccolo and D. Romaker for critically reviewing the manuscript. The β1-Integrin and Vimentin antibodies were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa. Drs P. Krieg, N. Papalopulu and P. Vize and the NIBB/NIG/NBRP Xenopus laevis EST project generously provided plasmids. This work was supported by grants from NIH/NIDDK (#5F30DK082121-02 to J.T.W. and #5R21DK077763-03 to O.W.). Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.042887/-/DC1
References
- Agrawal R., Tran U., Wessely O. (2009). The miR-30 miRNA family regulates Xenopus pronephros development and targets the transcription factor Xlim1/Lhx1. Development 136, 3927-3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asashima M., Ito Y., Chan T., Michiue T., Nakanishi M., Suzuki K., Hitachi K., Okabayashi K., Kondow A., Ariizumi T. (2009). In vitro organogenesis from undifferentiated cells in Xenopus. Dev. Dyn. 238, 1309-1320 [DOI] [PubMed] [Google Scholar]
- Barletta G. M., Kovari I. A., Verma R. K., Kerjaschki D., Holzman L. B. (2003). Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J. Biol. Chem. 278, 19266-19271 [DOI] [PubMed] [Google Scholar]
- Bernstein J., Cheng F., Roszka J. (1981). Glomerular differentiation in metanephric culture. Lab. Invest. 45, 183-190 [PubMed] [Google Scholar]
- Bouchard M., Souabni A., Mandler M., Neubuser A., Busslinger M. (2002). Nephric lineage specification by Pax2 and Pax8. Genes Dev. 16, 2958-2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan H. C., Nijjar S., Jones E. A. (1999). The specification and growth factor inducibility of the pronephric glomus in Xenopus laevis. Development 126, 5847-5856 [DOI] [PubMed] [Google Scholar]
- Carroll T. J., Vize P. D. (1996). Wilms' tumor suppressor gene is involved in the development of disparate kidney forms: evidence from expression in the Xenopus pronephros. Dev. Dyn. 206, 131-138 [DOI] [PubMed] [Google Scholar]
- Carroll T. J., Wallingford J. B., Vize P. D. (1999). Dynamic patterns of gene expression in the developing pronephros of Xenopus laevis. Dev. Genet. 24, 199-207 [DOI] [PubMed] [Google Scholar]
- Chan T. C., Ariizumi T., Asashima M. (1999). A model system for organ engineering: transplantation of in vitro induced embryonic kidney. Naturwissenschaften 86, 224-227 [DOI] [PubMed] [Google Scholar]
- Cheng H. T., Miner J. H., Lin M., Tansey M. G., Roth K., Kopan R. (2003). Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development 130, 5031-5042 [DOI] [PubMed] [Google Scholar]
- Cheng H. T., Kim M., Valerius M. T., Surendran K., Schuster-Gossler K., Gossler A., McMahon A. P., Kopan R. (2007). Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 134, 801-811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O., Tonissen K. F., Saha M. S., Krieg P. A. (1997). Neovascularization of the Xenopus embryo. Dev. Dyn. 210, 66-77 [DOI] [PubMed] [Google Scholar]
- Coffman C., Harris W., Kintner C. (1990). Xotch, the Xenopus homolog of Drosophila Notch. Science 249, 1438-1441 [DOI] [PubMed] [Google Scholar]
- Coolen M., Sii-Felice K., Bronchain O., Mazabraud A., Bourrat F., Retaux S., Felder-Schmittbuhl M. P., Mazan S., Plouhinec J. L. (2005). Phylogenomic analysis and expression patterns of large Maf genes in Xenopus tropicalis provide new insights into the functional evolution of the gene family in osteichthyans. Dev. Genes Evol. 215, 327-339 [DOI] [PubMed] [Google Scholar]
- Cui S., Schwartz L., Quaggin S. E. (2003). Pod1 is required in stromal cells for glomerulogenesis. Dev. Dyn. 226, 512-522 [DOI] [PubMed] [Google Scholar]
- Davidson E. H., McClay D. R., Hood L. (2003). Regulatory gene networks and the properties of the developmental process. Proc. Natl. Acad. Sci. USA 100, 1475-1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehbi M., Ghahremani M., Lechner M., Dressler G., Pelletier J. (1996). The paired-box transcription factor, PAX2, positively modulates expression of the Wilms' tumor suppressor gene (WT1). Oncogene 13, 447-453 [PubMed] [Google Scholar]
- Dent J. A., Polson A. G., Klymkowsky M. W. (1989). A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein Vimentin in Xenopus. Development 105, 61-74 [DOI] [PubMed] [Google Scholar]
- Devic E., Paquereau L., Vernier P., Knibiehler B., Audigier Y. (1996). Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech. Dev. 59, 129-140 [DOI] [PubMed] [Google Scholar]
- Doherty J. R., Johnson Hamlet M. R., Kuliyev E., Mead P. E. (2007). A flk-1 promoter/enhancer reporter transgenic Xenopus laevis generated using the Sleeping Beauty transposon system: an in vivo model for vascular studies. Dev. Dyn. 236, 2808-2817 [DOI] [PubMed] [Google Scholar]
- Edwards A., Daniels B. S., Deen W. M. (1999). Ultrastructural model for size selectivity in glomerular filtration. Am. J. Physiol. 276, F892-F902 [DOI] [PubMed] [Google Scholar]
- Eisen J. S., Smith J. C. (2008). Controlling morpholino experiments: don't stop making antisense. Development 135, 1735-1743 [DOI] [PubMed] [Google Scholar]
- Ellis L. C., Youson J. H. (1991). Ultrastructure of the pronephric kidney of embryos and prolarvae of the sea lamprey, Petromyzon marinus. Tissue Cell 23, 393-410 [DOI] [PubMed] [Google Scholar]
- Eremina V., Baelde H. J., Quaggin S. E. (2007). Role of the VEGF-a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol. 106, 32-37 [DOI] [PubMed] [Google Scholar]
- Gawantka V., Ellinger-Ziegelbauer H., Hausen P. (1992). Beta1-Integrin is a maternal protein that is inserted into all newly formed plasma membranes during early Xenopus embryogenesis. Development 115, 595-605 [DOI] [PubMed] [Google Scholar]
- Gerth V. E., Zhou X., Vize P. D. (2005). Nephrin expression and three-dimensional morphogenesis of the Xenopus pronephric glomus. Dev. Dyn. 233, 1131-1139 [DOI] [PubMed] [Google Scholar]
- Gonlusen G., Ergin M., Paydas S., Tunali N. (2001). The expression of cytoskeletal proteins (alpha-SMA, Vimentin, Desmin) in kidney tissue: a comparison of fetal, normal kidneys, and glomerulonephritis. Int. Urol. Nephrol. 33, 299-305 [DOI] [PubMed] [Google Scholar]
- Haldin C. E., Nijjar S., Masse K., Barnett M. W., Jones E. A. (2003). Isolation and growth factor inducibility of the Xenopus laevis Lmx1b gene. Int. J. Dev. Biol. 47, 253-262 [PubMed] [Google Scholar]
- Haldin C. E., Masse K. L., Bhamra S., Simrick S., Kyuno J., Jones E. A. (2008). The Lmx1b gene is pivotal in glomus development in Xenopus laevis. Dev. Biol. 322, 74-85 [DOI] [PubMed] [Google Scholar]
- Hammes A., Guo J. K., Lutsch G., Leheste J. R., Landrock D., Ziegler U., Gubler M. C., Schedl A. (2001). Two splice variants of the Wilms' tumor 1 gene have distinct functions during sex determination and nephron formation. Cell 106, 319-329 [DOI] [PubMed] [Google Scholar]
- Howland R. B. (1916). On the effect of removal of the pronephros of the amphibian embryo. Proc. Natl. Acad. Sci. USA 2, 231-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S., Yasuda K. (2001). Distinct roles of Maf genes during Xenopus lens development. Mech. Dev. 101, 155-166 [DOI] [PubMed] [Google Scholar]
- Johnstone D. B., Holzman L. B. (2006). Clinical impact of research on the podocyte slit diaphragm. Nat. Clin. Pract. Nephrol. 2, 271-282 [DOI] [PubMed] [Google Scholar]
- Jokelainen P. (1963). An electron microscopic study of the early development of the rat metanephric nephron. Acta Anat. 47, 1-73 [Google Scholar]
- Khokha M. K., Yeh J., Grammer T. C., Harland R. M. (2005). Depletion of three BMP antagonists from Spemann's organizer leads to a catastrophic loss of dorsal structures. Dev. Cell 8, 401-411 [DOI] [PubMed] [Google Scholar]
- Koster M., Dillinger K., Knochel W. (2000). Activin A signaling directly activates Xenopus winged helix factors XFD-4/4', the orthologues to mammalian MFH-1. Dev. Genes Evol. 210, 320-324 [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker A. G., Wiessner S., Jensen A. M., Drummond I. A. (2005). Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev. Biol. 285, 316-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg J. A., Sariola H., Loring J. M., Maeda M., Pelletier J., Housman D., Jaenisch R. (1993). WT-1 is required for early kidney development. Cell 74, 679-691 [DOI] [PubMed] [Google Scholar]
- Lindahl P., Hellstrom M., Kalen M., Karlsson L., Pekny M., Pekna M., Soriano P., Betsholtz C. (1998). Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125, 3313-3322 [DOI] [PubMed] [Google Scholar]
- Liu G., Clement L. C., Kanwar Y. S., Avila-Casado C., Chugh S. S. (2006). ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J. Biol. Chem. 281, 39681-39692 [DOI] [PubMed] [Google Scholar]
- Majumdar A., Lun K., Brand M., Drummond I. A. (2000). Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development 127, 2089-2098 [DOI] [PubMed] [Google Scholar]
- McLaughlin K. A., Rones M. S., Mercola M. (2000). Notch regulates cell fate in the developing pronephros. Dev. Biol. 227, 567-580 [DOI] [PubMed] [Google Scholar]
- Miner J. H., Morello R., Andrews K. L., Li C., Antignac C., Shaw A. S., Lee B. (2002). Transcriptional induction of slit diaphragm genes by Lmx1b is required in podocyte differentiation. J. Clin. Invest. 109, 1065-1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Hamada M., Morito N., Terunuma T., Hasegawa K., Zhang C., Yokomizo T., Esaki R., Kuroda E., Yoh K., et al. (2006). MafB is essential for renal development and F4/80 expression in macrophages. Mol. Cell. Biol. 26, 5715-5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya N., Uchiyama H., Asashima M. (1993). Induction of pronephric tubules by activin and retinoic acid in presumptive ectoderm of Xenopus laevis. Dev. Growth Differ. 35, 123-128 [DOI] [PubMed] [Google Scholar]
- Naylor R. W., Jones E. A. (2009). Notch activates Wnt-4 signalling to control medio-lateral patterning of the pronephros. Development 136, 3585-3595 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Faber J. (1994). Normal Table of Xenopus laevis New York: Garland Publishing; [Google Scholar]
- Osafune K., Nishinakamura R., Komazaki S., Asashima M. (2002). In vitro induction of the pronephric duct in Xenopus explants. Dev. Growth Differ. 44, 161-167 [DOI] [PubMed] [Google Scholar]
- Pavenstadt H., Kriz W., Kretzler M. (2003). Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253-307 [DOI] [PubMed] [Google Scholar]
- Perner B., Englert C., Bollig F. (2007). The Wilms tumor genes Wt1a and Wt1b control different steps during formation of the zebrafish pronephros. Dev. Biol. 309, 87-96 [DOI] [PubMed] [Google Scholar]
- Pozzi A., Jarad G., Moeckel G. W., Coffa S., Zhang X., Gewin L., Eremina V., Hudson B. G., Borza D. B., Harris R. C., et al. (2008). Beta1-Integrin expression by podocytes is required to maintain glomerular structural integrity. Dev. Biol. 316, 288-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaggin S. E. (2002). Transcriptional regulation of podocyte specification and differentiation. Microsc. Res. Tech. 57, 208-211 [DOI] [PubMed] [Google Scholar]
- Quaggin S. E., Kreidberg J. A. (2008). Development of the renal glomerulus: good neighbors and good fences. Development 135, 609-620 [DOI] [PubMed] [Google Scholar]
- Quaggin S. E., Schwartz L., Cui S., Igarashi P., Deimling J., Post M., Rossant J. (1999). The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis. Development 126, 5771-5783 [DOI] [PubMed] [Google Scholar]
- Raciti D., Reggiani L., Geffers L., Jiang Q., Bacchion F., Subrizi A. E., Clements D., Tindal C., Davidson D. R., Kaissling B., et al. (2008). Organization of the pronephric kidney revealed by large-scale gene expression mapping. Genome Biol. 9, R84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascle A., Suleiman H., Neumann T., Witzgall R. (2007). Role of transcription factors in podocytes. Nephron Exp. Nephrol. 106, e60-e66 [DOI] [PubMed] [Google Scholar]
- Reggiani L., Raciti D., Airik R., Kispert A., Brandli A. W. (2007). The prepattern transcription factor Irx3 directs nephron segment identity. Genes Dev. 21, 2358-2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B., De Robertis E. M. (2005). Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell 123, 1147-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R., Karnovsky M. J. (1974). Porous substructure of the glomerular slit diaphragm in the rat and mouse. J. Cell Biol. 60, 423-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr C., Prestel J., Heidet L., Hosser H., Kriz W., Johnson R. L., Antignac C., Witzgall R. (2002). The LIM-homeodomain transcription factor Lmx1b plays a crucial role in podocytes. J. Clin. Invest. 109, 1073-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotsalainen V., Ljungberg P., Wartiovaara J., Lenkkeri U., Kestila M., Jalanko H., Holmberg C., Tryggvason K. (1999). Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl. Acad. Sci. USA 96, 7962-7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht H. D., Drummond I. A., Madden S. L., Rauscher F. J., Sukhatme V. P. (1994). The Wilms' tumor suppressor gene WT1 is negatively autoregulated. J. Biol. Chem. 269, 6198-6206 [PubMed] [Google Scholar]
- Ryan G., Steele-Perkins V., Morris J. F., Rauscher F. J., 3rd, Dressler G. R. (1995). Repression of Pax-2 by WT1 during normal kidney development. Development 121, 867-875 [DOI] [PubMed] [Google Scholar]
- Sadl V., Jin F., Yu J., Cui S., Holmyard D., Quaggin S., Barsh G., Cordes S. (2002). The mouse Kreisler (Krml1/MafB) segmentation gene is required for differentiation of glomerular visceral epithelial cells. Dev. Biol. 249, 16-29 [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., De Robertis E. M. (1995). Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376, 333-336 [DOI] [PubMed] [Google Scholar]
- Saulnier D. M., Ghanbari H., Brandli A. W. (2002). Essential function of Wnt-4 for tubulogenesis in the Xenopus pronephric kidney. Dev. Biol. 248, 13-28 [DOI] [PubMed] [Google Scholar]
- Saxén L. (1987). Organogenesis of the Kidney Cambridge, UK: Cambridge University Press; [Google Scholar]
- Schwarz K., Simons M., Reiser J., Saleem M. A., Faul C., Kriz W., Shaw A. S., Holzman L. B., Mundel P. (2001). Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and Nephrin. J. Clin. Invest. 108, 1621-1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simrick S., Masse K., Jones E. A. (2005). Developmental expression of Pod1 in Xenopus laevis. Int. J. Dev. Biol. 49, 59-63 [DOI] [PubMed] [Google Scholar]
- Sive H. L., Grainger R. M., Harland R. M. (2000). Early Development of Xenopus laevis: A Laboratory Manual Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Stark K., Vainio S., Vassileva G., McMahon A. P. (1994). Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679-683 [DOI] [PubMed] [Google Scholar]
- Suleiman H., Heudobler D., Raschta A. S., Zhao Y., Zhao Q., Hertting I., Vitzthum H., Moeller M. J., Holzman L. B., Rachel R., et al. (2007). The podocyte-specific inactivation of Lmx1b, Ldb1 and E2a yields new insight into a transcriptional network in podocytes. Dev. Biol. 304, 701-712 [DOI] [PubMed] [Google Scholar]
- Taelman V., Van Campenhout C., Solter M., Pieler T., Bellefroid E. J. (2006). The Notch-effector HRT1 gene plays a role in glomerular development and patterning of the Xenopus pronephros anlagen. Development 133, 2961-2971 [DOI] [PubMed] [Google Scholar]
- Takahashi-Iwanaga H. (2002). Comparative anatomy of the podocyte: A scanning electron microscopic study. Microsc. Res. Tech. 57, 196-202 [DOI] [PubMed] [Google Scholar]
- Takemoto M., He L., Norlin J., Patrakka J., Xiao Z., Petrova T., Bondjers C., Asp J., Wallgard E., Sun Y., et al. (2006). Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J. 25, 1160-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. E., Wharram B. L., Goyal M., Wiggins J. E., Holzman L. B., Wiggins R. C. (1994). GLEPP1, a renal glomerular epithelial cell (podocyte) membrane protein-tyrosine phosphatase. Identification, molecular cloning, and characterization in rabbit. J. Biol. Chem. 269, 19953-19962 [PubMed] [Google Scholar]
- Torrey T. W. (1965). Morphogenesis of the vertebrate kidney. Organogenesis (ed. R. L. DeHaan and H. Ursprung), pp.559-579 New York: Rinehart and Winston; [Google Scholar]
- Tran U., Pickney L. M., Ozpolat B. D., Wessely O. (2007). Xenopus Bicaudal-C is required for the differentiation of the amphibian pronephros. Dev. Biol. 307, 152-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uochi T., Asashima M. (1996). Sequential gene expression during pronephric tubule formation in vitro in Xenopus ectoderm. Dev. Growth Differ. 38, 625-634 [DOI] [PubMed] [Google Scholar]
- Urban A. E., Zhou X., Ungos J. M., Raible D. W., Altmann C. R., Vize P. D. (2006). FGF is essential for both condensation and mesenchymal-epithelial transition stages of pronephric kidney tubule development. Dev. Biol. 297, 103-117 [DOI] [PubMed] [Google Scholar]
- Vize P., Woolf A., Bard J. (2003). The Kidney: From Normal Development to Congenital Diseases Amsterdam: Academic Press; [Google Scholar]
- Wagner N., Wagner K. D., Xing Y., Scholz H., Schedl A. (2004). The major podocyte protein Nephrin is transcriptionally activated by the Wilms' tumor suppressor WT1. J. Am. Soc. Nephrol. 15, 3044-3051 [DOI] [PubMed] [Google Scholar]
- Wallingford J. B., Carroll T. J., Vize P. D. (1998). Precocious expression of the Wilms' tumor gene xWT1 inhibits embryonic kidney development in Xenopus laevis. Dev. Biol. 202, 103-112 [DOI] [PubMed] [Google Scholar]
- Wang P., Pereira F. A., Beasley D., Zheng H. (2003). Presenilins are required for the formation of comma- and S-shaped bodies during nephrogenesis. Development 130, 5019-5029 [DOI] [PubMed] [Google Scholar]
- Wilm B., James R. G., Schultheiss T. M., Hogan B. L. (2004). The forkhead genes, Foxc1 and Foxc2, regulate paraxial versus intermediate mesoderm cell fate. Dev. Biol. 271, 176-189 [DOI] [PubMed] [Google Scholar]
- Wingert R. A., Selleck R., Yu J., Song H. D., Chen Z., Song A., Zhou Y., Thisse B., Thisse C., McMahon A. P., et al. (2007). The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 3, 1922-1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold B., Myers R. M. (2008). Sequence census methods for functional genomics. Nat. Methods 5, 19-21 [DOI] [PubMed] [Google Scholar]
- Zhou X., Vize P. D. (2004). Proximo-distal specialization of epithelial transport processes within the Xenopus pronephric kidney tubules. Dev. Biol. 271, 322-338 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.