Abstract
Toll-like receptor 4 (TLR4), a protein integral to innate immunity, is elevated in skeletal muscle of obese and type 2 diabetic humans and has been implicated in the development of lipid-induced insulin resistance. The purpose of this study was to examine the role of TLR4 as a modulator of basal (non-insulin-stimulated) substrate metabolism in skeletal muscle with the hypothesis that its activation would result in reduced fatty acid oxidation and increased partitioning of fatty acids toward neutral lipid storage. Human skeletal muscle, rodent skeletal muscle, and skeletal muscle cell cultures were employed to study the functional consequences of TLR4 activation on glucose and fatty acid metabolism. Herein, we demonstrate that activation of TLR4 with low (metabolic endotoxemia) and high (septic conditions) doses of LPS results in increased glucose utilization and reduced fatty acid oxidation in skeletal muscle and that these changes in metabolism in vivo occur in concert with increased circulating triglycerides. Moreover, animals with a loss of TLR4 function possess increased oxidative capacity in skeletal muscle and present with lower fasting levels of triglycerides and nonesterified free fatty acids. Evidence is also presented to suggest that these changes in substrate metabolism under metabolic endotoxemic conditions are independent of skeletal muscle-derived proinflammatory cytokine production. This report illustrates that skeletal muscle is a target for circulating endotoxin and may provide critical insight into the link between a proinflammatory state and dysregulated metabolism as observed with obesity, type 2 diabetes, and metabolic syndrome.
Keywords: toll-like receptor 4, skeletal muscle, obesity, glucose metabolism, fatty acid metabolism, human, mouse
skeletal muscle, by virtue of its contribution to total body mass, is a predominant site of substrate disposal. As such, dysregulated metabolism in this tissue can have profound deleterious effects on whole body energy homeostasis and is associated with metabolic disorders such as obesity, insulin resistance, and type 2 diabetes. Skeletal muscle from obese humans possesses an abnormal metabolic phenotype in that, despite chronic exposure to high lipid levels in a fasted state, glucose tends to be the preferred substrate for ATP production (32). This results in increased intramyocellular lipid accumulation (22, 29, 43), which contributes to defective insulin signaling (11, 12, 14, 22, 43). As such, a better understanding of the mechanism(s) behind the inability of skeletal muscle to adapt to lipotoxic conditions by increasing fatty acid oxidation (FAO), in pathological states, is warranted and has yet to be discerned.
Pathological states such as obesity, insulin resistance, and type 2 diabetes are associated with chronic activation of proinflammatory pathways (13, 36, 40, 50). Toll-like receptors are transmembrane receptors that play an important role in innate immunity and the induction of proinflammatory responses (64). Toll-like receptor 4 (TLR4) was identified as the first human homolog of the Drosophila Toll gene (46) and is well known as the receptor for lipopolysaccharide (LPS) (51). In addition to its location on immune cells, TLR4 is also abundant in adipose tissue, liver, and skeletal muscle (17, 24, 63). Expression in these tissues suggests that TLR4 is not only a common component of innate immunity but also perhaps a modulator in metabolic systems. Frost and colleagues (19, 39) were among the first to show that TLR4 is present in skeletal muscle and when activated induces a local inflammatory response. More recently, Reyna et al. (57) reported increased expression and protein content of TLR4 in skeletal muscle of obese and type 2 diabetic humans, which was associated with insulin resistance. Radin et al. (52) and Shi et al. (60) have shown that TLR4 is important to the development of fatty acid (FA)-induced insulin resistance in skeletal muscle. In light of the recently proposed phenomena termed “metabolic endotoxemia” (7), the importance of these findings may now be more pertinent. Metabolic endotoxemia refers to a condition of increased circulating endotoxin levels in the blood of humans and rodents in states of obesity, type 2 diabetes, or in response to high fat feeding (8). What sets metabolic endotoxemia aside from other situations of endotoxemia, such as sepsis, are that endotoxin levels are only modestly increased (0–200 pg/ml) (7). The observations that TLR4 is a necessary component in lipid-induced insulin resistance along with the idea that obesity is associated with a state of metabolic endotoxemia are the foundation of our hypothesis that endotoxin-mediated activation of TLR4 results in a shift in skeletal muscle substrate handling that favors glucose as an energy source over that of FAs.
Herein, we demonstrate that activation of TLR4 with low (metabolic endotoxemia) and high (septic conditions) doses of LPS results in increased glucose utilization and reduced FAO in skeletal muscle, and that these changes in metabolism in vivo occur in concert with increased circulating triglycerides. Moreover, animals with a loss of TLR4 function possess increased oxidative capacity in skeletal muscle and present with lower fasting levels of triglycerides and nonesterified free fatty acids (NEFAs). Evidence is also presented to suggest that these changes in substrate metabolism under metabolic endotoxemic conditions are independent of skeletal muscle-derived proinflammatory cytokine production. This report illustrates that skeletal muscle is a target for circulating endotoxin and may provide critical insight into the link between a proinflammatory state and dysregulated metabolism as observed with obesity, type 2 diabetes, and metabolic syndrome.
METHODS
Human subjects.
An Affymetrix Micro-Array (Human Genome U133A and U133B GeneChips; Affymetrix, Santa Clara, CA) that has been previously described (28) was revisited for this study. Subjects included in this analysis provided written informed consent under an approved protocol by East Carolina University Institutional Review Board and were described previously (28, 29).
Animals.
Animal studies were performed under an approved protocol by the Institutional Animal Care and Use Committee at Virginia Polytechnic Institute and State University. Multiple studies were conducted using 8-wk-old male C3H/HeJ (TLR4-mutant) and C3HeB/FeJ (control) mice that were purchased from the Jackson Laboratory (Bar Harbor, ME). The TLR-mutant mice possess a point mutation in the TLR4 receptor and do not have functional TLR4 signaling. The control mice possess functional TLR4 signaling and served as genetic background controls. The first experiments assessed basal FAO, citrate synthase (CS) activity, and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity in gastrocnemius whole muscle homogenates from overnight-fasted, control (n = 5), and TLR4-mutant (n = 5) mice. The second experiment assessed FAO, glucose oxidation, and enzyme activities of CS, β-HAD, and phosphofructokinase (PFK) in whole muscle homogenates prepared from red and white portions of gastrocnemius muscle harvested from control and TLR4-mutant mice 4 h following a single intraperitoneal injection of either saline (control, n = 6; TLR4-mutant), low-dose LPS (control, n = 4; TLR4 mutant, n = 4; 0.025 μg/mouse), or high-does LPS (control, n = 6; TLR4-mutant, n = 6; 25 μg/mouse). LPS from Escherichia coli 0111:B4 was used for all studies (catalog no. L2630; Sigma-Aldrich, St. Louis, MO). Mice for all studies were maintained on a normal chow diet and a 12:12-h light-dark cycle.
Cell culture.
Cell culture studies were conduced using C2C12 mouse and human primary myotubes. C2C12 were purchased from the American Type Culture Collection (Manassas, VA) and grown to ∼80% confluence in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Cells were grown to confluence and then differentiated into myotubes in DMEM containing 2% horse serum, 50 U/ml penicillin, and 50 μg/ml streptomycin (Invitrogen). All experiments were performed between days 4 and 6 of differentiation.
Cultures of primary human muscle cells were performed as previously described (28) and were obtained from subjects who provided written informed consent under approved protocols by the Pennington Biomedical Research Center and Virginia Polytechnic Institute and State University Institutional Review Boards. All experiments were performed on day 7 of differentiation.
A C2C12 cell line with stable overexpression of TLR4/MD2 was generated by transient transfection at ∼50% confluence with LyoVec transfection media (Invivogen, San Diego, CA) and pDUO plasmid, which coexpresses mouse TLR4/MD2 (Invivogen). Positively transfected cells were selected and maintained using blasticidin (Invivogen) at dosages of 30 and 10 μg/ml. TLR4 overexpression was verified by quantitative RT-PCR (qRT-PCR) and Western blotting as described under RNA extraction and qRT-PCR and Western blotting.
LPS treatment in cell culture experiments.
Cell culture experiments were performed with 50 pg/ml and 500 ng/ml concentrations of LPS (E. coli 0111:B4, catalog no. L2630; Sigma-Aldrich) unless otherwise stated. All LPS preparations were made using endotoxin-free water. In a majority of the studies, treatment time was 2 h, and all cell culture plates were washed three times with PBS before initiating assessment of glucose and FA metabolism or collection for any other assays described below. A time course study using low (50 pg/ml) and high (500 ng/ml) concentrations of LPS was performed in human primary muscle cells in which glucose and FA metabolism were measured following 2, 6, 12, and 24 h of LPS treatment. To ensure there were no confounding effects of impure LPS, a series of experiments were performed with ultra pure LPS (500 ng/ml, E. coli 0111:B4; Invivogen) and lipid A (1 μg/ml, synthetic monophosphoryl lipid A from E. coli; Invivogen). Studies were also conducted with 500 ng/ml of LPS in C2C12 cells in the presence and absence of 20 μM of IκB kinase (IKK) inhibitor, parthenolide (Sigma Aldrich), to determine whether changes in substrate metabolism were dependent on the activation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB).
FA metabolism.
FAO was assessed in cell culture (C2C12 and human primary myotubes) and whole muscle homogenates by measuring and summing 14CO2 production and 14C-labeled acid-soluble metabolites from the oxidation of [1-14C]palmitic acid as previously described (12, 28). Neutral lipids were extracted as previously described (28, 29), and incorporation of [1-14C]palmitic acid was measured using an AR 2000 TLC plate scanner (Bioscan, Washington, DC).
Glucose oxidation.
Glucose oxidation was assessed in cell culture (C2C12 and human primary myotubes) and whole muscle homogenates by measuring 14CO2 production from the oxidation of [U-14C]glucose (Perkin-Elmer, Waltham, MA) as previously described (12, 28) with the exception that glucose was substituted for BSA-bound palmitic acid.
Glucose uptake.
Basal (non-insulin-stimulated) glucose uptake was assessed in C2C12 cells in Krebs-Ringer HEPES buffer (in mM: 136 NaCl, 4.7 KCl, 1.25 MgSO4, 1.2 CaCl, and 20 HEPES, pH 7.4) with the addition of 10 μM 2-deoxyglucose (1 μCi/ml 2-deoxy-[3H]glucose) and 10 μM cytochalasin B (control for extracellular binding). After 10 min of incubation, plates were placed on ice, washed three times with ice-cold PBS, and harvested in 400 μl of 0.2 M NaOH for cell lysis. Glucose uptake was calculated based on specific activity and expressed relative to protein content.
Cytokine release.
Cytokine release from human myotubes was measured in response to 2 h treatment with 50 pg/ml and 500 ng/ml of LPS. After 2 h, the LPS media was removed, a sample was retained for cytokine measures, cells were washed three times with PBS, and fresh DMEM was added. Media was then sampled at 3, 6, 12, and 24 h after the addition of fresh media, and concentrations of interleukin 6 (IL-6), monocyte chemoattractant protein 1 (MCP1), and tumor necrosis factor-α (TNF-α) were measured using the Bioplex suspension array system (Bio-Rad).
Serum glucose, free fatty acid, and triacylglycerol analyses.
Serum was separated from blood that was collected, via postmortem cardiac puncture, from control and TLR4-mutant mice 4 h after intraperitoneal injections of either saline (control, n = 4; TLR4-mutant, n = 4) or a 25 μg/mouse dose of LPS (control, n = 4; TLR4-mutant, n = 4). Serum NEFA concentrations were determined by using the Free Fatty Acids, Half-micro test kit (Roche) per instructions provided by the manufacturer. Serum glucose concentrations were quantified by using the YSI 2300 STAT Plus Glucose and Lactate analyzer (YSI Life Sciences, Yellow Springs, OH). Serum triacylglycerides (TAGs) were measured using the Triacylglycerol-GPO Reagent Kit for TECO Diagnostics (TECO Diagnostics, Anaheim, CA).
RNA extraction and qRT-PCR.
RNA was extracted using an RNeasy Mini Kit (Qiagen) and DNase I treatment (Qiagen, Valencia, CA), according to the manufacturer's instructions. qRT-PCR was performed using an ABI PRISM 7900 Sequence Detection System instrument and TaqMan Universal PCR Master Mix used according to the manufacturer's specifications (Applied Biosystems, Foster City, CA). Target gene expression in rodent skeletal muscle and cell culture was normalized to β-actin RNA levels. Target gene expression in human skeletal muscle primary cell culture was normalized to cyclophilin B RNA levels. Primers and 5# FAM-labeled TaqMan probes were purchased as prevalidated assays (ABI). Relative quantification of target genes was calculated using the 2−ΔCT method. Derivation of the 2−ΔCT equation has been described in Applied Biosystems User Bulletin no. 2 (P/N 4303859).
Western blotting.
Western analysis was performed using cell lysates harvested in Mammalian Cell Lysis Buffer (Sigma Aldrich). Proteins (30 μg) were separated using a 10% Criterion-Tris·HCl gel (Bio-Rad, Hercules, CA) and subsequently transferred to a polyvinylidene difluoride membrane (Bio-Rad). Blots were probed with primary antibodies against β-actin (1:1,000; Cell Signaling, Danvers, MA), peroxisome proliferator-activated receptor (PPAR)α (1:1,000; Abcam, Cambridge, MA) and PPARδ (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), liver X receptor (LXR) α and LXRβ (both 5 μg/μl; Novus Biologicals, Littleton, CO), CCAAT-enhancer-binding protein (C/EBP) β and C/EBPδ (both 1:1,000; Cell Signaling), and TLR4 (1:1,000; Cell Signaling) followed by anti-rabbit, mouse, or goat secondary antibodies (1:10,000; Jackson ImmunoResearch Laboratories, West Grove, PA). Proteins were visualized using Super-Signal Chemiluminescent Substrate (Pierce, Rockville, IL) and a ChemiDoc XRS Imaging System (Bio-Rad).
Enzyme activity.
Enzyme activities were assessed in cell lysates (5-fold dilution) and muscle homogenates (20-fold dilution). Sample buffer consisted of 0.1 mol/l KH2PO4/Na2PHO4 and 2 mmol/l EDTA, pH 7.2. PFK, CS, and β-HAD activities were determined spectrophotometrically as previously described (25).
Statistics.
Results were analyzed with two-tailed Student's t-tests or two-way ANOVA with Tukey's post hoc tests (multiple comparisons). Results were expressed as means ± SE. The level of significance was set at P < 0.05.
RESULTS
Activation of TLR4 in skeletal muscle has functional consequences on glucose and FA metabolism.
Data from a previously described (28) Affymetrix data set from analysis of rectus abdominus muscle from nonobese and obese humans demonstrate that TLR4 mRNA levels were significantly higher in skeletal muscle from obese individuals relative to nonobese controls (P < 0.05; Supplemental Fig. 1A). These data support the recent report from Reyna et al. (47), but it is important to note that there was a lack of sample to confirm expression levels by quantitative real-time PCR. As such, the results are presented in Supplemental Fig. 1 (Supplemental data for this article may be found in the American Journal of Physiology: Endocrinology and Metabolism wesbsite.). Affymetrix expression levels of TLR4 were significantly associated with body mass index (Supplemental Fig. 1B; r = 0.58, P = 0.004), partitioning of FA toward neutral lipid synthesis (Supplemental Fig. 1C; r = 0.42, P = 0.04) and FAO (Supplemental Fig. 1D; r = −0.46, P = 0.03).
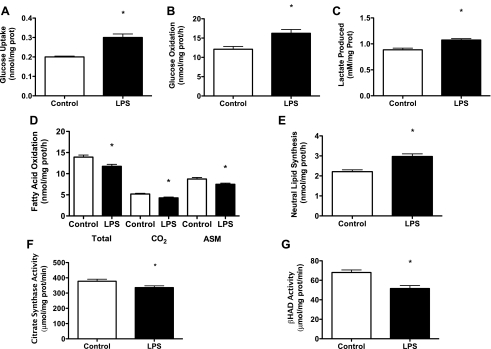
These data from humans prompted experiments to assess the functional significance of TLR4 activation using skeletal muscle cull cultures by measuring substrate (glucose and FA) metabolism and enzyme function in response to LPS. Experiments were first carried out in C2C12 cells with high doses of LPS (500 ng/ml) for 2 h. These studies showed that 2 h of LPS treatment caused increases in glucose uptake and oxidation by 33 and 25% (both P < 0.05; Fig. 1, A and B), respectively, which was accompanied by significant increases in lactate concentrations in the incubation media (P < 0.05; Fig. 1C). FAO was reduced by 20% (P < 0.05; Fig. 1D), and partitioning of FA toward neutral lipid synthesis was increased by 26% (P < 0.05; Fig. 1E). Decreases in FAO were accompanied by reductions in CS (P < 0.05; Fig. 1F) and β-HAD (P < 0.05; Fig. 1G) enzyme activities.
Fig. 1.
Activation of toll-like receptor 4 (TLR4) in C2C12 skeletal muscle cells modulates glucose and fatty acid (FA) metabolism. Radiolabeled substrates (glucose and palmitate) were used to measure glucose and FA metabolism in C2C12 cells following 2 h of lipopolysaccharide (LPS) treatment (500 ng/ml). In response to LPS, there was an increase in glucose uptake (A), glucose oxidation (B), and lactate presence (C) in the media. LPS treatment also resulted in a decrease in total FA oxidation {complete (CO2) and incomplete [acid soluble metabolites (ASMs)]} (D), increased FA partitioning to neutral lipid depots (E), and reductions in citrate synthase (CS; F) and β-hydroxyacyl-CoA dehydrogenase (β-HAD; G) activities. Data are presented as means ± SE. *P < 0.05, control vs. LPS.
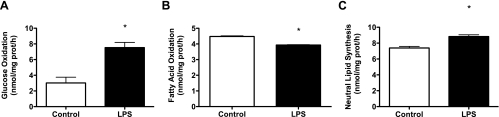
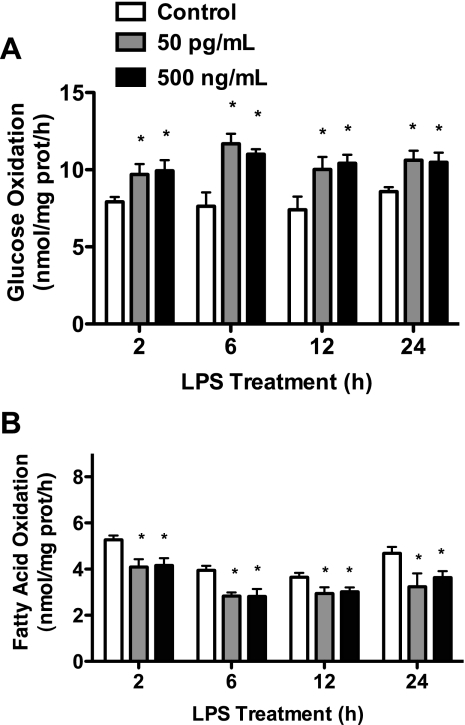
To ensure that TLR4-mediated changes in substrate metabolism were not isolated to C2C12 muscle cells and relevant to human skeletal muscle, experiments were repeated using human primary skeletal muscle cells with the same LPS concentration (500 ng/ml). Figure 2, A–C, demonstrates the identical LPS-mediated shift in substrate metabolism in human primary cells. We next performed experiments to assess the time course (2–24 h) of LPS-mediated metabolic effects and whether LPS doses comparable to metabolic endotoxemia (50 pg/ml) would elicit the same changes as observed with 500 ng/ml. These studies were conducted in human primary myotubes with 2-, 6-, 12-, and 24-h treatments with 50 pg/ml and 500 ng/ml of LPS followed by measures of glucose and FAO. These studies revealed that 50 pg/ml of LPS elicited the identical effects as 500 ng/ml of LPS with no evidence of dose or time effects (Fig. 3, A and B).
Fig. 2.
Activation of TLR4 in human primary skeletal muscle cells modulates glucose and FA metabolism. Radiolabeled substrates (glucose and palmitate) were used to measure glucose and FA metabolism in human primary skeletal muscle cells following 2 h of LPS treatment (500 ng/ml). In response to LPS, there was an increase in glucose oxidation (A), a decrease in FA oxidation (B), and an increase partitioning of FAs toward neutral lipid depots (C). Data are presented as means ± SE. *P < 0.05, control vs. LPS.
Fig. 3.
LPS dose and time course experiments. Radiolabeled substrates (glucose and palmitate) were used to measure glucose and FA metabolism in C2C12 cells treated with either 50 or 500 ng/ml of LPS for 2, 6, 12, and 24 h. In response to either dose of LPS, glucose oxidation was increased (A) and FA oxidation was reduced (B) with no dose effects. Data are presented as means ± SE. *P < 0.05, control vs. LPS.
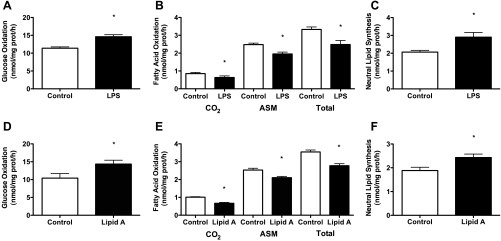
To assess the possibility of confounding effects of impure LPS, a series of experiments were conducted in C2C12 cells using ultrapure LPS (500 ng/ml; Invivogen) and lipid A (1 μg/ml; Invivogen). Lipid A is the active constituent of LPS that is responsible for binding and activating TLR4 (53). Treatments with ultrapure LPS, relative to control, significantly increased glucose oxidation (+22–27%) and partitioning of FA to neutral lipid pools (+19–27%) and decreased FAO (−32–56%) (Fig. 4, A–C). Treatments with lipid A, relative to control, also significantly increased glucose oxidation (+27–33%) and partitioning of FA to neutral lipid pools (+21–25%), and decreased FAO (−25–35%) (Fig. 4, D–F).
Fig. 4.
Activation of TLR4 via ultra pure LPS and lipid A also modulates glucose and FA metabolism. C2C12 cells were treated with ultra pure LPS (500 ng/ml) or lipid A (1 μg/ml), the active constituent of LPS, for 2 h. Both ultra pure LPS and lipid A resulted in an increase in glucose oxidation (A and D), a decrease in FA oxidation (B and E), and increased FA partitioning to neutral lipid depots (C and F). Data are presented as means ± SE. *P < 0.05, control vs. LPS.
Gain or loss of TLR4 function exacerbates or abolishes, respectively, the LPS-induced shift in skeletal muscle substrate metabolism in vitro.
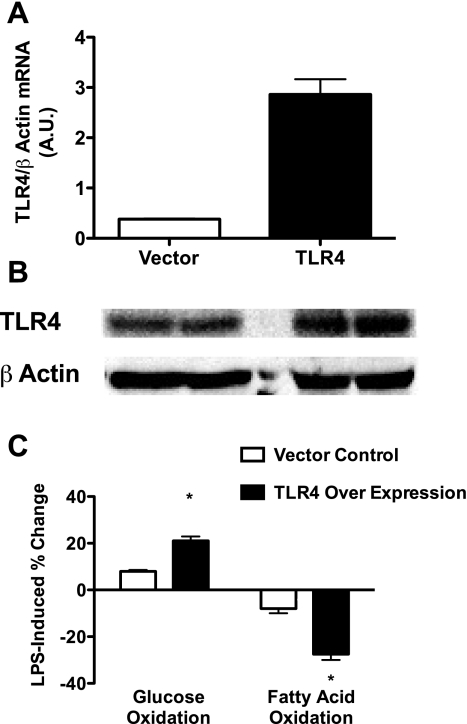
To determine if the LPS-induced shift in substrate metabolism was heightened with increased protein content of TLR4, a C2C12 muscle cell line with a stable overexpression of TLR4/MD2 (C2C12-TLR4/MD2) was generated. The C2C12-TLR4/MD2 cells exhibited a 7.5- and 2-fold increase in TLR4 mRNA (Fig. 5A) and protein (Fig. 5B) levels, respectively, relative to empty vector controls. The increases and decreases in glucose and FAO in response to LPS were significantly more robust in the C2C12-TLR4/MD2 cells relative to empty vector controls (Fig. 5C).
Fig. 5.
Overexpression of TLR4 in C2C12 skeletal muscle cells results in an enhanced LPS-mediated shift in substrate metabolism. C2C12 cells were generated with a stable overexpression of the TLR4/MD2 and treated (500 ng/ml) with LPS for 2 h, and oxidation of [U-14C]glucose and [1-14C]palmitic acid was assessed. Relative to empty vector controls, the stable cell line possessed higher TLR4 mRNA (A) and protein (B) levels, and the LPS-induced percent changes in glucose and FA metabolism were more robust (C). Data are expressed as means ± SE. *P < 0.05, control vs. LPS.
LPS-induced shift in skeletal muscle substrate metabolism is partially because of activation of NF-κB.
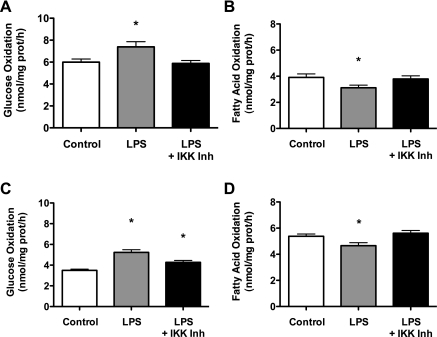
To determine whether the effects of LPS on metabolism were dependent on the activation of NF-κB, cells were treated with 50 pg/ml and 500 ng/ml of LPS for 2 h in the presence and absence of 20 μM of the IKK inhibitor parthenolide. IKK phosphorylates IκBα, the regulatory subunit of NF-κB, and is therefore required for nuclear translocation of NF-κB. The presence of parthenolide either partially or completely attenuated the effects of both the 50 pg/ml (Fig. 6, A and B) and 500 ng/ml (Fig. 6, C and D) LPS treatments on glucose oxidation and FAO. The data suggest that the metabolic perturbations induced by TLR4 activation in skeletal muscle are due in part to NF-κβ activation.
Fig. 6.
The effect of TLR4 activation on substrate metabolism is partially due to activation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB). C2C12 cells were treated with either 50 pg/ml (A and B) or 500 ng/ml (C and D) of LPS for 2 h in the presence and absence 20 μM of the IκB kinase (IKK) inhibitor parthenolide. The presence of parthenolide either partially or completely blocked the effects of LPS on glucose oxidation and FA oxidation. Data are expressed as means ± SE. *P < 0.05, control vs. LPS.
High dose of LPS, but not low dose, results in increased expression of proinflammatory cytokines in human primary myotubes.
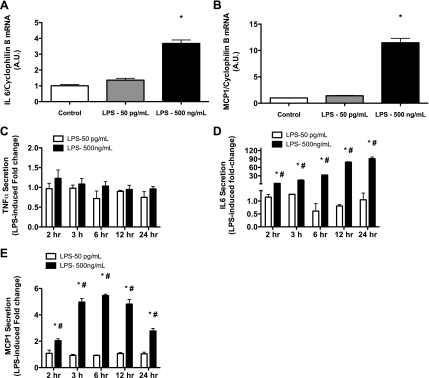
Human primary myotubes were treated with 50 pg/ml and 500 ng/ml of LPS for 2 h and then harvested for gene expression analysis of TNF-α, IL-6, and MCP1. No changes in TNF-α mRNA levels were detected with either the high or low doses of LPS (data not shown). mRNA levels of IL-6 and MCP1 were not affected by 50 pg/ml of LPS but were increased ∼4- and ∼10-fold, respectively, in response to 500 ng/ml of LPS (Fig. 7, A and B). Human primary myotubes were then treated with 50 pg/ml and 500 ng/ml of LPS for 2 h, and, at the 2-h time point, media was collected to measure TNF-α, IL-6, and MCP1 concentrations. Cells were washed three times with PBS, fresh media was added, and media was then sampled at 3, 6, 12, and 24 h after the initial treatment with LPS. We did not observe any changes in cytokine secretion at any time point in response to 50 pg/ml of LPS (Fig. 7, C–E). No changes in TNF-α secretion were observed at any time point following 500 ng/ml of LPS treatment (Fig. 7C). IL-6 and MCP1 secretion were both significantly increased in a time-dependent manner in response to 500 ng/ml of LPS. Relative to untreated cells, media concentrations of IL-6 were increased 3-, 15-, 33-, 79-, and 91-fold at 2, 3, 6, 12, and 24 h post-LPS treatment, respectively (Fig. 7D). MCP1 concentrations in the media were increased by 2-, 5-, 5.5-, 5-, and 3-fold at the same time points, respectively (Fig. 7E).
Fig. 7.
High dose of LPS, but not low dose, results in increased expression of proinflammatory cytokines in human primary myotubes. Human primary myotubes were treated with either 50 pg/ml or 500 ng/ml of LPS for 2 h and then harvested for gene expression analysis of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP1). mRNA levels of IL-6 and MCP1 were increased ∼4- and ∼5-fold, respectively, in response to 500 ng/ml of LPS (A and B); however, TNF-α mRNA remained unchanged. There was no effect 50 pg/ml on IL-6, MCP1, or TNF-α mRNA. Human primary myotubes were then treated with either 50 pg/ml or 500 ng/ml of LPS for 2 h. At the 2-h time point, medium was collected to measure TNF-α, IL-6, and MCP1 concentrations. Cells were washed, and medium was then sampled at 3, 6, 12, and 24 h after the initial treatment with LPS. There was no effect of either low- or high-dose LPS on TNF-α concentration at any time point following treatment (C). Media concentrations of IL-6 (D) and MCP1 (E) were significantly increased at all time points following the high-dose LPS treatment. There was no effect on IL-6 or MCP1 concentrations at any time point following low-dose LPS treatment. Data are expressed as fold change relative to control treatments ± SE. P < 0.05, control vs. LPS (*) and low- vs. high-dose LPS (#).
TLR4 activation does not alter protein content of metabolic transcription factors.
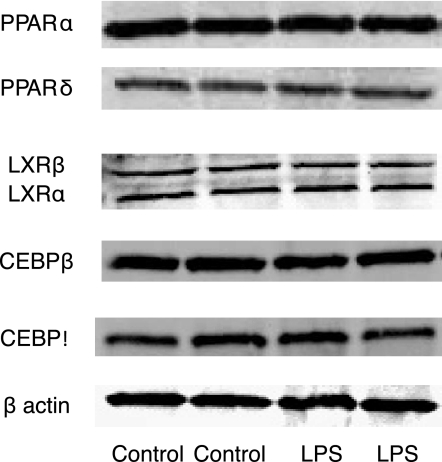
Previous work has demonstrated that LPS modulates lipid metabolism in cardiac myocytes (16), which was accompanied by decreases in protein content of PPARα and PPARδ, upstream transcription factors of FA metabolism transcripts. In contrast, we observed LPS-induced changes in FA metabolism in skeletal muscle cell cultures with no concomitant changes in PPARα or PPARδ protein content (Fig. 8). TLR4 activation also did not alter protein content of LXRα or LXRδ or C/EBPβ or C/EBPδ.
Fig. 8.
Potential mechanism(s) for TLR4 regulation of substrate metabolism. C2C12 cells were treated with LPS as described above, cell lysates were harvested, and Western blotting was performed. Treatment with LPS resulted in no changes in peroxisome proliferator-activated receptor (PPAR)α (57 kDa) and PPARβ (50 kDa), liver X receptor (LXR)α (50 kDa) and LXRβ (56 kDa), CCAAT-enhancer-binding protein (C/EPB)β (37 kDa) and C/EBPδ (29 kDa), and β-actin (45 kDa).
Absence of TLR4 signaling is associated with increased capacity for basal FAO in skeletal muscle and reduced serum FA and triglyceride concentrations.
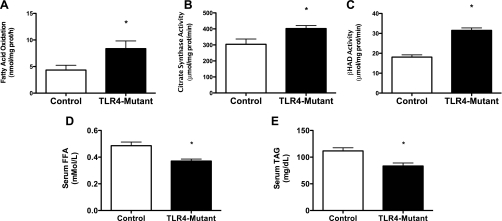
Eight-week-old control (C3HeB/FeJ, n = 9) and TLR4-mutant (C3H/HeJ, n = 8) male mice were fasted overnight and killed, gastrocnemius muscles were harvested, and whole homogenates were prepared for measures of FAO, CS activity, and β-HAD activity. Blood was also taken to measure fasting serum NEFAs and triglycerides. The TLR4-mutant animals, which lack functional TLR4 signaling, possessed significantly higher rates of palmitic acid oxidation, CS activity, and β-HAD activity relative to control mice (Fig. 9, A–C). These findings were accompanied by significantly lower circulating levels of serum NEFAs and triglycerides (Fig. 9, D and E) These data support the notion that increased TLR4 activity in skeletal muscle may contribute to a reduced capacity for FAO in skeletal muscle, which may then contribute to reduced clearance of circulating NEFAs and triglycerides.
Fig. 9.
Loss of TLR4 function is associated with an increased capacity to oxidize FAs in skeletal muscle. Gastrocnemius skeletal muscles were extracted from control (C3HeB/FeJ, n = 9) and TLR4-mutant (C3H/HeJ, n = 8) mice, and whole muscle homogenates were prepared for measures of [1-14C]palmitic acid oxidation and enzyme activities of CS and β-HAD. FA oxidation (A), CS activity (B), and β-HAD activity (C) were all higher in the TLR4-mutant mice relative to control mice. Serum free fatty acids (D) and triacylglycerols (E) were lower in the TLR4-mutant mice. Data are presented as means ± SE. *P < 0.05.
Gain or loss of TLR4 function exacerbates or abolishes, respectively, the LPS-induced shift in skeletal muscle substrate metabolism in vivo.
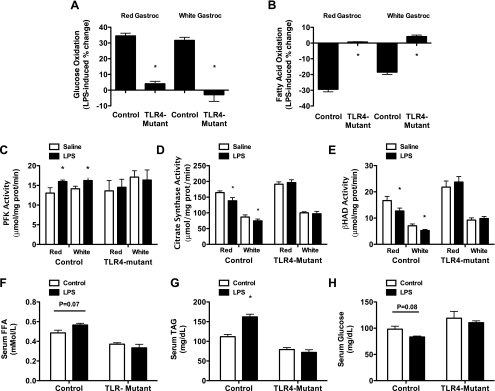
To confirm the cell culture experiments in an in vivo model and determine if the LPS-mediated changes in metabolism were occurring through TLR4, acute (4 h) LPS (25 μg/mouse) experiments were conducted in 8-wk-old, fasted, control (C3HeB/FeJ; saline, n = 6; LPS, n = 6) and TLR4-mutant (C3H/HeJ; saline, n = 6; LPS, n = 6) mice. After the intraperitoneal injections of saline or LPS (4 h), mice were killed, gastrocnemius was harvested and separated into red and white portions, and whole homogenates were prepared for measures of FAO, PFK activity, CS activity, and β-HAD activity. Blood was also collected for measures of NEFAs, TAGs, and glucose. In control animals, LPS induced significant increases and reductions in glucose (Fig. 10A) and FAO (Fig. 10B), respectively, which was abolished in TLR-mutant animals. Additionally, LPS increased PFK (Fig. 10C) enzyme activity and reduced CS (Fig. 10D) and β-HAD (Fig. 10E) enzyme activities in control animals with no effect in TLR4-mutant animals. These changes in skeletal muscle metabolism in control animals were accompanied by an increase in serum TAGs (P < 0.05; Fig. 10G) with a tendency for increases in serum NEFAs (P = 0.07; Fig. 10F) and reductions in serum glucose (P = 0.08; Fig. 10H). No changes in blood glucose, NEFAs, and triglycerides were observed in the TLR4-mutant animals in response to LPS. It is important to note that the increase in serum NEFAs in control mice but not TLR4-mutant mice in response to LPS is consistent with results from Hoch et al. (26) who demonstrated that activation of TLR4 stimulated lipolysis in adipocytes. As such, the tendency for higher concentrations of NEFAs in the control animals in response to LPS cannot be solely attributed to a reduction in FAO in skeletal muscle.
Fig. 10.
LPS-induced shift in muscle substrate metabolism occurs in vivo and is TLR4 dependent. At 8 wk of age, control (C3HeB/FeJ, n = 14) and TLR4-mutant (C3H/HeJ, n = 14) mice were injected with either saline (n = 7/group) or 1 mg/kg (∼25 μg/mouse) of LPS (n = 7/group) and killed 4 h postinjection. Gastrocnemius skeletal muscle was harvested and dissected into red and white portions. LPS treatment resulted in robust increases and decreases in LPS-induced percent changes in glucose (A) and FA (B) oxidation, respectively, which was completely blocked in the TLR4-mutant animals. These effects coincided with an increase in phosphofructokinase (PFK) activity (C) and decreased in CS (D) and β-HAD (E) activities in control mice, which were also blocked in the TLR4-mutant animals. Serum triacylglycerols (G) were also significantly increased following LPS injection in the control animals, which was blocked in the TLR4-mutant animals. Finally, although not significant, there was a trend for a decrease in serum free fatty acid (F) and an increase in plasma glucose (H) in the control animal. Data are presented as means ± SE. *P < 0.05.
DISCUSSION
There is mounting evidence that the immune response is important in the pathophysiology of various obesity-associated metabolic disorders (4, 61). Previous research has demonstrated the presence of the TLR4 pathway in skeletal muscle (17, 39), and a focus has been placed on the role of innate immunity and inflammation on the development of insulin resistance (52, 60). While this research is of utmost importance, we contend that it is also vitally important to understand the signaling pathways that contribute to conditions of maladaptation in skeletal muscle when chronically heightened circulating lipid levels are not met with increased FA utilization as reported in skeletal muscle of obese humans in a fasted state (32, 62, 65). Such a maladaptation can lead to increased intramuscular lipid accumulation, which is a well-established contributor to skeletal muscle insulin resistance (3, 6, 30, 32, 33). The results in this report confirm work from others (17–19, 38, 39, 47–49, 54–56, 68) that skeletal muscle is a target of circulating endotoxin but also highlights that activation of TLR4 signaling in skeletal muscle causes a shift in substrate handling with preferential oxidation of glucose over that of FAs. These results are important and perhaps expand the relevance of the recent work of Cani et al. (7) who describe a phenomenon that has been termed metabolic endotoxemia, which persists in states of obesity, type 2 diabetes, and following high-fat feeding.
Conditions of severe illness and/or infection are characterized by alterations in energy expenditure and metabolism (2, 35). LPS, a type of bacterial endotoxin, is found in the outer membrane of gram-negative bacteria and is made up of a polysaccharide and lipid A. Earlier studies conducted in the sixties and seventies used high doses of LPS for the purpose of understanding the physiological consequences of sepsis and septic shock. These studies demonstrated that LPS treatment to induce septic shock also resulted in alterations in mitochondrial respiration (44, 45, 68). Additionally, these earlier studies identified that lipid A was the active component of LPS and was required for the observed metabolic effects (31, 44). More recent studies in immune cells demonstrated that stimulation with LPS resulted in an increased reliance on glycolysis (20, 58). The current study extends these previous findings to demonstrate that 1) the LPS-induced shift in basal metabolism away from FAO toward glucose oxidation is relevant in skeletal muscle; 2) these effects are observed using much lower doses of LPS than previously described; and 3) most importantly, the LPS-induced effects on skeletal muscle are dependent on the presence of TLR4. Based on these findings, it is important to better understand the role of TLR4 in altered skeletal muscle substrate utilization not only in the obese state but also in the progression of other proinflammatory human conditions such as infection, sepsis, cancer, and cardiovascular disease. It is also equally important to study the metabolic effects of TLR4 activation in other tissues such as liver, adipose tissue, and the pancreas.
The mechanism(s) contributing to the TLR4-mediated shifts in metabolism in skeletal muscle still remains an area in need of clarity and a direction of future study. The initiation of TLR4 signaling results in the activation of a myriad of transcription factors responsible for pro- and anti-inflammatory responses. NF-κB is one such transcription factor and is known to stimulate the production of the cytokines TNF-α, IL-6, and MCP1. Previous studies have found that these cytokines illicit a metabolic response in skeletal muscle, but the precise affect remains equivocal due to disparity in models, doses, and time course of treatments (1, 5, 9, 15, 21, 23, 34, 41, 42). One finding of the current work is that pharmacological blockade of NF-κB either partially or completely prevents the changes in substrate metabolism in response to both low (50 pg/ml) and high (500 ng/ml) doses of LPS in C2C12 muscle cells. Perhaps a more significant finding is that the dose of LPS that is synonymous with metabolic endotoxemia (50 pg/ml) results in alterations in substrate metabolism, but occurs with no change in mRNA and secreted levels of TNF-α, IL-6, and MCP1 in human primary muscle cells, whereas, with a high dose of LPS (500 pg/ml), there is the exact magnitude of change in substrate metabolism as observed with 50 pg/ml of LPS, but it is accompanied with robust changes in mRNA and secretion of IL-6 and MCP1. The fact that both treatments illicit changes in substrate handling but only the high dose leads to inductions of IL-6 and MCP1 implies a disconnect between TLR4-mediated effects on metabolism and local cytokine production in skeletal muscle. It is also important to mention that, in our hands, we see no changes in TNF-α mRNA levels, protein levels, or secreted protein in skeletal muscle cell cultures in response to 2 h of low- or high-dose treatments of LPS. We also observed no changes in TNF-α mRNA and protein levels in skeletal muscle samples extracted from mice 4 h after receiving intraperitoneal injections of low or high doses of LPS. This is in agreement with a recent report (27) but in conflict with others showing changes in TNF-α in skeletal muscle following an endotoxin challenge (37, 39). The disparity in our studies and those of others is unclear but may be due to dosage and time course of treatments.
We also did not observe any changes in protein content of the transcription factors PPARα, PPARδ, LXRα, LXRδ, C/EBPβ, or C/EBPδ following 2 h of LPS stimulation, which is not surprising considering the acute nature of the treatment period used. We did observe consistent decreases in CS and β-HAD activities following LPS stimulation in both skeletal muscle cell cultures and skeletal muscle extracted from mice. It is important to consider that cells and skeletal muscle were collected, extracts were prepared without phosphates inhibitors, and enzyme function was measured under neutral conditions. As such, the likelihood of altered pH, covalent modification, or allosteric modification contributing the changes in enzyme activity in this ex vivo preparation is remote. Because of the acute treatment period, it is also improbable that reductions in enzyme protein content caused these changes. A plausible explanation may be that redox regulation (e.g., oxidative damage) is contributing to altered enzyme function. It is well established that reactive oxygen species interact with proteins causing modifications and malfunction (10, 59, 66, 67), and this may be a fruitful area of future study with regard to TLR4-mediated alterations in substrate metabolism.
In conclusion, we report that activation of TLR4 with LPS doses as low as those reported in states of metabolic endotoxemia causes a shift in substrate metabolism in skeletal muscle that favors glucose oxidation over FAs. These effects are TLR4 dependent and appear to be disconnected to local skeletal muscle cytokine production. These findings may provide the critically needed link between a proinflammatory state and dysregulated metabolism as observed with obesity, type 2 diabetes, and metabolic syndrome.
GRANTS
This work was funded by grants from the American Diabetes Association (1-JF-05-24, M. Hulver) and the National Institutes of Health (RO1 DK-078765 and R56 DK-078765, M. Hulver; RO1 DK-56112, principal investigator J. A. Houmard, funded human transcriptional profiling; and RO1 AR-049881, principal investigator R. Grange, which funded K. Voelker's time).
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
REFERENCES
- 1.Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 20: 3364–3375, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Black PR, Brooks DC, Bessey PQ, Wolfe RR, Wilmore DW. Mechanisms of insulin resistance following injury. Ann Surg 196: 420–435, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep 6: 177–181, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Boos CJ, Lip GY. Is hypertension an inflammatory process? Curr Pharm Des 12: 1623–1635, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bruce CR, Dyck DJ. Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-α. Am J Physiol Endocrinol Metab 287: E616–E621, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Bruce CR, Mertz VA, Heigenhauser GJ, Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes 54: 3154–3160, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55: 2688–2697, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chinopoulos C, Tretter L, Adam-Vizi V. Depolarization of in situ mitochondria due to hydrogen peroxide-induced oxidative stress in nerve terminals: inhibition of α-ketoglutarate dehydrogenase. J Neurochem 73: 220–228, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Cortright RN, Azevedo JL, Jr, Zhou Q, Sinha M, Pories WJ, Itani SI, Dohm GL. Protein kinase C modulates insulin action in human skeletal muscle. Am J Physiol Endocrinol Metab 278: E553–E562, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Cortright RN, Sandhoff KM, Basilio JL, Berggren JR, Hickner RC, Hulver MW, Dohm GL, Houmard JA. Skeletal muscle fat oxidation is increased in African-American and white women after 10 days of endurance exercise training. Obesity (Silver Spring) 14: 1201–1210, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Davi G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, Nutini M, Sensi S, Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. J Am Med Assoc 288: 2008–2014, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Dohm GL, Tapscott EB, Pories WJ, Dabbs DJ, Flickinger EG, Meelheim D, Fushiki T, Atkinson SM, Elton CW, Caro JF. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest 82: 486–494, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J 16: 1335–1347, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Feingold K, Kim MS, Shigenaga J, Moser A, Grunfeld C. Altered expression of nuclear hormone receptors and coactivators in mouse heart during the acute-phase response. Am J Physiol Endocrinol Metab 286: E201–E207, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am J Physiol Regul Integr Comp Physiol 283: R698–R709, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide stimulates nitric oxide synthase-2 expression in murine skeletal muscle and C2C12 myoblasts via Toll-like receptor-4 and c-Jun NH2-terminal kinase pathways. Am J Physiol Cell Physiol 287: C1605–C1615, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Frost RA, Nystrom GJ, Lang CH. Multiple Toll-like receptor ligands induce an IL-6 transcriptional response in skeletal myocytes. Am J Physiol Regul Integr Comp Physiol 290: R773–R784, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Nogales P, Almeida A, Fernandez E, Medina JM, Bolanos JP. Induction of glucose-6-phosphate dehydrogenase by lipopolysaccharide contributes to preventing nitric oxide-mediated glutathione depletion in cultured rat astrocytes. J Neurochem 72: 1750–1758, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Glund S, Deshmukh A, Long YC, Moller T, Koistinen HA, Caidahl K, Zierath JR, Krook A. Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes 56: 1630–1637, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 49: 467–472, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Gray SR, Ratkevicius A, Wackerhage H, Coats P, Nimmo MA. The effect of interleukin-6 and the interleukin-6 receptor on glucose transport in mouse skeletal muscle. Exp Physiol 94: 899–905, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Deviere J, Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology 43: 989–1000, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes Res 13: 574–581, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Hoch M, Eberle AN, Peterli R, Peters T, Seboek D, Keller U, Muller B, Linscheid P. LPS induces interleukin-6 and interleukin-8 but not tumor necrosis factor-α in human adipocytes. Cytokine 41: 29–37, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Huey KA, Fiscus G, Richwine AF, Johnson RW, Meador BM. In vivo vitamin E administration attenuates interleukin-6 and interleukin-1beta responses to an acute inflammatory insult in mouse skeletal and cardiac muscle. Exp Physiol 93: 1263–1272, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab 2: 251–261, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ, MacDonald KG, Cline GW, Shulman GI, Dohm GL, Houmard JA. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab 284: E741–E747, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Hulver MW, Saleh O, MacDonald KG, Pories WJ, Barakat HA. Ethnic differences in adiponectin levels. Metabolism 53: 1–3, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Kato M. Site of action of lipid A on mitochondria. J Bacteriol 112: 268–275, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277: E1130–E1141, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr 22: 325–346, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Kelly M, Gauthier MS, Saha AK, Ruderman NB. Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes 58: 1953–1960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 45: 1169–1196, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Kriketos AD, Greenfield JR, Peake PW, Furler SM, Denyer GS, Charlesworth JA, Campbell LV. Inflammation, insulin resistance, and adiposity: a study of first-degree relatives of type 2 diabetic subjects. Diabetes Care 27: 2033–2040, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Krogh-Madsen R, Plomgaard P, Akerstrom T, Moller K, Schmitz O, Pedersen BK. Effect of short-term intralipid infusion on the immune response during low-dose endotoxemia in humans. Am J Physiol Endocrinol Metab 294: E371–E379, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Lang CH. Neural regulation of the enhanced uptake of glucose in skeletal muscle after endotoxin. Am J Physiol Regul Integr Comp Physiol 269: R437–R444, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Lang CH, Silvis C, Deshpande N, Nystrom G, Frost RA. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor α, interleukin-1beta, -6, and high-mobility-group protein-1 in skeletal muscle. Shock 19: 538–546, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Lemieux I, Pascot A, Prud'homme D, Almeras N, Bogaty P, Nadeau A, Bergeron J, Despres JP. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol 21: 961–967, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Li YP, Reid MB. Effect of tumor necrosis factor-α on skeletal muscle metabolism. Curr Opin Rheumatol 13: 483–487, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Lo YT, Tzeng TF, Liu IM. Role of tumor suppressor PTEN in tumor necrosis factor α-induced inhibition of insulin signaling in murine skeletal muscle C2C12 cells. Horm Metab Res 39: 173–178, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Malenfant P, Joanisse DR, Theriault R, Goodpaster BH, Kelley DE, Simoneau JA. Fat content in individual muscle fibers of lean and obese subjects. Int J Obes Relat Metab Disord 25: 1316–1321, 2001 [DOI] [PubMed] [Google Scholar]
- 44.McGivney A, Bradley SG. Action of bacterial endotoxin and lipid A on mitochondrial enzyme activities of cells in culture and subcellular fractions. Infect Immun 25: 664–671, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGivney A, Bradley SG. Action of bacterial lipopolysaccharide on the respiration of mouse liver mitochondria. Infect Immun 27: 102–106, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388: 394–397, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Memon RA, Feingold KR, Moser AH, Fuller J, Grunfeld C. Regulation of fatty acid transport protein and fatty acid translocase mRNA levels by endotoxin and cytokines. Am J Physiol Endocrinol Metab 274: E210–E217, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Memon RA, Fuller J, Moser AH, Smith PJ, Feingold KR, Grunfeld C. In vivo regulation of acyl-CoA synthetase mRNA and activity by endotoxin and cytokines. Am J Physiol Endocrinol Metab 275: E64–E72, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Myrvold HE, Enger E, Haljamae H. Early effects of endotoxin on tissue phosphagen levels in skeletal muscle and liver of the dog. Eur Surg Res 7: 181–192, 1975 [DOI] [PubMed] [Google Scholar]
- 50.Poitou C, Coussieu C, Rouault C, Coupaye M, Cancello R, Bedel JF, Gouillon M, Bouillot JL, Oppert JM, Basdevant A, Clement K. Serum amyloid A: a marker of adiposity-induced low-grade inflammation but not of metabolic status. Obesity (Silver Spring) 14: 309–318, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Radin MS, Sinha S, Bhatt BA, Dedousis N, O'Doherty RM. Inhibition or deletion of the lipopolysaccharide receptor Toll-like receptor-4 confers partial protection against lipid-induced insulin resistance in rodent skeletal muscle. Diabetologia 51: 336–346, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem 71: 635–700, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raymond RM, Harkema JM, Emerson TE., Jr Direct effects of gram-negative endotoxin on skeletal muscle glucose uptake. Am J Physiol Heart Circ Physiol 240: H342–H347, 1981 [DOI] [PubMed] [Google Scholar]
- 55.Raymond RM, Harkema JM, Emerson TE., Jr Insulin-like action of E. coli endotoxin in promoting skeletal muscle glucose uptake in the dog. Adv Shock Res 6: 141–161, 1981 [PubMed] [Google Scholar]
- 56.Raymond RM, Rosenfeld GA, Emerson TE., Jr Direct effects of insulin and endotoxin on glucose uptake by skeletal muscle during high cardiac index sepsis in the dog. Surg Gynecol Obstet 155: 881–887, 1982 [PubMed] [Google Scholar]
- 57.Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 57: 2595–2602, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuster DP, Brody SL, Zhou Z, Bernstein M, Arch R, Link D, Mueckler M. Regulation of lipopolysaccharide-induced increases in neutrophil glucose uptake. Am J Physiol Lung Cell Mol Physiol 292: L845–L851, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Schwarze SR, Weindruch R, Aiken JM. Oxidative stress and aging reduce COX I RNA and cytochrome oxidase activity in Drosophila. Free Radic Biol Med 25: 740–747, 1998 [DOI] [PubMed] [Google Scholar]
- 60.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J 13: 2051–2060, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun 346: 739–745, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 21: 335–376, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, Swanson MS, Houmard JA. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282: E1191–E1196, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Tretter L, Adam-Vizi V. Inhibition of α-ketoglutarate dehydrogenase due to H2O2-induced oxidative stress in nerve terminals. Ann NY Acad Sci 893: 412–416, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of [α]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci 20: 8972–8979, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trumbeckaite S, Opalka JR, Neuhof C, Zierz S, Gellerich FN. Different sensitivity of rabbit heart and skeletal muscle to endotoxin-induced impairment of mitochondrial function. Eur J Biochem 268: 1422–1429, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.