Abstract
Biomarkers providing in vivo quantification of the basic elements of CF lung disease are needed. We questioned whether the absorption of a small, radiolabeled, hydrophilic molecule (Indium 111 DTPA) would be increased in CF airways. DTPA clearance has been used previously to assess epithelial permeability and may also be useful for quantifying liquid absorption.
The absorptive clearance rate of DTPA was quantified in 10 CF and 11 control subjects using a novel aerosol technique. Subjects inhaled an aerosol containing non-absorbable Technetium-99m sulfur colloid (Tc-SC) particles and Indium-111 DTPA (In-DTPA). Tc-SC clearance from the lung is exclusively mucociliary while In-DTPA is cleared by both absorption and mucociliary clearance. The difference between the In-DTPA and Tc-SC clearance rates estimates In-DTPA absorption.
Tc-SC (mucociliary) clearance was similar in central and peripheral zones in CF and non-CF. Total In-DTPA clearance was increased in both zones in CF. The absorptive component of In-DTPA clearance was increased in the airway-dominated central lung zones in CF (42 vs. 32 %/hr, p=0.03).
The absorption of In-DTPA is increased in the CF airway. Further study is needed to understand the relative roles of fluid absorption, inflammation, and other mechanisms potentially affecting epithelial permeability and DTPA absorption.
Keywords: Aerosol, clearance, cystic fibrosis, DTPA, epithelial permeability
INTRODUCTION
There is a substantial need for new biomarkers in the study of cystic fibrosis (CF) lung disease [1]. Conventional endpoints, such as rate of FEV1 decline, require prolonged trials and large sample sizes to demonstrate therapeutic efficacy. Given the small number of CF patients, preliminary efficacy studies are cost prohibitive, highlighting the need for rapid outcome measures, such as biomarkers. Ideally such biomarkers would provide a quantitative window to the most basic aspects of CF pathophysiology, allowing for the development and evaluation of therapies prior to large scale clinical trials. The basic defect of CF lung disease occurs in the airways where dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) and epithelial sodium (ENaC) channels is thought to create an ionic gradient that causes excessive liquid absorption across the epithelium [2–7]. This results in a dehydrated airway surface liquid (ASL) layer, defective mucociliary clearance, and an increased proclivity for infection and inflammation.
Aerosol-based methods have been developed to measure mucociliary clearance in the lung and used to demonstrate the efficacy of inhaled osmotic therapies [8–10]. We have developed an aerosol technique to measure both mucociliary clearance and the absorptive clearance of a hydrophilic small molecule (Diethylene triamine pentaacetic acid - DTPA) in whole, central, and peripheral lung regions.
We hypothesized that the absorptive clearance of DTPA would occur at an increased rate in the CF airway based on several potential contributory mechanisms. Pro-inflammatory cytokines have been shown to increase airway tight junction permeability, potentially resulting in increased DTPA absorption rates with inflammation [11]. Epithelial damage and denudation have been shown to directly increase DTPA absorption [12]. And finally, bulk liquid flux across the epithelium caused by osmotic gradients, has been shown to alter DTPA absorption rates [13]. Presumably this occurs through a solvent drag mechanism that affects the amount of DTPA available at the epithelial surface for paracellular absorption. Liquid hyperabsorption after volume addition has been well described in in vitro models of the CF airway [14,15], and may contribute to increased DTPA absorption rates in the CF airway after aerosol volume addition. Measurements of DTPA absorption in the airways may be useful for detecting the acute changes in liquid absorption that would be expected after successful treatment with an osmotic or a channel modulating therapy. The same biomarker, if applied longitudinally, might be useful for quantifying the decreases in epithelial permeability which might be expected with an anti-inflammatory therapy or with a healing epithelium.
MATERIALS AND METHODS
In this pilot study we compared measurements of mucociliary clearance and DTPA absorptive clearance in whole, central, and peripheral lung zones of 10 CF subjects and 11 healthy controls using a dual isotope technique (clinicaltrials.gov NCT00248755 and NCT00541190).
Aerosol delivery
Subjects were enrolled in two sequential groups (n=11: 6 controls, 5 CF; n=10: 5 controls, 5 CF). Both groups inhaled the test aerosols, which contained Technetium 99m sulfur colloid (Tc-SC) and Indium 111 DTPA (In-DTPA) in normal saline, from a Whisper-Jet nebulizer (Vital Signs Colorado, Inc., Englewood, CO) driven by 8 LPM of oxygen. Group 1 inhaled for 8 minutes without delivery controls. Group 2 inhaled for 4 minutes using visual feedback to control inhalation flow rate (0.5 LPS) and audible feedback to maintain a set respiratory pattern. This change in delivery technique was made to improve inter-subject dosing uniformity. The breathing pattern was intended to provide both central and peripheral lung dosing. Subjects were seated during aerosol delivery. More detail on aerosol delivery is included in the methods supplement.
Imaging and image analysis
Subjects were imaged continuously for 60 minutes after aerosol delivery while lying recumbent. Independent windows (based on photon energy level) were used to depict the clearance of Tc-99m and In-111. Counts from the clearance images were corrected for decay time, background, and isotope spillover, and fit to single exponential curves. These curves were evaluated at t=60 minutes and those values reported. (Details are included in the supplement.) As In-DTPA can be cleared from the lung via both absorption and mucociliary clearance mechanisms, the absorptive clearance rate was defined as the difference between 60 minute clearance rates of In-DTPA and Tc-SC.
Xenon-133 equilibrium images were used to define the lung outlines. Only the right lung was used to avoid interference from the stomach. Posterior images were used. The whole lung region was defined with a lung-shaped outline while central clearance was measured within a rectangular zone with one-half the height and one-half the width of a rectangle containing the whole lung outline. The peripheral zone was the difference between the whole and central lung zones.
We estimated the radiation exposure associated with aerosol delivery and Xenon inhalation to be approximately 68 mREM (0.68 mSv) - effective dose equivalent.
Statistical methods
Measurements of Tc-S C , In-DTPA total, and In-DTPA absorptive clearance were compared by zone in CF and control subjects using non-parametric (Mann-Whitney) tests. A series of independent linear regressions was performed comparing measurements of aerosol dose and distribution to clearance rates to determine if differences in aerosol delivery techniques or normal inter-subject dosing variations might have affected the study results. Deposited counts of each isotope in each zone were included in this analysis along with central/peripheral dose ratios for both In-DTPA and TC-SC (c/p In and c/p Tc).
RESULTS
Subject characteristics
Testing groups 1 and 2 were combined for preliminary analysis. Two subjects (one CF, one control) were identified as obvious outliers with whole lung Tc-SC clearance rates more than two standard deviations above the averages of the group: 25% and 32% respectively vs. the CF mean of 10 ± 7% and the control mean of 9 ± 9% (± SD; n=21). These outliers were excluded from further analyses. Subject data is presented in Table 1 (n=19). The CF subjects exhibited well maintained pulmonary function, as summarized in Table 2 with other potentially pertinent clinical information regarding medication histories. The ratios of central to peripheral aerosol deposition are also included in Table 1. No significant differences in these ratios were observed when comparing CF and control subjects (c/p Tc: p=0.62, c/p In: p=0.41; Mann-Whitney). Values of c/p In were slightly increased vs. c/p Tc in 15/19 subjects (p=0.004 by Wilcoxin signed-rank).
TABLE 1.
Subject characteristics and central to peripheral (c/p) deposition ratios. All mean ± S.D. Comparing c/p ratios in CF vs. controls: c/p Tc99m p=0.62; c/p In111: p=0.41 by Mann-Whitney.
| Group | n = | Sequential Group 1 |
Sequential Group 2 |
FEV1%p | FEV1%p (max-min) |
FVC%p | FEF25–75%p | Age | c/p ratio Tc99m |
c/p ratio In111 |
|---|---|---|---|---|---|---|---|---|---|---|
| CF | 9 | 5 | 4 | 86 ± 15 | 57–104 | 95 ± 9 | 70 ± 30 | 28 ± 12 | 0.64 ± 0.17 | 0.73 ± 0.22 |
| Control | 10 | 5 | 5 | 99 ± 8 | 86–107 | 98 ± 10 | 103 ± 18 | 30 ± 7 | 0.59 ± 0.16 | 0.63 ± 0.20 |
TABLE 2.
Pulmonary function values, ages, sexes, whole lung (Tc-SC) mucociliary and central lung absorptive clearance rates, and medications of individual cystic fibrosis subjects. Clearance values represent % cleared over 60 minutes.
| CF subject number |
FEV1%p (%) |
FVC%p (%) |
25–75%p (%) |
age (yrs) |
Sex | whole Tc (%/60 min) |
cen absorp (%/60 min) |
HS* | Tobi | Pulmozyme | Albuterol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 83 | 85 | 75 | 56 | M | 8 | 52 | 3% | − | + | + |
| 2 | 73 | 92 | 37 | 19 | F | 5 | 25 | - | − | + | + |
| 3 | 104 | 111 | 89 | 20 | M | 11 | 49 | 3% | − | + | − |
| 4 | 74 | 92 | 41 | 33 | F | 10 | 46 | 7% | + | + | + |
| 5 | 57 | 81 | 23 | 25 | F | 13 | 43 | 3% | − | + | + |
| 6 | 100 | 100 | 109 | 19 | F | 6 | 58 | - | + | + | + |
| 7 | 98 | 99 | 99 | 22 | M | 12 | 41 | - | + | + | + |
| 8 | 92 | 91 | 86 | 31 | M | 11 | 35 | - | − | − | + |
| 9 | 92 | 101 | 71 | 26 | M | 0 | 29 | - | + | + | + |
HS=inhaled hypertonic saline (% listed), Tobi=Tobramycin Solution for Inhalation, Pulmozyme = Dornase Alpha.
subjects did not inhale hypertonic saline for at least 48 hours prior to study.
Mucociliary (Tc-SC) clearance rates are similar in CF and control groups
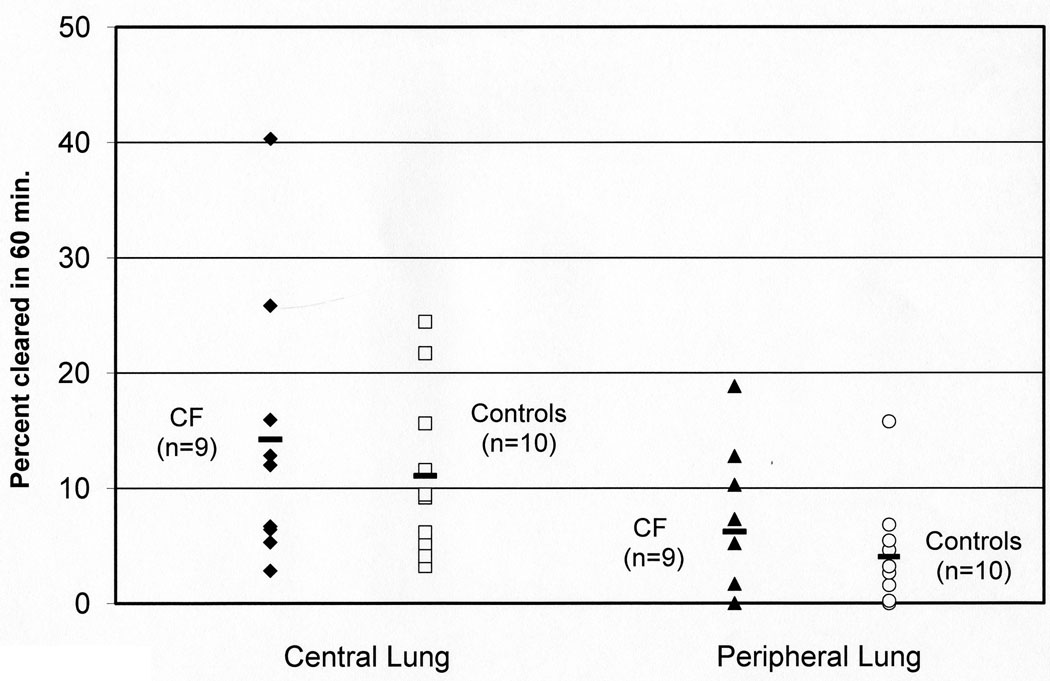
Average clearance rates for Tc-SC and In-DTPA are reported by zone in Table 3. Mucociliary (Tc-SC) clearance rates were similar in CF and control subjects in whole, central, and peripheral lung zones as shown in Figure 1.
TABLE 3.
Measurements of Technetium 99m sulfur colloid (Tc-SC) and total Indium 111 DTPA (In-DTPA) clearance, and the absorptive component of In-DTPA clearance in CF and control subjects. Data is reported as percent cleared over 60 minutes. All mean ± S.D. All p-values by Mann-Whitney test.
| CF (n=9) |
Controls (n= 10) |
p (n=19) |
|
|---|---|---|---|
| Tc-SC (mucociliary) whole lung clearance | 8 ± 4 | 7 ± 4 | 0.41 |
| Tc-SC (mucociliary) central lung clearance | 14 ± 12 | 11 ± 7 | 0.51 |
| Tc-SC (mucociliary) peripheral lung clearance | 6 ± 7 | 4 ± 5 | 0.62 |
| In-DTPA whole lung clearance | 56 ± 14 | 44 ± 8 | 0.04 |
| In-DTPA central lung clearance | 56 ± 16 | 43 ± 9 | 0.05 |
| In-DTPA peripheral lung clearance | 56 ± 13 | 44 ± 8 | 0.04 |
| In-DTPA absorptive whole lung clearance | 48 ± 12 | 37 ± 8 | 0.04 |
| In-DTPA absorptive central lung clearance | 42 ± 11 | 32 ± 7 | 0.03 |
| In-DTPA absorptive peripheral lung clearance | 50 ± 13 | 40 ± 9 | 0.14 |
FIGURE 1.
Central and peripheral lung clearance rates of Technetium 99m sulfur colloid (Tc-SC) in CF and control subjects. This represents the rate of mucociliary clearance. Data is reported as percent cleared over 60 minutes. Central lung p=0.51, peripheral lung p=0.62 by Mann-Whitney test.
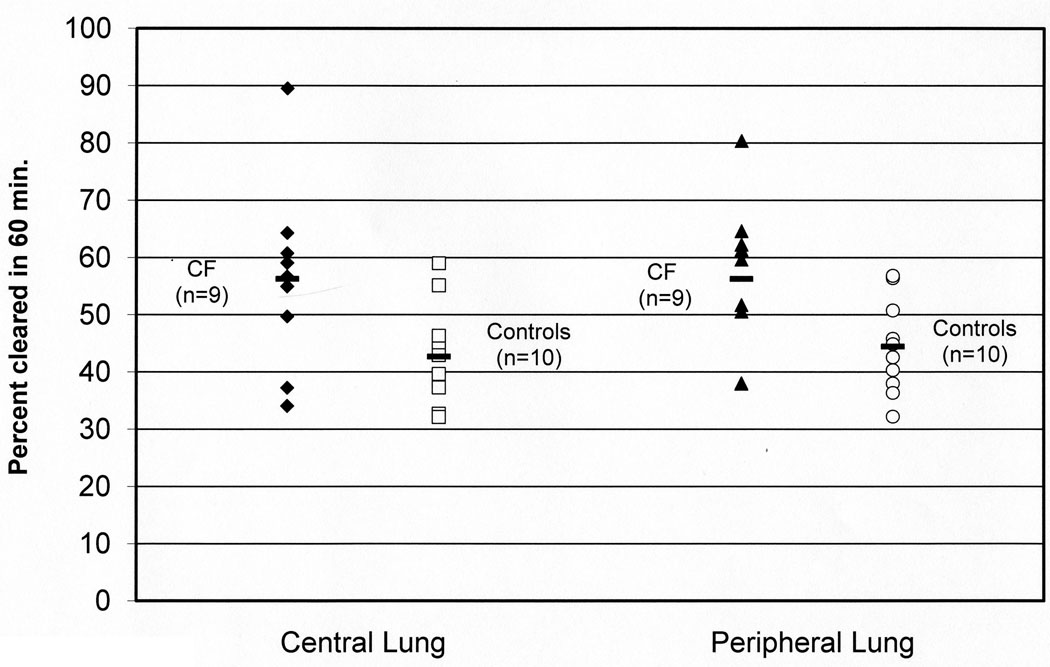
Total In-DTPA clearance rates are increased in CF
In contrast to measurements of Tc-SC clearance, clearance rates of In-DTPA were significantly increased in CF subjects in all zones, as shown in Table 3 and Figure 2. These total In-DTPA clearance rates, which include both mucociliary and absorptive components, were similar within the groups across the central and peripheral lung zones.
FIGURE 2.
Central and peripheral lung clearance rates of Indium 111 DTPA (In-DTPA) in CF and control subjects. In-DTPA is cleared through both adsorptive and mucociliary routes. Data is reported as percent cleared over 60 minutes. Central lung p=0.05, peripheral lung p=0.04 by Mann-Whitney test.
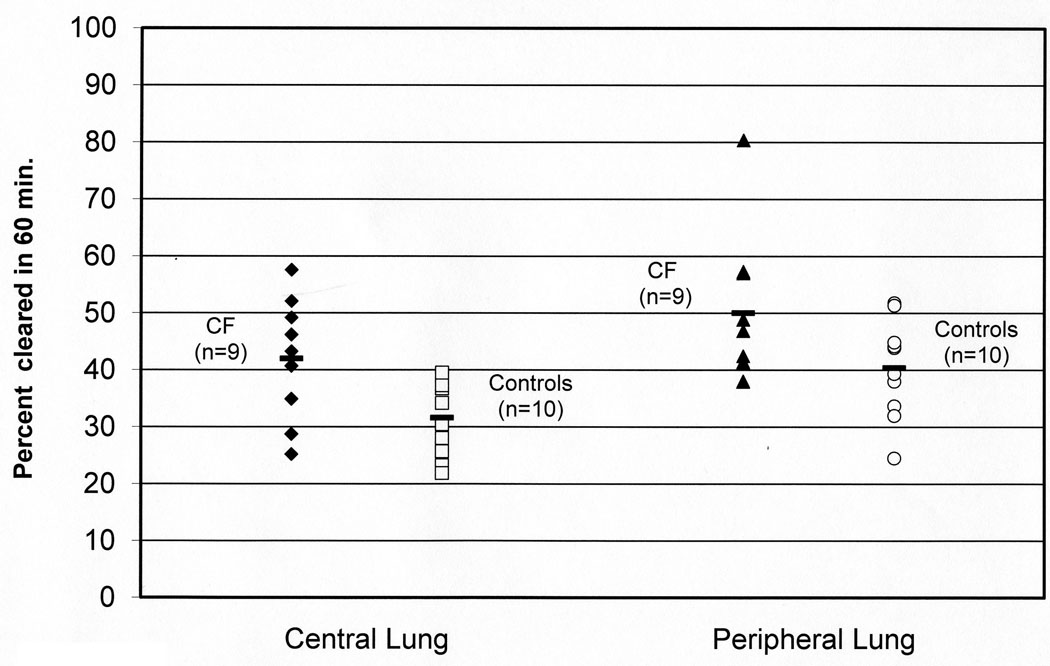
Absorptive clearance rates are increased in whole and central zones in CF
The absorptive component of In-DTPA clearance, as defined as the In-DTPA clearance rate minus the Tc-SC clearance rate, was significantly increased in CF in whole and central zones, as shown in Table 3 and Figure 3. There was a trend towards increased absorptive clearance in the peripheral zones in CF, but the difference was not statistically significant (p=0.14).
FIGURE 3.
The absorptive component of In-DTPA clearance in central and peripheral lung zones in CF and control subjects. Absorptive clearance is calculated as the difference between the total In-DTPA clearance rate and the Tc-SC (mucociliary) clearance rate in each subject. Data is reported as percent cleared over 60 minutes. Central lung p=0.03, peripheral lung p=0.14 by Mann-Whitney test.
The effect of outliers
Inclusion of the two outliers did not affect any previously reported trends but did reduce overall statistical significance. When the outliers were included (n=21), the average whole lung In-DTPA clearance rate was 55 ± 14%/hr in CF vs. 45 ± 8%/hr in controls, p=0.09, ± S.D. Average central lung In-DTPA clearance was 55 ± 16%/hr in CF vs. 44 ± 10%/hr in controls, p=0.07, and the average peripheral lung In-DTPA clearance rate was 55 ± 13%/hr in CF vs. 46 ± 9%/hr in controls, p=0.08. The average whole lung absorptive clearance rate was 45 ± 15%/hr in CF vs. 36 ± 8%/hr in controls, p=0.09. Average central lung absorptive clearance was 39 ± 15%/hr in CF vs. 30 ± 9%/hr in controls, p=0.07, a n d the average peripheral lung absorptive clearance rate was 47 ± 15%/hr in CF vs. 40 ± 8%/hr in controls, p=0.23.
Linear regression modeling to determine the effects of dosing variability
Linear regression was used to determine whether differences in deposited dose caused by aerosol delivery technique or normal inter-subject differences might have affected measurements of clearance. No significant correlations were found between Tc-SC clearance and any dosing variable. Central to peripheral dose ratios were not predictive of In-DTPA or Tc-SC clearance. The deposited dose of In-DTPA significantly predicted absorptive clearance rate in all zones and total In-DTPA clearance in whole and peripheral zones. Absorptive clearance rate was inversely related to In-DTPA dose with higher doses clearing more slowly on a percent per hour basis. Central lung absorptive clearance was significantly increased in CF subjects when compared to controls using a linear regression model that included the effects of deposited In-DTPA dose (p=0.05).
DISCUSSION
Mucociliary clearance plays a vital host defense role in the lungs by providing rapid mechanical clearance of deposited particulates and pathogens. In the current study no deficits in mucociliary clearance rate were detected in the CF subjects. Donaldson et al demonstrated deficits in 24 hour clearance in CF that were not detectable through similar 60 minute measurements [9]. Both studies included CF groups with well preserved pulmonary function. These studies imply that baseline deficits in mucociliary clearance may not be detectable in more healthy CF subject groups, at least not using 60 minute measurements.
In contrast, In-DTPA clearance was significantly increased in CF in all lung zones. It was notably rapid with 56% of deposited material being cleared within one hour in CF and 44% in the controls. This clearance includes both mucociliary and absorptive components which can be differentiated through our technique. Absorption was the dominant component, comprising 86% of total whole-lung clearance in CF and 84% in controls. Absorptive clearance was significantly increased in the airway dominated central lung zones in CF and also in the whole lung zones.
Whole lung DTPA absorption has been previously used as a gauge of alveolar epithelial permeability [16–19]. In the CF airways, epithelial injury and increases in tight junction permeability associated with infection and inflammation would be expected to increase baseline permeability [11,12]. DTPA is a small hydrophilic molecule, and it is likely that it remains in an aqueous solution and moves in parallel to fluid flux in the airway. In vivo animal data supports the notion that DTPA is a marker of fluid flux. DTPA absorption across an isolated trachea was accelerated or slowed when the airway lumen was bathed with hypotonic or hypertonic fluid (saline or mannitol), respectively [13]. Numerous studies have demonstrated excessive airway surface liquid absorption in the CF airway [14,15], and the hyperabsorption of the liquid associated with the aerosol could provide the mechanism for the increased rates of DTPA absorption noted in the CF subjects.
The data from our pilot clinical study does not allow us to differentiate liquid absorptive effects vs. effects associated with epithelial permeability. Measurements of DTPA absorption in the airways may be useful for detecting both immediate changes in liquid absorption, as might be found after treatment with an osmotic or a channel modulating therapy, and longer term changes in epithelial permeability as might be found after anti-inflammatory treatments or with a healing epithelium. Longer term effects would logically require longitudinal measurements. Both of these applications require further study with the next logical step being assessment of response to a known therapy.
Differences in aerosol deposition pattern have been shown to affect measurements of mucociliary clearance [8]. In this study two different aerosol delivery techniques were used. (Details included in methods supplement.) In any study a certain amount of dosing variability can be expected, and when comparing subjects with lung disease to controls, true dose matching is even less likely. Differences in dosing can be accurately depicted through a series of variables considering radioactive counts of each isotope in each lung zone, and the ratio of these doses in central vs. peripheral zones. The use of linear regression models provided us a means to determine and correct for differences in aerosol deposition, ultimately allowing us to combine subject groups receiving the aerosols through different techniques. These regression models demonstrated an important inverse relationship between deposited In-DTPA dose and absorption rate. Higher deposited doses of In-DTPA resulted in proportionally slower percent per hour absorptive clearance rates in all zones, surprisingly indicating a finite capacity for DTPA transport even across the large surface area of the peripheral lung. After correction for dosing effects, significant increases in central lung absorptive clearance were still noted in the CF subjects.
One limitation of our technique is our inability to truly segregate alveolar and airway zones. Results from the central zones were undoubtedly influenced by alveolar tissue overlaying the airways. However comparisons of the central and peripheral zones are still useful for demonstrating the relative effects of the airways. A comparison of the central and peripheral zones used in our analysis demonstrated expected effects. Mucociliary clearance was 2–3 fold higher in the central zones while absorptive clearance was significantly increased in peripheral zones, giving some indication that our designated zones are anatomically distinct. Another study limitation was the presence of two outliers, removed from the analyses based on whole lung mucociliary clearance measurements that were more than two standard deviations above the mean for the group. We believe that technique related issues (i.e. the inadvertent counting of esophageal material) must have produced the errant results, though this could not be absolutely determined, and these outliers may indicate that a higher level of variability would be demonstrated in a larger population.
This study demonstrates a basic physiological difference in the CF airway that is easily measurable at a macroscopic level, apparent in subjects with well maintained pulmonary function, and likely related to the basic defects of CF lung disease. Increased airway epithelial permeability may contribute to the increased absorption rates demonstrated in the CF subjects. Liquid hyperabsorption may also contribute at a significant level. We believe that our aerosol technique can be further developed into a biomarker to independently quantify either of these mechanisms, providing indication of therapeutic efficacy ahead of currently available indicators. Importantly, these studies can be performed with low to minimal radiation exposures making multiple measurements and application in pediatric populations feasible.
Supplementary Material
ACKNOWLEDGEMENTS
Thanks to David Rowlands M.D. for his thoughtful review of the manuscript.
FUNDING:
Funded by NIH K25HL081533 and P30DK072506, and the Cystic Fibrosis Foundation Research Development Program.
Footnotes
COMPETING INTERESTS:
None
References
- 1.Mayer-Hamblett N, Ramsey BW, Kronmal RA. Advancing outcome measures for the new era of drug development in cystic fibrosis. Proc Am Thorac Soc. 2007;4:370–377. doi: 10.1513/pats.200703-040BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsh AJ. Altering airway surface liquid volume: inhalation therapy with amiloride and hyperosmotic agents. Adv Drug Deliv Rev. 2002;54:1445–1462. doi: 10.1016/s0169-409x(02)00161-8. [DOI] [PubMed] [Google Scholar]
- 3.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 5.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 6.Frizzell RA, Pilewski JM. Finally, mice with CF lung disease. Nat Med. 2004;10:452–454. doi: 10.1038/nm0504-452. [DOI] [PubMed] [Google Scholar]
- 7.Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev. 1999;79:S215–S255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson SH, Corcoran TE, Laube BL, Bennett WD. Mucociliary clearance as an outcome measure for cystic fibrosis clinical research. Proc Am Thorac Soc. 2007;4:399–405. doi: 10.1513/pats.200703-042BR. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 10.Robinson M, Daviskas E, Eberl S, Baker J, Chan HK, Anderson SD, Bye PT. The effect of inhaled mannitol on bronchial mucus clearance in cystic fibrosis patients: a pilot study. Eur Respir J. 1999;14:678–685. doi: 10.1034/j.1399-3003.1999.14c30.x. [DOI] [PubMed] [Google Scholar]
- 11.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218–3234. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells UM, Woods AJ, Hanafi Z, Widdicombe JG. Tracheal epithelial damage alters tracer fluxes and effects of tracheal osmolaity in sheep in vivo. J Appl Physiol. 1995;78:1921–1930. doi: 10.1152/jappl.1995.78.5.1921. [DOI] [PubMed] [Google Scholar]
- 13.Wells UM, Hanafi Z, Widdicombe JG. Osmolality alters tracheal blood flow and tracer uptake in anesthetized sheep. J Appl Physiol. 1994;77:2400–2407. doi: 10.1152/jappl.1994.77.5.2400. [DOI] [PubMed] [Google Scholar]
- 14.Chambers LA, Rollins BM, Tarran R. Liquid movement across the surface epithelium of large airways. Respir Physiol Neurobiol. 2007;159:256–270. doi: 10.1016/j.resp.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blouquit S, Regnier A, Dannhoffer L, Fermanian C, Naline E, Boucher R, Chinet T. Ion and fluid transport properties of small airways in cystic fibrosis. Am J Respir Crit Care Med. 2006;174:299–305. doi: 10.1164/rccm.200506-987OC. [DOI] [PubMed] [Google Scholar]
- 16.Brown MA, Lantz RC, Sobonya R, Devine LC, Lentz LA, Lemen RJ. Aerosolized lipopolysaccharide increases pulmonary clearance of 99mTc-DTPA in rabbits. Am Rev Respir Dis. 1992;146:1462–1468. doi: 10.1164/ajrccm/146.6.1462. [DOI] [PubMed] [Google Scholar]
- 17.Coates G, O'Brodovich H, Dolovich M. Lung clearance of 99mTc-DTPA in patients with acute lung injury and pulmonary edema. J Thorac Imaging. 1988;3:21–27. doi: 10.1097/00005382-198807000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Groth S. Pulmonary clearance of 99mTc-DTPA. An index of alveolar epithelial permeability. Dan Med Bull. 1991;38:189–203. [PubMed] [Google Scholar]
- 19.Mason GR, Peters AM, Bagdades E, Myers MJ, Snook D, Hughes JM. Evaluation of pulmonary alveolar epithelial integrity by the detection of restriction to diffusion of hydrophilic solutes of different molecular sizes. Clin Sci (Lond) 2001;100:231–236. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.