Abstract
Angiogenesis occurs through a convergence of diverse signaling mechanisms with prominent pathways that include autocrine effects of endothelial nitric oxide (NO) synthase (eNOS)-derived NO and vascular endothelial growth factor (VEGF). However, the redundant and distinct roles of NO and VEGF in angiogenesis remain incompletely defined. Here, we use the partial hepatectomy model in mice genetically deficient in eNOS to ascertain the influence of eNOS-derived NO on the angiogenesis that accompanies liver regeneration. While sinusoidal endothelial cell (SEC) eNOS promotes angiogenesis in vitro, surprisingly the absence of eNOS did not influence the angiogenesis that occurs after partial hepatectomy in vivo. While this observation could not be attributed to induction of alternate NOS isoforms, it was associated with induction of VEGF signaling as evidenced by enhanced levels of VEGF ligand in regenerating livers from mice genetically deficient in eNOS. However, surprisingly, mice that were genetically heterozygous for deficiency in the VEGF receptor, fetal liver kinase-1, also maintained unimpaired capacity for liver regeneration. In summary, inhibition of VEGF- and NO-dependent angiogenesis does not impair liver regeneration, indicating signaling redundancies that allow liver regeneration to continue in the absence of this canonical vascular pathway.
Keywords: endothelial nitric oxide synthase, vascular endothelial growth factor, nitric oxide, fetal liver kinase-1, angiogenesis, partial hepatectomy
nitric oxide (NO) is a key molecule for angiogenesis because of its pleotrophic effects on vascular endothelial cells (35, 53). Indeed, impaired angiogenesis can be evidenced in mice with genetic deletion of endothelial NO synthase (eNOS) in several mouse models of systemic and peripheral angiogenesis, including hindlimb ischemia (32, 51). In liver, NO is derived from eNOS expressed within liver sinusoidal endothelial cells (SEC), thereby providing the basis for an autocrine angiogenic loop for angiogenesis. Importantly, vascular endothelial growth factor (VEGF) is a canonical agonist for eNOS activation although the eNOS-dependent and eNOS-independent actions of VEGF are not yet fully defined (5, 27, 33).
VEGF is a potent endothelial cell mitogen/motogen and dominant angiogenic growth factor (15, 16, 26). In liver, VEGF, like NO, has been shown to have stimulatory effects on SEC proliferation (50). Interestingly, the pathways through which VEGF promotes angiogenesis have not been fully delineated, especially with regard to cross talk and redundancy with the NO system.
Liver regeneration is critical, not only postoperatively after hepatic surgical resection, but also in the recovery process that follows a variety of hepatic insults/injuries (40). A critical component of the liver regeneration response is thought to be dependent on angiogenesis since expansion of avascular islands of hepatocytes is associated with an angiogenic switch (1, 2, 37); however, the overall requirement of angiogenesis for liver regeneration is not fully defined. Thus partial hepatectomy is a compelling model in which to ascertain angiogenic cross talk in vivo, especially because of the well-defined timeline of molecular and cellular events that occur in the first several days in response to 70% hepatic resection (14, 30, 39). In this study, we use this model to explore the influence of eNOS and VEGF on angiogenesis in vivo. Surprisingly, we demonstrate that, while critical for angiogenesis in vitro, neither eNOS deficiency nor partial fetal liver kinase-1 (Flk-1) deficiency adversely affects liver regeneration in vivo. Thus these studies highlight the redundancy of signaling pathways that allows liver regeneration to be maintained in the absence of these canonical angiogenic pathways.
MATERIALS AND METHODS
Animal studies.
The animal protocols in this study were submitted and approved by the Mayo Clinic Rochester (Rochester, MN) Institutional Animal Care and Use Committee. All animal procedures were performed in accordance with the Institutional Animal Care and Use Committee guidelines of Mayo Clinic. eNOS wild-type (+/+) and eNOS knockout (−/−) mice (male C57BL/6 mice, 12–14 wk old; Jackson laboratories, Bar Harbor, ME) along with Flk-1+/− and Flk-1+/+ mice (male CD1 mice; Jackson Laboratories) (46) were maintained under standard 12:12-h light-dark cycles with free access to chow and water. Surgical resection of ∼70% of the liver was performed as described by Rai et al. (36) with minor modifications. Sham-operated mice underwent identical abdominal incision and liver mobilization. Subsequently, eNOS+/+ and eNOS−/− mice were killed at 2, 4, 6, or 8 days and Flk-1+/− and Flk-1+/+ mice were killed at 2 and 4 days after surgery at which time the percent of liver weight recovery was determined (as below). Next, aliquots of liver tissue from each mouse were fixed for histology or alternatively frozen for subsequent biochemical and molecular analyses.
Calculation of liver regeneration.
At the time of partial hepatectomy, the resected liver mass represented 70% of the total liver weight. This was used to calculate the total liver weight as resected liver = 0.70 × (total liver weight). At death, the regenerated liver was weighed, divided by the initial total weight, and expressed as the percentage of the liver that had regenerated (36). In experiments involving the inhibition of NOS activity in vivo, mice (male C57BL6) were injected with either vehicle (normal saline) or NG-nitro-l-arginine methyl ester (l-NAME, 100 mg/kg body wt ip) (6) 24 h before hepatectomy and immediately following the procedure. Mice were killed 24 h following the procedure, and the liver tissue was harvested for immunohistochemical analysis.
Cell culture and adenoviral transduction.
Human hepatic SEC (HHSEC; Cell Science) were grown in endothelial cell medium supplemented with endothelial cell growth supplement, 10% FBS, and 1% streptomycin/penicillin. To transduce HHSEC with adenoviral vectors for eNOS (AdeNOS) or LacZ control (AdLacZ), HHSEC were grown to confluence in 100-mm dishes and infected with 25 multiplicity of infection of virus, which achieved ∼90% transduction efficiency with minimal toxicity. In all experiments, cells were used between 32 and 36 h postviral transduction.
Proliferation assay.
The relative proliferation rates of AdeNOS- and AdLacZ-transduced HHSEC were determined by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay on 96-well plates as described previously (24).
Vascular tube formation assay.
AdeNOS- and AdLacZ-transduced HHSEC were placed on 100 μl matrigel after 30 min of preincubation at 37°C. Cells were incubated for 18 h at 37°C and 5% CO2, imaged using a ×4 objective, and analyzed using Image-Pro Plus software for quantification of tube formation as described previously (42).
Immunohistochemistry.
Tissue sections (5 μm) were cut from optimum cutting temperature blocks and stained with hematoxylin and eosin and antibodies to Ki-67. Proliferation index of sinusoidal lining cells (SLC) and hepatocytes was determined by calculating the fraction of Ki-67-positive cells to the total number of cells within the given field. SLC and hepatocytes were differentiated by their prominent difference in morphology. For Ki-67 immunostaining, the slides were fixed in ice-cold acetone (10 min), blocked with 10% FBS, and then incubated with rabbit polyclonal anti Ki-67 antibody (1:200) overnight at 4°C. Donkey anti-rabbit Alexafluor 488 was used for detection of Ki-67 positive staining cells, and 4′,6-diamidino-2-phenylindole was used to visualize the nucleus.
Quantitative real-time PCR and mouse endothelial biology PCR array.
Total RNA was extracted from liver tissue using an RNeasy kit according to the manufacturer's instruction (QIAGEN, Valencia, CA) as we have previously described (10). In brief, 1.5 μg of total RNA was used for the cDNA synthesis using the Random Hexamer Primer of SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). For TaqMan-based real-time PCR analysis, 25 ng of each cDNA was added to the Taqman Universal PCR Master Mix along with 900 nM of each primer and 200 nM of probe according to the manufacturer's instruction (Applied Biosystems). Real-time fluorescence monitoring was performed with the Applied Biosystems 7500 Real Time PCR System instrument. Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used in the same reaction as a normalization control. Levels of VEGF, neuronal NOS (nNOS), and inducible NOS (iNOS) mRNA were depicted as fold difference of partial hepatectomy liver compared with sham-operated liver samples after normalization. The cDNA from SEC isolated from sham-operated and hepatectomized eNOS−/− and eNOS+/+ mice was also analyzed on a mouse endothelial cell biology RT2 Profiler PCR array (SABioscience, Fredrick, MD) using the RT2 ProfilerPCR Array Data Analysis software.
Western blot analysis.
Frozen liver tissue samples were homogenized in a lysis buffer as previously described (10). Samples containing equal amounts of protein were separated by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with antibody specifically recognizing VEGF (Santa Cruz, Santa Cruz, CA) or β-actin control (Sigma, St. Louis, MO).
NOS activity assay.
NOS activity was measured as described previously (20). Briefly, detergent-soluble lysates were incubated for 20 min with a buffer containing 1 mM NADPH, 3 μM tetrahydrobiopterin, 10 nM calmodulin, 0.25 mM CaCl2, 10 μM l-arginine, and 0.2 μCi l-[3H]arginine at 37°C. Samples were run in duplicate. The reaction was terminated by the addition of 1 ml of cold stop buffer (20 mM HEPES, 2 mM EDTA, and 2 mM EGTA, pH 5.5), and the reaction mix was applied to a Dowex AG 50WX-8 resin column. Radiolabeled counts per minute of l-citrulline generation were measured and used to determine l-NAME-inhibited NOS activity (20).
Statistical analysis.
Experiments were performed in triplicates with a minimum of three independent experiments. Data are depicted as means ± SE. Comparisons were performed via Student's t-test or one-way ANOVA when comparing more than two sample groups, with statistical significance set at P < 0.05.
RESULTS
NO promotes angiogenesis in vitro.
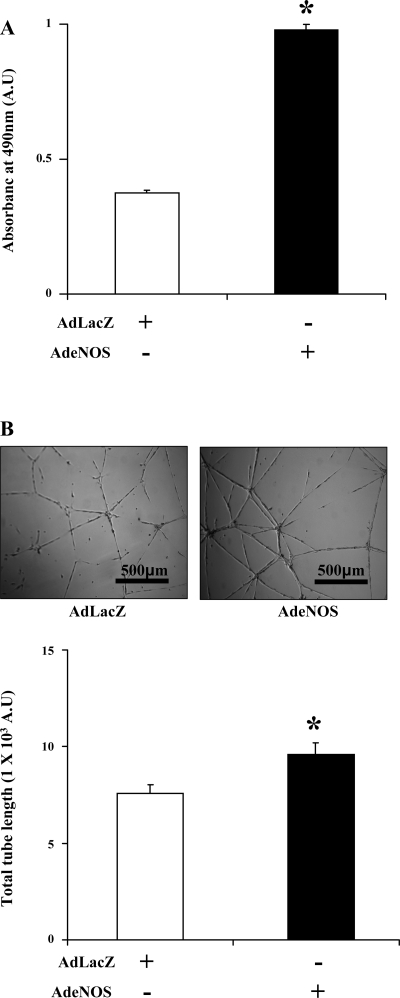
First, to ascertain the effect of eNOS on angiogenic responses in SEC in vitro, HHSEC were transduced with AdeNOS or AdLacZ and assayed for proliferation and tubulogenesis, the latter of which is an in vitro correlate of angiogenesis. The AdeNOS construct prominently increases eNOS protein levels in transduced cells (11). HHSEC transduced with AdeNOS showed a significantly higher proliferative index compared with the AdLacZ-transduced group as assessed by MTS assay (Fig. 1A). To examine the angiogenic effect of eNOS in HHSEC, AdeNOS- and AdLacZ-transduced cells were plated on a matrigel-coated four-well glass chamber and allowed to form tubes for 18 h in basal media, and the total tube length was then calculated using Image-Pro Plus software. Again, eNOS overexpression significantly promoted tube formation compared with the AdLacz-transduced control group (Fig. 1B). Thus these studies, consistent with prior ones (34), indicate that eNOS and the NO system promote EC-based angiogenesis in vitro, and this concept was pursued further using the in vivo hepatectomy model as described below, to ascertain the effects of eNOS on angiogenesis in setting of liver regeneration in vivo.
Fig. 1.
Endothelial (e) nitric oxide synthase (NOS) overexpression increases proliferation and tube formation in human hepatic (HH) sinusoidal endothelial cells (SEC). A: HHSEC were transduced with adenoviral vectors for eNOS (AdeNOS) or LacZ control (AdLacZ). Following transduction, 5 × 103 HHSEC were plated on a 96-well plate and serum starved for 10 h, and, subsequently, the proliferation index was evaluated using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. HHSEC transduced with AdeNOS showed significantly increased proliferation compared with the AdLacZ-transduced group (n = 3 separate experiments, each in triplicate *P < 0.05). B: to assess tube formation, HHSEC were transduced with AdeNOS and AdLacZ and were then plated on matrigel-coated four-chamber slides for 18 h in basal endothelial cell medium. Tubulogenesis was measured using Image-Pro Plus software. Cells transduced with AdeNOS showed a greater increase in total tube length compared with the AdLacz-transduced group (scale bar represents the tube size, n = 3 separate experiments with 15 representative images taken and analyzed from each group in each experiment; *P < 0.05).
Kinetic profiles of proliferation of SLC and hepatocytes after partial hepatectomy.
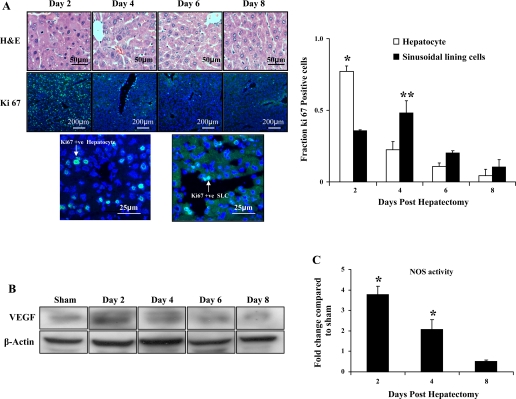
As an initial step to ascertain the time of the angiogenic switch in the partial hepatectomy model, we measured proliferation kinetics of hepatocytes compared with SLC, which are comprised predominantly of SEC in this model. The mice (C57BL6, n = 6/group) were killed at days 2, 4, 6, and 8 after partial hepatectomy, and the remnant lobes of the liver were harvested, embedded, and sectioned to stain for Ki-67, a standard marker of cellular proliferation. Fractions of Ki-67-positive staining among the hepatocytes and among the SLC were used to determine the rate of proliferation. Although the peak proliferation was observed at day 2 for the hepatocytes, SLC proliferation lagged behind at day 4 (Fig. 2A, left and right), indicating that the angiogenic switch occurred after the initial burst of hepatocyte proliferation in response to partial hepatectomy. Increased VEGF protein levels (Fig. 2B) in day 2 samples coincided with peak hepatocyte proliferation, indicating that angiogenesis in the regenerating model may be driven by hepatocyte-derived angiogenic factors such as VEGF. Interestingly, this peak also coincided with the peak of NOS activity from liver lysates; NOS activity peaked at day 2 after which it gradually decreased to levels similar to sham mice (Fig. 2C).
Fig. 2.
Sinusoidal lining cell and hepatocyte proliferation kinetics after partial hepatectomy: Wild-type C57BL/6J mice (n = 6/group) underwent partial hepatectomy; mice were killed at 0, 2, 4, 6, and 8 days following the procedure. The remnant liver was weighed and embedded in optimum-cutting temperature medium for subsequent sectioning. A: left, photomicrographs along with scale bars of hematoxylin and eosin (H&E) and Ki-67 staining of remnant liver sections show the proliferation pattern at different time points. Images at bottom convey the different morphological pattern of hepatocyte and sinusoidal lining staining. Right, Ki-67-positive cells among both the hepatocyte and sinusoidal lining cells were counted and expressed as a fraction of the total cells in that respective cell population. Although proliferation of hepatocytes was significantly greater than sinusoidal lining cells at day 2, this pattern was reversed at day 4 [P < 0.05, hepatocyte vs. sinusoidal lining cell at day 2 (*) and sinusoidal lining cell vs. hepatocyte at day 4 (**)]. B: Western blot analysis of liver tissue lysates was performed to determine changes in vascular endothelial growth factor (VEGF)-A expression at day 2, day 4, day 6, and day 8 in mice posthepatectomy and in sham-operated mice; β-actin served as a loading control. VEGF-A expression was highest at day 2. C: conversion of l-[3H]arginine to l-[3H]citrulline was used to assess the NOS activity in liver samples obtained from C57BL/6J mice 2, 4, and 8 days following partial hepatectomy (n = 4 in each group) and was compared with sham-operated mice. Peak in NOS activity was observed at day 2.
Absence of eNOS does not affect the regenerative process.
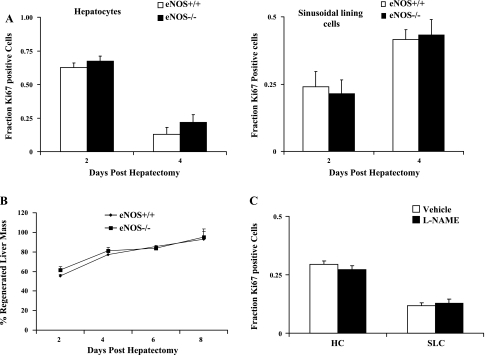
Next, to directly test the influence of eNOS-derived NO on angiogenesis and liver regeneration in vivo, we performed partial hepatectomy or sham surgery in eNOS−/− mice or age- and sex-matched littermate control mice. At days 2 (n = 4 for eNOS−/− and n = 6 for eNOS+/+), 4 (n = 6 for eNOS−/− and n = 6 for eNOS+/+), 6 (n = 5 for eNOS−/− and n = 6 for eNOS+/+), and 8 (n = 4 for eNOS−/− and n = 6 for eNOS+/+) after surgery, mice were killed, and the liver was harvested for measurement of regeneration as well as complementary biochemical and histological analyses. Surprisingly, despite the prominent angiogenic effects of eNOS on angiogenesis in vitro, eNOS−/− mice and their controls showed similar regeneration kinetics following the procedure. Analyses to examine the pattern of proliferation of parenchymal cells and SLC from harvested tissues using Ki-67 also showed no substantive differences between eNOS−/− mice and their controls (Fig. 3A). Similarly, there was no significant difference in the percentage of the liver mass that regenerated following hepatectomy between either group (Fig. 3B). Additionally, to further exclude the role of eNOS in the regenerative process, we injected C57BL6 mice (n = 4/group) with either vehicle (normal saline) or l-NAME (100 mg/kg ip). Because l-NAME is a nonspecific NOS inhibitor, we used a regimen involving acute dosage (6) wherein mice were injected 24 h before the hepatectomy and immediately following the surgery so as to minimize the iNOS inhibition that has been previously shown to inhibit the posthepatectomy liver regeneration (36). Mice were killed 24 h after the resection. Immunohistochemical analyses of hepatocytes and SLC proliferation using Ki-67 showed that there was no significant difference between the vehicle-treated group and the l-NAME-treated group (Fig. 3C). These studies indicate that eNOS-derived NO is not required for the angiogenesis that accompanies liver regeneration.
Fig. 3.
Liver regeneration is not impaired following eNOS inhibition. Partial hepatectomy was conducted in eNOS wild-type (+/+) and eNOS knockout (−/−) mice; restituted liver mass was measured, and tissue was retrieved at specific time points after surgery for immunohistochemical analyses. A: immunostaining with Ki-67 was used as a proliferation marker to determine the fraction of positively staining cells in the hepatocyte population (left) or the sinusoidal lining cell (SLC) population (right) in both groups at different time points. No differences were observed between eNOS+/+ and eNOS+/+ mice (day 2, n = 4 for eNOS−/− and n = 6 for eNOS+/+; day 4, n = 6 for eNOS−/− and n = 6 for eNOS+/+; day 6, n = 5 for eNOS−/− and n = 6 for eNOS+/+; day 8, n = 4 for eNOS−/− and n = 6 for eNOS+/+; P > 0.05). B: the remnant liver removed at day 2, day 4, day 6, and day 8 was used to calculate the restituted liver mass according to the formula mentioned in materials and methods, and the two groups were compared for each time point. There was no significant difference in regenerating liver mass in eNOS−/− compared with eNOS+/+ mice (P > 0.05). C: administration of NG-nitro-l-arginine methyl ester (l-NAME, 100 mg/kg body wt ip) did not significantly alter the proliferation pattern of hepatocytes and SLC compared with the vehicle-treated group (n = 4 mice/group, P > 0.05).
Upregulation of VEGF expression in eNOS−/− mice following partial hepatectomy may compensate for deficiency of eNOS.
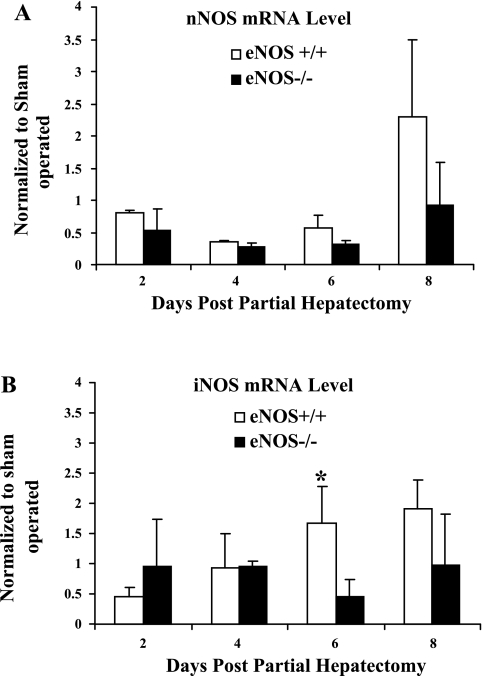
We next sought to elucidate a variety of potential mechanisms that could maintain angiogenesis and liver regeneration in vivo despite eNOS deficiency. First, to determine whether alternative NOS isoforms may compensate for the lack of eNOS in the knockout mice in vivo, we compared the mRNA levels of nNOS and iNOS in eNOS−/− and littermate control mice (Fig. 4, A and B). Although the patterns of expression of both NOS isoforms did change during the course of liver regeneration, there was no significant difference in the expression of the isoforms between wild-type and eNOS−/− mice at the various time points. These results indicate that alternative NOS isoform expression is not likely a compensatory mechanism to account for angiogenesis and liver regeneration that progress unabated in eNOS−/− mice.
Fig. 4.
Alternative NOS isoforms do not compensate for eNOS deficiency after hepatectomy. Total RNA extracted from the harvested liver of eNOS−/− and eNOS+/+ mice following partial hepatectomy was used to compare the expression of neuronal NOS (nNOS; A) and inducible NOS (iNOS, B) between eNOS−/− and wild-type control mice at different time points using qPCR. The mRNA levels of nNOS were not statistically significant at any time point under consideration between the two groups; however, there was an increase in iNOS mRNA levels in eNOS+/+ mice compared with eNOS−/− at day 6 (*P < 0.05; n = 3 animals/group).
Next, to further ascertain the pattern of expression of genes that might play an important role in SEC biology following hepatectomy, we isolated SEC from eNOS−/− and eNOS+/+ mice at days 2 and 4 postresection and looked at the gene profile using endothelial cell biology RT2 Profiler PCR array [Supplemental Table 1 (Supplemental material for this article may be found on the American Journal of Physiology: Regulatory, Integrative and Comparative Physiology website.)]. An interesting observation was the upregulation of Birc2 (cIap1), an anti-apoptotic protein that has previously been shown to play an important role in endothelial cell survival and vessel integrity (41), in the eNOS knockout mice compared with the wild-type mice under basal conditions. However, following hepatectomy, the knockout mice showed a reversal of Birc2 expression level, a phenomenon that highlights the potential influence of EC apoptotic factors on angiogenesis.
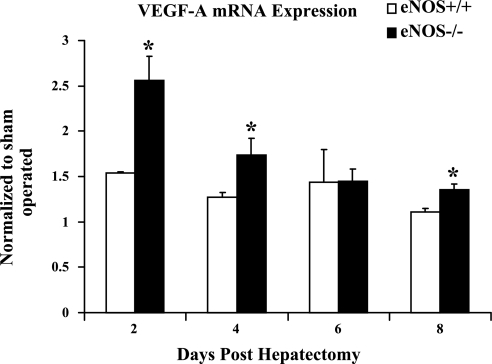
Finally, since 2 days following partial hepatectomy there is a peak in hepatocyte proliferation with an accompanying increase in VEGF protein levels, we also examined VEGF mRNA levels to test for differences in eNOS−/− and wild-type mice after partial hepatectomy. Indeed, VEGF mRNA levels in both wild-type and eNOS−/− mice were increased compared with the sham-operated animals, with eNOS−/− mice displaying significantly higher VEGF mRNA levels at days 2, 4, and 8 (Fig. 5). Similar results were not seen with the VEGF receptor, Flk-1 (Supplemental Fig. 1). These results indicate that increased VEGF expression that occurs in eNOS−/− mice after partial hepatectomy could compensate for deficiency of eNOS-derived NO.
Fig. 5.
VEGF mRNA levels increase in eNOS−/− mice to a greater extent than wild-type mice posthepatectomy: VEGF mRNA levels were measured in the wild-type and eNOS−/− mice after partial hepatectomy. eNOS−/− animals showed a significant increase in VEGF mRNA levels compared with the wild-type mice at days 2, 4, and 8 (n = 3 animals/group; *P < 0.05).
Mice genetically heterozygous for Flk-1 have normal regenerative capacity.
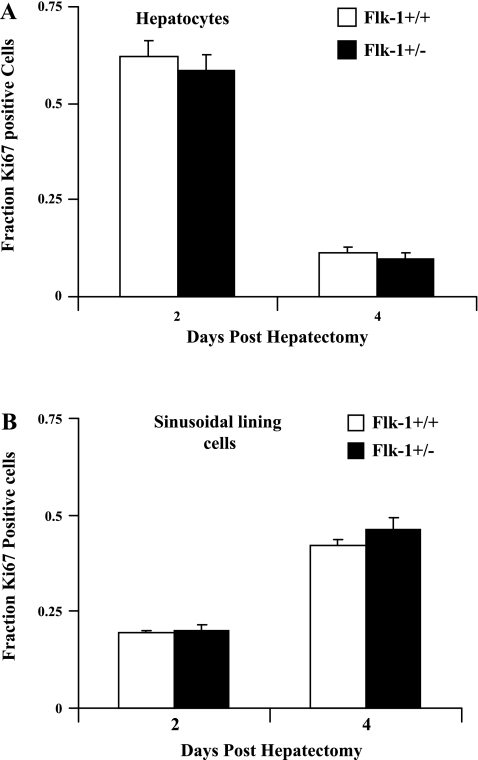
Because we saw an upregulation of VEGF in eNOS knockout mice and a number of changes in SEC gene profile, which could potentially contribute to the normal angiogenic response in these animals following hepatectomy, we next looked at the regenerative process in mice heterozygous for Flk-1 (VEGF-R2) and their wild-type littermate controls (n = 3 for each group at each time point). Heterozygous mice were chosen for study because homozygous deletion of Flk-1 is lethal (43). Interestingly, even though the VEGF-Flk-1 axis is a well-known canonical signaling pathway involved in endothelial cell angiogenesis (17, 23, 49), there was no statistically significant difference in the proliferation of the SLC nor parenchymal cells as assessed by Ki-67 immunostaining of frozen sections between the Flk-1+/− mice and their controls in response to partial hepatectomy (Fig. 6A). These data indicate that a partial reduction of Flk-1 levels does not compromise the liver regenerative process.
Fig. 6.
Normal regeneration in mice genetically heterozygous for fetal liver kinase-1 (Flk-1). Flk-1+/+ and Flk-1+/− mice were subjected to partial hepatectomy, and the remnant liver was removed at day 2 (n = 3 for Flk-1+/− and n = 3 for Flk-1+/+) and day 4 (n = 3 for Flk-1+/− and n = 3 for Flk-1+/+) for analysis following surgery. Immunostaining of 5-μm section of the regenerated liver sections with Ki-67 was used to determine the fraction of positively staining cells in the hepatocyte population (A) or the SLC population (B) in both groups at the different time points. There were no significant differences in proliferation observed between the Flk-1+/− and Flk-1+/+ mice in hepatocyte or SLC populations (P > 0.05).
DISCUSSION
The influence of angiogenesis on liver function and pathobiology is an area of increasing interest (3, 8). Indeed, angiogenesis in liver is important, not only for liver regeneration but also in liver cirrhosis, its ensuing complications of portal hypertension, hepatocellular cancer, and recovery from liver injury or resection (9, 13, 29). However, although broadly termed as angiogenesis, each of these processes maintains distinct signaling interactions between SEC and other cell types within the sinusoidal microenvironment that culminate in changes in vascular structure and function specific to the final biological response (i.e., tumor growth, fibrosis, regeneration) (25, 38). Interestingly, the time kinetics of these biological responses varies as well, since angiogenesis associated with fibrosis is prolonged, whereas angiogenesis associated with liver regeneration is largely completed within 1 wk, concurrent with the completion of the regeneration process (30). Both NO and VEGF are dominant signal transduction molecules that are paramount for angiogenesis because of their stimulatory effects on SEC. In this context, the present findings demonstrating the dispensability of eNOS and Flk-1 for liver regeneration are surprising. However, the redundant and distinct roles of angiogenic molecules vary with experimental context. For example, eNOS-deficient mice also do not demonstrate prominent defects in portal pressure or in hepatic tumor angiogenesis (11, 21). Although future studies will be necessary to delineate the precise role of eNOS and Flk-1 in the angiogenic cascade in liver, these results, in total, support the concept of redundancy in angiogenic function in liver, which allows biological functions to continue despite absence of single key angiogenic molecules.
Liver is unique in its immense capacity to regenerate following injury or resection. In rodent models, 70% liver resection is followed by an initial phase of hepatocyte proliferation that is followed by a secondary SEC proliferative response, with this angiogenic switch thought to be required for the culminating wave of hepatocyte proliferation that leads to full restitution of hepatic mass (44). During the preangiogenic phase of regeneration, hepatocytes form avascular clusters (28) that are then infiltrated by the proliferating SEC that restore the normal lobular architecture of this nascently regenerating liver. However, the lack of changes in liver regeneration in eNOS−/− and Flk-1+/− mice do suggest that angiogenesis may not be the key driver in the liver regeneration process (although our observations in Flk-1+/− mice could also be attributable to the available Flk-1 protein levels generated by the single allele).
VEGF is one of the most potent angiogenic factors because of its prominent mitogenic and motogenic effects on endothelial cells (19). Indeed, homozygous or heterozygous deletion of VEGF is embryologically lethal in mice (7). Furthermore, prior studies in the liver regeneration model have revealed an essential role of VEGF for coordination of angiogenesis and liver regeneration (37, 47). Interestingly, eNOS-derived NO is a putative second messenger for VEGF-induced proliferation and migration of endothelial cells because of direct activation of eNOS by VEGF (31, 52). For example, pharmacological inhibition of NOS abrogates VEGF-induced proliferation and migration of endothelial cells in vitro (34), and delivery of dominant-negative eNOS constructs abolishes VEGF function in vivo in animal models such as hindlimb ischemia (51). This VEGF-induced activation of eNOS is thought to occur through pathways that include cAMP-dependent protein kinase/protein kinase B (12, 18) and calcium/calmodulin (4). However, VEGF may stimulate endothelial cell proliferation through pathways independent of eNOS (5, 33). Indeed, a recent study showed that eNOS is dispensable in a diabetic mouse model of endothelial cell proliferation in the glomerulus because of high VEGF levels in these mice despite the absence of eNOS (33). These results are reminiscent of the findings in our current study whereby redundant pathways are likely to be responsible for the dispensability of eNOS for angiogenesis and liver regeneration in the setting of eNOS deficiency. Indeed, VEGF also appears to compensate for fibroblast growth factor (FGF) deficiency in FGF−/− mice undergoing regeneration after partial hepatectomy (45). Similarly, the dispensability of Flk-1 in the regeneration process may be the result of redundant effects of alternative VEGF receptors or alternatively adequate signaling through the protein derived from the single Flk-1 allele present in heterozygous mice.
NO is generated through one of three isoforms [eNOS, iNOS, or nNOS (22)]. Although NO from both iNOS and eNOS have been implicated in hepatocyte responses during regeneration (36, 48), little attention has been paid to the role of NO in the angiogenic response that accompanies regeneration. Upon first evaluation of our data, we initially envisioned that the regenerative response that occurs unabated in eNOS-deficient mice may be because of compensation by iNOS, resulting from the essential role of iNOS in liver regeneration previously defined in studies by Rai et al. (36). In that study, the effect of iNOS on regeneration had been attributed mainly to the protective effect that iNOS may impart on hepatocytes against tumor necrosis factor-dependent induction of caspases, rather than the result of a direct effect of iNOS on angiogenesis per se (35). In our study, iNOS was not further increased in eNOS−/− mice beyond the increase that was observed in response to partial hepatectomy in control mice. Furthermore, the peak of iNOS activity did not occur until day 6, well after the angiogenic switch accompanying liver regeneration had already transpired. Thus, in the partial hepatectomy model, although iNOS-derived NO prominently influences hepatocyte biology, its effects on angiogenesis are probably limited. These observations highlight a key emerging concept pertaining to NO; effects of NO generated from different isoforms may show widely divergent biological effects because of kinetics of NO generation, proximity of NO gradients to various cell types, and the tissue redox state of the NO microenvironment.
In summary, the present study demonstrates that, although eNOS is a powerful angiogenic factor for SEC in vitro, it is not required for the EC proliferative wave that accompanies liver regeneration in vivo likely because of compensatory and redundant angiogenic mechanisms.
GRANTS
This work was funded through the Mayo Clinic and the National Institutes of Health [Grants DK-59615 and HL-86990 to V. Shah and a Silvio Conte National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Center Grant]. D. Langer was funded through an NIDDK Training Grant (V. Shah) and A. Das through the Pilot Feasibility Award by Mayo Clinic Centre on Cell Signaling in Gastroenterology.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
REFERENCES
- 1.Assy N, Spira G, Paizi M, Shenkar L, Kraizer Y, Cohen T, Neufeld G, Dabbah B, Enat R, Baruch Y. Effect of vascular endothelial growth factor on hepatic regenerative activity following partial hepatectomy in rats. J Hepatol 30: 911–915, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Bockhorn M, Goralski M, Prokofiev D, Dammann P, Grunewald P, Trippler M, Biglarnia A, Kamler M, Niehues EM, Frilling A, Broelsch CE, Schlaak JF. VEGF is important for early liver regeneration after partial hepatectomy. J Surg Res 138: 291–299, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bosch J. Vascular deterioration in cirrhosis: the big picture. J Clin Gastroenterol 41, Suppl 3: S247–S253, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Brock TA, Dvorak HF, Senger DR. Tumor-secreted vascular permeability factor increases cytosolic Ca2+ and von Willebrand factor release in human endothelial cells. Am J Pathol 138: 213–221, 1991 [PMC free article] [PubMed] [Google Scholar]
- 5.Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A. Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am J Pathol 159: 993–1008, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantre D, Schuett H, Hildebrandt A, Dold S, Menger MD, Vollmar B, Eipel C. Nitric oxide reduces organ injury and enhances regeneration of reduced-size livers by increasing hepatic arterial flow. Br J Surg 95: 785–792, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Chaparro M, Sanz-Cameno P, Trapero-Marugan M, Garcia-Buey L, Moreno-Otero R. Mechanisms of angiogenesis in chronic inflammatory liver disease. Ann Hepatol 6: 208–213, 2007 [PubMed] [Google Scholar]
- 9.Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology 35: 1010–1021, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Das A, Fernandez-Zapico ME, Cao S, Yao J, Fiorucci S, Hebbel RP, Urrutia R, Shah VH. Disruption of an SP2/KLF6 repression complex by SHP is required for farnesoid X receptor-induced endothelial cell migration. J Biol Chem 281: 39105–39113, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Decker NK, Abdelmoneim SS, Yaqoob U, Hendrickson H, Hormes J, Bentley M, Pitot H, Urrutia R, Gores GJ, Shah VH. Nitric oxide regulates tumor cell cross-talk with stromal cells in the tumor microenvironment of the liver. Am J Pathol 173: 1002–1012, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 13.El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology 27: 1554–1562, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 43: S45–S53, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 18: 4–25, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376: 66–70, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273: 30336–30343, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Hendrickson H, Chatterjee S, Cao S, Morales Ruiz M, Sessa WC, Shah V. Influence of caveolin on constitutively activated recombinant eNOS: insights into eNOS dysfunction in BDL rat liver. Am J Physiol Gastrointest Liver Physiol 285: G652–G660, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Iwakiri Y, Cadelina G, Sessa WC, Groszmann RJ. Mice with targeted deletion of eNOS develop hyperdynamic circulation associated with portal hypertension. Am J Physiol Gastrointest Liver Physiol 283: G1074–G1081, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J 298: 249–258, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroll J, Waltenberger J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem 272: 32521–32527, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Langer DA, Das A, Semela D, Kang-Decker N, Hendrickson H, Bronk SF, Katusic ZS, Gores GJ, Shah VH. Nitric oxide promotes caspase-independent hepatic stellate cell apoptosis through the generation of reactive oxygen species. Hepatology 47: 1983–1993, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JS, Kang Decker N, Chatterjee S, Yao J, Friedman S, Shah V. Mechanisms of nitric oxide interplay with Rho GTPase family members in modulation of actin membrane dynamics in pericytes and fibroblasts. Am J Pathol 166: 1861–1870, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306–1309, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Farre A, Sanchez de Miguel L, Caramelo C, Gomez-Macias J, Garcia R, Mosquera JR, de Frutos T, Millas I, Rivas F, Echezarreta G, Casado S. Role of nitric oxide in autocrine control of growth and apoptosis of endothelial cells. Am J Physiol Heart Circ Physiol 272: H760–H768, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Hernandez A, Delgado FM, Amenta PS. The extracellular matrix in hepatic regeneration. Localization of collagen types I, III, IV, laminin, and fibronectin. Lab Invest 64: 157–166, 1991 [PubMed] [Google Scholar]
- 29.Medina J, Arroyo AG, Sanchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology 39: 1185–1195, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Michalopoulos GK. Liver regeneration. J Cell Physiol 213: 286–300, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol Heart Circ Physiol 270: H411–H415, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest 101: 2567–2578, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa T. Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: an explanation for the paradoxical effects of VEGF in renal disease. Am J Physiol Renal Physiol 292: F1665–F1672, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 100: 3131–3139, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pipili-Synetos E, Sakkoula E, Maragoudakis ME. Nitric oxide is involved in the regulation of angiogenesis. Br J Pharmacol 108: 855–857, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A, Liew FY, Zaragoza C, Lowenstein C, Diehl AM. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci USA 95: 13829–13834, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redaelli CA, Semela D, Carrick FE, Ledermann M, Candinas D, Sauter B, Dufour JF. Effect of vascular endothelial growth factor on functional recovery after hepatectomy in lean and obese mice. J Hepatol 40: 305–312, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Rieder H, Armbrust T, Meyer zum Buschenfelde KH, Ramadori G. Contribution of sinusoidal endothelial liver cells to liver fibrosis: expression of transforming growth factor-beta 1 receptors and modulation of plasmin-generating enzymes by transforming growth factor-beta 1. Hepatology 18: 937–944, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Ross MA, Sander CM, Kleeb TB, Watkins SC, Stolz DB. Spatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liver. Hepatology 34: 1135–1148, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Rutherford A, Chung RT. Acute liver failure: mechanisms of hepatocyte injury and regeneration. Semin Liver Dis 28: 167–174, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Santoro MM, Samuel T, Mitchell T, Reed JC, Stainier DY. Birc2 (cIap1) regulates endothelial cell integrity and blood vessel homeostasis. Nat Genet 39: 1397–1402, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterology 135: 671–679, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376: 62–66, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Shimizu H, Miyazaki M, Wakabayashi Y, Mitsuhashi N, Kato A, Ito H, Nakagawa K, Yoshidome H, Kataoka M, Nakajima N. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol 34: 683–689, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Sturm J, Keese M, Zhang H, Bonninghoff R, Magdeburg R, Vajkoczy P, Dono R, Zeller R, Gretz N. Liver regeneration in FGF-2-deficient mice: VEGF acts as potential functional substitute for FGF-2. Liver Int 24: 161–168, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Thirunavukkarasu M, Addya S, Juhasz B, Pant R, Zhan L, Surrey S, Maulik G, Menon VP, Maulik N. Heterozygous disruption of Flk-1 receptor leads to myocardial ischaemia reperfusion injury in mice: application of affymetrix gene chip analysis. J Cell Mol Med 12: 1284–1302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Buren G, 2nd, Yang AD, Dallas NA, Gray MJ, Lim SJ, Xia L, Fan F, Somcio R, Wu Y, Hicklin DJ, Ellis LM. Effect of molecular therapeutics on liver regeneration in a murine model. J Clin Oncol 26: 1836–1842, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Vazquez-Chantada M, Ariz U, Varela-Rey M, Embade N, Martinez-Lopez N, Fernandez-Ramos D, Gomez-Santos L, Lamas S, Lu SC, Martinez-Chantar ML, Mato JM. Evidence for LKB1/AMP-activated protein kinase/ endothelial nitric oxide synthase cascade regulated by hepatocyte growth factor, S-adenosylmethionine, and nitric oxide in hepatocyte proliferation. Hepatology 49: 608–617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 269: 26988–26995, 1994 [PubMed] [Google Scholar]
- 50.Yamane A, Seetharam L, Yamaguchi S, Gotoh N, Takahashi T, Neufeld G, Shibuya M. A new communication system between hepatocytes and sinusoidal endothelial cells in liver through vascular endothelial growth factor and Flt tyrosine kinase receptor family (Flt-1 and KDR/Flk-1). Oncogene 9: 2683–2690, 1994 [PubMed] [Google Scholar]
- 51.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci USA 102: 10999–11004, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 99: 2625–2634, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest 94: 2036–2044, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.