Abstract
Insulin-like growth factor I (IGF-I) coordinates proliferation and differentiation in a wide variety of cell types. The igf1 gene not only produces IGF-I, but also generates multiple carboxy-terminal extensions, the E-peptides, through alternative splicing leading to different isoforms. It is not known if the IGF-I isoforms share a common pathway for their actions, or if there are specific actions of each protein. Viral administration of murine IGF-IA, IGF-IB, and mature IGF, which lacked an E-peptide extension, was utilized to identify IGF-I isoform-specific responsive genes in muscles of young growing mice. Microarray analysis revealed responses that were driven by increased IGF-I regardless of the presence of E-peptide, such as Bcl-XL. In contrast, distinct expression patterns were observed after viral delivery of IGF-IA or IGF-IB, which included matrix metalloproteinase 13 (MMP13). Expression of Bcl-XL was prevented when viral administration of the IGF-I isoforms was performed into muscles of MKR mice, which lack functional IGF-I receptors on the muscle fibers. However, MMP13 expression persisted under the same conditions after viral injection of IGF-IB. At 4 mo after viral delivery, expression of IGF-IA or IGF-IB promoted muscle hypertrophy, but viral delivery of mature IGF-I failed to increase muscle mass. These studies provide evidence that local production of IGF-I requires the E-peptides to drive hypertrophy in growing muscle and that both common and unique pathways exist for the IGF-I isoforms to promote biological effects.
Keywords: alternative splicing, Bcl-XL, matrix metalloproteinase 13, MKR mouse
insulin-like growth factor-I (IGF-I) is central to tissue growth in vertebrate species. The igf1 gene, which encodes IGF-I, undergoes alternative splicing in mammals and fish, producing multiple isoforms. In all cases, the splice forms retain a highly conserved 70-residue sequence for mature IGF-I, and it is this protein that is widely recognized as the primary agent responsible for promoting proliferation and differentiation via the IGF-I receptor in a variety of cell types. However, alternative splicing also produces divergent peptides at the carboxyl terminus, called the E peptides, which share only 50% homology (1, 4, 29). There are two IGF-I different isoforms found in rodents, dogs, sheep, and cattle, called IGF-IA and IGF-IB (29, 42); three isoforms have been observed in humans and nonhuman primates (IGF-IA, IGF-IB, IGF-IC) (47); and at least four have been identified in fish (41). In mammals, IGF-IA is virtually identical. Rodent IGF-IB and human IGF-IC bear high homology and have also been called mechanogrowth factor (MGF) (50). Human IGF-IB appears to be unique to primates, for it has not been found in rodents or fish.
Activities of synthetic peptides similar to two of the alternative splicing products include cell proliferation, migration, and survival and have been observed in a number of different conditions. Increased proliferation occurs in neuroblastoma cells, bronchial epithelial cells, and myoblasts after exposure to human E-peptides from IGF-IB or IGF-IC (EB and EC, respectively) (25, 43, 50). Human EC has also been shown to enhance the migration of myoblasts after transplantation into muscle and to protect neurons from ischemic brain injury (12, 33). No independent activity of EA has been reported. In addition to direct actions, the E peptides appear to modulate IGF-I activity in vitro, for expression of pro-IGF-I, which retains the E peptides, affords enhanced uptake of IGF-I compared with expression of mature IGF-I alone (38). Thus the E peptides have both direct and indirect biological actions that contribute to the growth-promoting effects of IGF-I.
Because the role of IGF-I has been well described for skeletal muscle growth, this tissue has served as a model system to understand the actions and regulation of the IGF-I isoforms. To date, infusion of recombinant IGF-I that consists solely of the mature protein, and transgenic or viral expression of IGF-IA have consistently caused increased muscle mass (2, 5, 7, 10, 35, 40). However, the hypertrophic effects of rodent IGF-IB (homologous to human IGF-IC) requires growing muscle or an active population of satellite cells (5). Finally, cardiac-specific transgenic expression of human IGF-IB, which is the unique isoform, causes cardiomyocyte proliferation resulting in cardiomegaly (39). Therefore, even though these isoforms share a common sequence for mature IGF-I, there is clear divergence in the effects of this growth factor on muscle tissue that is dependent on the E peptide extension.
Previous studies have measured the expression, signal transduction pathways, and the physiological effects of the IGF-I isoforms and E peptides in muscle, but little is known about the changes in gene expression caused by these isoforms. Given that there are key differences in the ultimate effects of the growth factors, it is likely that the divergence occurs at the transcriptional level before increases in tissue mass or cell number. Recent correlation of human IGF-IC expression to the onset of satellite cell proliferation and the myogenic program in response to muscle damage has been observed, but no causative role between IGF-IC and stimulation of the myogenic program could be established (31). To determine if the IGF-I isoforms render specific patterns of expression, viral delivery of murine IGF-IA, IGF-IB, and mature IGF-I was performed into the hindlimb skeletal muscles of young growing mice. The goal of this study was to identify genes that are specific for mature IGF-I or the IGF-I isoforms and to evaluate the ability of local production of mature IGF-I for promoting muscle hypertrophy.
MATERIALS AND METHODS
Animals.
All experiments were approved by the university animal care committee. The animals utilized for this study included C57Bl/6 (C57) mice 2–3 wk of age at the start of the experiment. Also utilized was the MKR mouse, which harbors a dominant negative IGF-I receptor under the muscle creatine kinase (MCK) promoter (18). The MKR mice were backcrossed for 10 generations onto the C57 strain before their use in this study.
Viral constructs.
Murine class I Igf-1A and Igf-1B (GenBank AY878192 and AY878193, respectively) were used to generate recombinant adeno-associated virus serotype 2/8 (rAAV) as previously described (5). In addition, a third construct was generated that expressed only mature IGF-I without either E-peptide (IGF-Istop) (38). Transgene expression was regulated by the myosin light chain kinase 1/3 promoter/enhancer (14). An rAAV expressing beta-galactosidase (βGal) regulated by the chicken beta-actin promoter was also utilized as a control for validation of gene expression. Vector production was performed at the University of Pennsylvania Vector Core.
Injections.
The anterior hindlimb muscles of animals were injected with 1 × 1011 rAAV particles diluted in 75–100 μl phosphate-buffered saline (PBS) or PBS alone as previously described (5). Mice were killed at 1 and 4 mo postinjection for analysis. At 1 mo postinjection, the tibialis anterior (TA) and extensor digitorum longus (EDL) muscles were dissected, washed in PBS, blotted, weighed, and frozen rapidly in liquid nitrogen for subsequent measurements. At 4 mo postinjection, the EDL was also utilized for isolated force measurements as previously described (5).
Expression profiling.
To identify differences between IGF-I isoforms at the molecular level, expression profiling was performed as previously described (17) on total RNA isolated from the TA muscles of mice injected with rAAV IGF-IA, IGF-IB, IGF-Istop, or saline (n = 3 per condition) at 1 mo postinjection. Profiling measurements were completed at the Penn Microarray Core Facility utilizing Affymetrix Mouse 430 2A chips. All protocols were conducted as described in the Affymetrix GeneChip Expression Analysis Technical Manual. The Affymetrix Microarray Suite 5.0 algorithm in GeneChip Operating Software (GCOS) was used to quantify expression levels for targeted genes; default values provided by Affymetrix were applied to all analysis parameters. The sample quality for the profiling experiment is shown in Supplemental Table 1, available with the online version of this article.
Only transcripts that received a present or marginal call in all TA samples were utilized for comparisons between tissues. For those transcripts present in all samples, comparisons of the probe set signals were performed between the viral-injected and PBS-injected TA muscles using significance analysis of microarrays (SAM) (46) to detect >1.5-fold changes among samples with a false discovery rate < 7%. Gene ontology of the differentially expressed transcripts was performed using Affymetrix analysis tools (NetAffx).
Quantitative RT-PCR.
Total RNA was isolated as described above from TA muscles of additional C57 mice. RNA integrity was confirmed by gel electrophoresis. Equal amounts of total RNA from each sample were subjected to single-strand reverse transcription (Applied Biosystems, Foster City, CA). The resultant cDNA was utilized for quantitative RT-PCR (qRT-PCR) with oligonucleotides specific for genes listed in Table 1 using the Roche Lightcycler system, and reagents (LightCycler FastStart DNA MasterPLUS SYBR Green I; Roche Applied Science, Indianapolis, IN) as previously described (5). Controls included RNA not subjected to reverse transcription, and water only.
Table 1.
Validation of microarray results by qRT-PCR
| Bcl2l1 (NM_009743) | Mmp13 (NM_008607) | |
|---|---|---|
| Oligonucleotides (5′-3′) | ||
| Sense | gctgggacacttttgtggat | agttgacaggctccgagaaa |
| Antisense | tgtctggtcacttccgactg | cacatcaggcactccacatc |
| Band, bp | 150 | 112 |
| Expression difference (vs. control) | ||
| AAVIGF-IA | 3.01 ± 0.78* | 5.19 ± 2.06* |
| AAVIGF-IB | 6.94 ± 1.82* | 4.34 ± 0.78* |
| AAVIGF-Istop | 3.54 ± 0.48* | 1.92 ± 0.76 |
| AAVβGal | 1.17 ± 0.25 | 1.85 ± 0.72 |
Data are represented as means ± SE for n = 4 samples per muscle.
P < 0.05 vs. PBS-injected control.
Immunoblotting.
Muscles were removed from liquid nitrogen and utilized for immunoblotting as previously described (n = 4 per condition) (5, 17). Primary antibodies included those for phosphorylated and total Akt (nos. 9271, 9272, Cell Signaling, Beverly, MA); Bcl-XL (no. 2764, Cell Signaling); MMP13 (IM78, Calbiochem); and tubulin (T5168, Sigma-Aldrich, St Louis, MO).
Detection of IGF-I protein.
Frozen TA muscles were homogenized in PBS, and frozen at −80°C overnight. The TA homogenates were thawed and centrifuged at 5000 g for 5 min. The supernatants were utilized for measurements of total IGF-I as previously described (5, 38) in a commercially available ELISA kit specific for rodent IGF-I (R&D Systems, Minneapolis, MN), and calculations of IGF-I content were based on a standard curve generated from recombinant mouse IGF-I. Measurements were acquired on a microtiter-plate reader (Dynatech Laboratories, Chantilly, VA) at 450 nm. All samples were measured in duplicate.
Zymography.
Muscles were homogenized in buffer containing 100 mM Tris·HCl, pH 7.6, 200 mM NaCl, 100 mM CaCl2 and 1% Triton X-100 and then centrifuged at 12,000 rpm for 15 min. Fifty micrograms of the muscle homogenate was incubated with equal volume of nonreducing sample buffer containing 62.5 mM Tris·HCl, 10% glycerol, 2% SDS, and 0.01% bromophenol blue and then subjected to gel electrophoresis on zymogram gels. Gels consisted of 10% polyacrylamide impregnated with 1 mg/ml collagen (Sigma) (19). After electrophoresis, gels were washed twice for 1 h at room temperature in 100 ml of 2.5% Triton X-100 and then incubated 24 h at 37°C in substrate buffer containing 50 mM Tris·HCl, 5 mM CaCl2, 200 mM NaCl, and 0.02% Brij 45. Thereafter, the gels were stained with 0.25% Coomassie Blue R-250 (in 50% methanol, 10% acetic acid) for 2 h and destained with aqueous 10% methanol and 10% acetic acid. Image analysis was performed on the Kodak mm4000 detection system (Eastman Kodak, Rochester, NY).
Statistics.
Data are presented as means ± SE. Paired t-tests were utilized for comparisons between AAV-injected and contralateral control samples. One-way ANOVA followed by Tukey post hoc analysis was utilized for comparisons between IGF-I isoform treatments and mouse strains. Statistical significance was accepted for P < 0.05.
RESULTS
Microarray analysis identifies IGF-I isoform-specific genes.
Expression profiling was used as an initial screen to identify genes responsive to the specific IGF-I isoforms. The quality of the microarray data is summarized in Supplemental Table 1. Ratios of 3′:5′ for beta-actin and GAPDH were less than 2.0 for all samples. The percentage of transcripts expressed in all samples utilized for analysis exceeded 70% of the total number of probe sets. Of more than 22,000 probe sets represented on the chip, ∼17,000 transcripts were present or marginal in all TA samples.
Comparison of signal strength was performed between all viral injection samples and control samples. Of these, 223 transcripts were expressed at >1.50-fold differences (both increasing and decreasing) compared with PBS-injected controls (see Supplemental Fig. 1 available with the online version of this article). The transcripts were also classified by the viral construct(s) that caused changes in expression (Fig. 1). There were a total of 79 transcripts that increased or decreased in response to viral delivery of IGF-Istop, IGF-IA, or IGF-IB, regardless of the isoform expressed. These included increased expression of Bcl2l1, which encodes Bcl-XL, an inhibitor of programmed cell death (3). There were 109 transcripts that increased or decreased when IGF-IA or IGF-IB was expressed, but not after viral delivery of IGF-Istop (mature IGF-I). The most dramatic change observed in this group was Mmp13, which encodes matrix metalloproteinase 13 (MMP13), where there was more than a 10-fold increase in expression after viral expression of IGF-IA or IGF-IB. Validation of these of transcripts was performed by qRT-PCR (Table 1). Changes in expression were similar to those observed by microarray; injection of rAAV βGal did not cause any significant change in Bcl2l1 or Mmp13 expression. For further measurements, Bcl-XL was used to represent genes responsive to all IGF-I constructs, and MMP13 was used to represent genes responsive to IGF-IA and IGF-IB.
Fig. 1.
Distribution of changing transcripts with respect to IGF viral construct from microarray analysis. Viral delivery of either IGF-I isoform or mature IGF-I (IGFStop) caused changes in 79 transcripts. However, 109 transcripts changed only when the viral construct retained the E peptide (IGF-IA and IGF-IB), and not in response to mature IGF-I expression. A complete list of all changing transcripts is shown in Supplemental Fig. 1, available with the online version of this article.
Transgenic inhibition of the IGF-I receptor identifies genes that do not require receptor signaling.
The same viral constructs were injected into both C57 and MKR mice to determine the dependence of gene expression on the presence of functional IGF-I receptors in skeletal muscle. At 1 mo after viral injection, muscles were examined for IGF-I production, signal transduction through the IGF-I receptor, and protein levels of MMP13 and Bcl-XL.
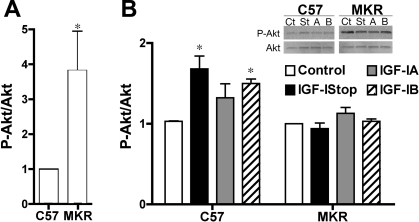
Total IGF-I content in the muscle tissue increased significantly in all rAAV-injected muscles compared with untreated controls (Fig. 2). Injection of constructs harboring IGF-Istop resulted in the highest level of IGF-I content in both C57 and MKR muscles. Signal transduction via the IGF-I receptor was intact in the C57 animals, where rAAV-injected muscles showed a 30–70% increase in phosphorylated Akt (P-Akt) compared with uninjected muscles, as shown in Fig. 3. In contrast, injection of the same rAAVs into MKR muscles did not cause increases in P-Akt, although basal P-Akt in MKR muscles was >2-fold higher than that in C57 muscles. Because MKR mice lack functional IGF-I receptors only in skeletal muscle, we concluded that the actions of IGF-I after viral injection of all IGF-I isoforms were mediated predominantly through IGF-IR on the skeletal muscle fibers.
Fig. 2.
IGF content in skeletal muscles 1 mo after recombinant adeno-associated virus serotype 2/8 (rAAV) injection. Total IGF-I was measured by ELISA for n = 4 muscles per condition. IGF-I content was significantly increased in tibialis anterior (TA) muscles by all IGF viral constructs compared with strain-matched controls (*P < 0.05 vs. control by 1-way ANOVA followed by Tukey test). Injection of rAAV IGF-Istop, which produced mature IGF-I, resulted in higher IGF-I levels than that measured in muscles injected with rAAV IGF-IA (†P < 0.05). Data are presented as means ± SE.
Fig. 3.
Phosphorylated Akt (P-Akt) levels as an indicator of IGF-I receptor signal transduction. A: relative basal P-Akt levels are 4-fold higher in untreated MKR muscles compared with C57 muscles. *P < 0.05 by unpaired t-test. B: relative P-Akt levels after viral injection of IGF-I isoforms. Levels are comparisons between injected and uninjected limbs for n = 4 animals per condition. Inset shows an immunoblot for phosphorylated and total Akt 1 mo after viral injection for all IGF viral constructs: Ct, Control; St, IGF-Istop; A, IGF-IA; B, IGF-IB. Injection of rAAV expressing IGF-Istop, IGF-IA, or IGF-IB caused a 30–70% increase in P-Akt compared with uninjected controls in C57 mice. However, the same injections into MKR mice did not result in increased P-Akt. Data are presented as means ± SE; *P < 0.05 by unpaired t-test between treated and untreated muscles for each IGF-I construct.
Immunoblotting was utilized to determine the levels of Bcl-XL and MMP13 after rAAV injection of the IGF isoforms (Fig. 4). In muscles of C57 mice, rAAV injection resulted in a twofold increase in Bcl-XL regardless of IGF-I isoform (Fig. 4, A and B). However, this increase was ablated when the same injections were performed into muscles of MKR mice. In addition, Bcl-XL levels were significantly lower in untreated MKR mouse muscles compared with those from untreated C57 mice. The levels of MMP13 showed a different response to rAAV injection (Fig. 4, A and C). The muscles from C57 mice displayed an increase in MMP13 when injected with rAAV IGF-IA or rAAV IGF-IB, but there was no increased MMP13 after injection with rAAV IGF-Istop. Further, only rAAV IGF-IB injections resulted in a significant increase in MMP13 in muscles from MKR mice. MMP13 levels in muscles from MKR mice injected with rAAV IGF-IB appeared lower than the same condition in C57 mice, but the difference was not significant.
Fig. 4.
Immunoblot analysis of IGF-responsive genes. A: representative blot of Bcl-XL and matrix metalloproteinase 13 (MMP13) protein in control and rAAV-injected muscles 1 mo after injection. B: Bcl-XL protein significantly increased after viral delivery of IGF-Istop (St), IGF-IA (A), and IGF-IB (B) in C57 muscles but did not change in MKR muscle after the same treatment. Untreated control MKR muscles had significantly less Bcl-XL than untreated C57 muscles. C: a significant increase of MMP13 occurred in C57 muscles after viral injection of IGF-IA or IGF-IB but not after injection of IGF-Istop. Only injection of rAAV IGF-IB resulted in increased MMP13 in MKR muscles. *P < 0.05 vs. strain-matched controls; †P < 0.05 MKR vs. C57 for untreated muscles (1-way ANOVA followed by Tukey test, n = 3–4 for each condition).
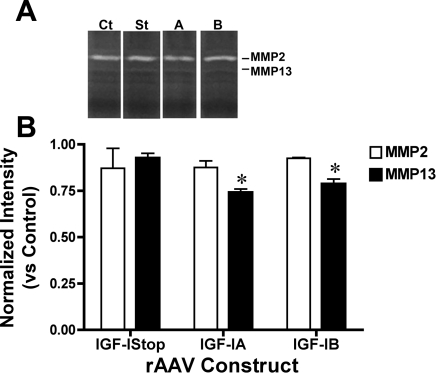
Collagen zymography was used to determine if increased MMP13 protein after viral injection led to a change in MMP13 activity (Fig. 5). The predominant band on the zymography gels was MMP2, which showed no change in any of the treated or control samples. MMP13 activity was ∼10% of the level of MMP2 in all of the samples. Comparisons of treatment groups to uninjected controls revealed that there was a decrease in MMP13 activity after injection of rAAV IGF-IA or rAAV IGF-IB, but not after injection of rAAV IGF-Istop. Thus, even though there was an increased production of MMP13 protein by the IGF-IA and IGF-IB constructs, this did not lead to enhanced MMP13 activity.
Fig. 5.
MMP activity after rAAV injection. A: collagen zymography of muscle lysates from C57 muscles after viral injection of IGF-Istop (St), IGF-IA (A), IGF-IB (B), or PBS (Ct). B: MMP13 activity decreased after viral injection of IGF-IA or IGF-IB compared with saline-injected controls. MMP2 activity was not affected by viral injection. *P < 0.05 vs. strain-matched controls (1-way ANOVA followed by Tukey test on n = 3 samples).
Intact IGF-I receptors are required for muscle hypertrophy.
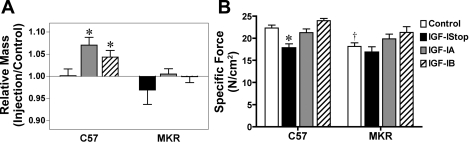
To determine if the IGF-I isoforms led to an increase in muscle mass and force production, muscles were analyzed at 4 mo of age after injection of rAAV at 2 wk of age (Fig. 6). As shown previously (5), viral expression of IGF-IA and IGF-IB caused a significant increase in relative muscle mass compared with the mass in the contralateral control limbs. Hypertrophy was blocked in the MKR mice treated with the same rAAV. Specific force did not change between control muscles and those injected with IGF-IA or IGF-IB in both C57 and MKR mice, However, there was a significant decrease in force production between control C57 and MKR muscles, similar to previous reports (23). These results demonstrate that the IGF-I receptors on the muscle fibers are required for IGF-I-mediated hypertrophy.
Fig. 6.
Muscle mass and force generation 4 mo after viral injection. A: viral injection of IGF-IA and IGF-IB resulted in a significant increase in muscle mass compared with the contralateral control muscle in C57 mice but did not cause hypertrophy after injection into muscles of MKR mice. Injection of IGF-Istop did not cause increase muscles mass in either mouse strain. B: specific tetanic force in C57 muscles was not affected by viral injection of IGF-IA or IGF-IB but decreased after injection of IGF-Istop. No change in specific force was observed in MKR muscles after viral injection compared with controls. Without viral injection, the MKR muscles were weaker than those from C57 mice. *P < 0.05 vs. strain-matched controls; †P < 0.05 MKR vs. C57 for untreated muscles (1-way ANOVA followed by Tukey test, n = 4–8 for each condition).
Viral expression of IGF-Istop does not cause muscle hypertrophy.
We extended our previous examination of IGF-I mediated hypertrophy to the effects of mature IGF-I (IGF-Istop). Unlike viral expression of either IGF-I isoform, there was no hypertrophy observed in C57 muscles after viral expression of IGF-Istop, which encodes mature IGF-I without any E peptide (Fig. 6A). Further, the IGF-Istop-injected muscles produced significantly less force than controls (Fig. 6B). Therefore, it appears that muscle-specific expression of IGF-I in the absence of E-peptides is ineffective at causing functional hypertrophy.
DISCUSSION
The goal of this study was to determine if the IGF-I isoforms promoted a pattern of gene expression that differed from mature IGF-I alone. We found unique patterns of gene expression by microarray after viral injection of IGF-IA and IGF-IB compared with injection of mature IGF-I. From this group of genes, we used Bcl2l1 to represent genes responsive to all IGF-I viral injections, and used Mmp13 to represent genes that were responsive only after viral injection of IGF-IA or IGF-IB, but not after injection of mature IGF-I. Specification of the genes that required an intact IGF-I signaling pathway was performed using the MKR mouse, which lacks functional IGF-I receptors on skeletal muscle fibers. Viral injection of the IGF-I isoforms into the MKR mouse ablated changes in Bcl2l1/Bcl-XL; however, changes in MMP13 persisted only after viral injection of IGF-IB. Finally, we found that only viral delivery of IGF-IA or IGF-IB promoted functional hypertrophy in muscles of C57 mice, whereas delivery of mature IGF-I failed to cause an increase in muscle mass. To our knowledge, this is the first study to demonstrate that there are both common and divergent patterns of gene expression and physiological effects regulated by the IGF-I isoforms.
IGF-I-dependent effects.
The IGF-I dependent effects in this study encompassed cell survival and several genes involved in proliferation and differentiation. These effects have been well documented in previous studies examining the effects of increased IGF-I. Microarray analysis of C2C12 cells treated with recombinant IGF-I demonstrated that activation of the PI3K/Akt pathway is critical for the regulation of growth promoting genes (26), for pharmacological inhibition of this pathway prevents upregulation of the muscle hypertrophy program. In our study, we utilized transgenic inhibition of muscle IGF-I receptors, which effectively blocked Akt phosphorylation after viral expression of any IGF-I constructs. Not only did this prevent hypertrophy, but it also prevented upregulation of Bcl-XL. These results do not preclude the activation of the PI3K/Akt pathway through other receptors or complexes, leading to hypertrophy or changes in Bcl-XL. For example, functional overload can still result in hypertrophy in the MKR mice and activation of the PI3K/Akt pathway, possibly through focal adhesion kinase, even though IGF-I receptor activity is blocked (44). Further, Akt phosphorylation is significantly higher in MKR muscles compared with wild-type controls (Fig. 3), indicating that the intracellular components of the pathway are intact and must be activated by proteins other than the IGF-I receptor. However, it appears that the dominant pathway mediating growth effects of IGF-I in vivo is consistent with the observations in culture and requires the IGF-I receptor.
Since it is clear that the IGF-I receptor is required for IGF-I-mediated hypertrophy, we were surprised by the lack of hypertrophy after viral delivery of mature IGF-I, even though there was increased phosphorylation of Akt and more IGF-I was produced by this viral construct compared with IGF-IA or IGF-IB. This counters previous results using infusion of recombinant IGF-I (2, 40), where an increase of circulating or exogenous IGF-I does lead to an increase of muscle mass. Because the ELISA assay of IGF-I protein measure the content of the muscle tissue, we cannot distinguish between protein that has been secreted from the muscle fibers and that remaining within the muscle fibers. Thus, even though we detected two to three times more IGF-I protein after viral expression of mature IGF-I, the entire pool of IGF-I may not be available in the extracellular space to bind and activate IGF-I receptors. From our previous study, and those before us, it is clear that pro-IGF-I can be secreted (11, 15, 38, 48), and that the secretion of IGF-I is highly regulated by chaperone proteins, such as GRP94 (36). However, it is not clear if the pro-IGF-I species are the predominant forms needed for secretion. Our results support that it is possible for mature IGF-I to be secreted from muscle fibers in vivo, consistent with our previous in vitro study (38). More importantly, our results suggest there are key differences in the growth factor expressed and produced locally compared with the growth factor delivered by the circulation. Distinctions between paracrine and endocrine actions of IGF-I have been proposed in the characterization of transgenic mice expressing IGF-I specifically in skeletal muscle (35), and by comparison of local and systemic routes of administration (40), but the mechanisms underlying the specification of action by local and circulating IGF-I have eluded the field. In culture, we observed that secretion of IGF-I from cells after transfection was not dependent on the IGF-I construct; however, the entry of IGF-I (an indirect indicator of receptor binding) was enhanced when IGF-IA or IGF-IB was expressed compared with transfection of mature IGF-I (38). In the present study, we have now observed that viral expression of IGF-IA or IGF-IB promotes the hypertrophic response of muscle in vivo, an effect that is distinctly absent with viral expression of mature IGF-I (Fig. 6). In addition, expression of mature IGF-I led to a loss of specific force in C57 muscles, suggesting that this form of the protein counters the expected functional gains by IGF-I. One possibility is that if a significant portion of mature IGF-I is retained in the muscle fibers, then it may impair the generalized increase in protein synthesis/secretion normally stimulated by IGF-I activity. Microarray analysis identified a twofold downregulation of Fbxo32 (aka MAFbx) only after viral expression of IGF-IA (Supplemental Fig. 1), which is similar to the change observed in myotubes exposed to IGF-I (26). However, neither IGF-IB nor mature IGF-I affected expression of this atrophy-associated gene. Because the apparent difference between these IGF-I constructs lies in the E-peptide extensions, another plausible explanation is that the E-peptides enhance the effects of local IGF-I, yet direct evidence of this has yet to be obtained. These changes may reflect coordination between mature IGF-I and the E-peptides that are produced after viral expression, or activity driven by the E peptides; these and other possibilities should be directly addressed in future studies.
IGF-I isoform vs. E peptide effects in muscle.
In the MKR mice, transgenic expression of the dominant negative IGF-IR is driven by the MCK promoter, which is highly active in the muscles utilized for this study (EDL and TA) (18). Therefore, the transgene effectively blocks IGF-I receptor and hybrid receptor (heterodimers of IGF-I receptor and insulin receptors) in muscle tissue, as shown by the inhibition of receptor signaling (Fig. 3). However, other cell types, such as fibroblasts and monocytes harboring intact IGF-I signaling, still reside in muscle, and may be the source of increased MMP13 production after viral delivery of IGF-IB. Indeed, previous studies have shown that IGF-IA (mIGF-1) is a chemoattractant for multiple cell types involved in the inflammatory response during muscle regeneration (37), and so a recruitment mechanism for a specific cell type expressing MMP13 could explain the IGF-IB response in our study. Observations of skeletal muscle specific expression of MMP13 were reported in a wound healing study (49), supporting the possibility that muscle may be at least one source of MMP13.
Another cell source may be satellite cells, which do not express MCK until after the inception of differentiation (9, 16), and so the transgene may not block initial IGF-I receptor signaling. However, satellite cells respond to IGF-I only after activation (8), so there is only a narrow temporal window in which satellite cells can respond to IGF-I before the onset of expression of the dominant negative transgene, which is regulated by the MCK promoter. Given the level of MMP13 production in the MKR mouse after injection of rAAV IGF-IB, this subpopulation of satellite cells would need to produce MMP13 several orders of magnitude higher than we measured in the bulk tissue to be the sole source of this protein.
Alternatively, IGF-IB, or the EB peptide alone, acts through an independent receptor to cause increased MMP13. Several in vitro studies have shown that rodent EB and its homologs retain activity in the presence of neutralizing antibodies for IGF-IR (25, 50), but the identity of the E-peptide “receptor” is unknown. Our results could be explained by a similar mechanism, although we have no definitive proof at this point. Future studies are needed to address the source of the increased MMP13, and to determine the active component of IGF-IB that is driving these changes.
The fact that IGF-IA and IGF-IB increased muscle mass in C57 mice, but failed to do so in MKR mice, supports that IGF-I receptors on muscle fibers are required for hypertrophy mediated by IGF-I, regardless of which isoform is expressed. While Goldspink and Yang (20) have proposed that IGF-IB/MGF is the more potent form of the growth factor for driving muscle hypertrophy, we have not observed a significant difference in the effects of IGF-IA or IGF-IB in young murine muscle (5). If anything, IGF-IB appears to have no effect on muscle mass in mature animals. Other effects of the E peptide extensions, including increased proliferation (43, 50) or potentially the change in MMP13, may not require the IGF-I receptor. Generating the E peptides independently of IGF-I will help to clarify these unique properties.
Roles of Bcl-XL and MMP13 in skeletal muscle.
The rationale for utilizing Bcl-XL and MMP13 as IGF-I dependent and independent genes was based on the robust and consistent response 1 mo after viral delivery of the mature IGF-I, IGF-IA, and IGF-IB, even though there are hundreds of genes that respond to viral expression of the IGF-I isoforms (Fig. 1 and Supplemental Fig. 1). However, these proteins may also delineate distinct functions of the isoforms in addition to their roles as biomarkers. Bcl-XL is an antiapoptotic protein that is normally found in skeletal muscle and throughout myogenesis (13). Although more attention has been placed on Bcl-2 in this gene family for muscle, activation of Bcl-XL can occur via the paired box transcription factor Pax3 (30), and the prosurvival properties appear beneficial for muscle disease (24, 32, 45). Loss of Bcl-XL through gene targeting is embryonic lethal (34), but the availability of tissue-specific Bcl-XL deficiency (22) could be utilized to determine the specific role of this protein in skeletal muscle.
MMP13 is a member of the collagenase sub-family, which also includes MMP1 and MMP8. MMP13 is a very potent degrading enzyme of the extracellular matrix (ECM) and a key activator of other MMPs. It cleaves the interstitial collagens I, II, III, and IV. It also has potent activity against aggrecan, perlecan, fibronectin, fibrillin, and potentially biglycan (27). All of these are components of the skeletal muscle ECM. In addition to its role in ECM degradation, MMP13 is pivotal in the MMP activation cascade. Specifically, MMP13 is activated by MMP2, MMP3, and MMP14 (a membrane-bound MMP), and in turn, can activate MMP2 and MMP9. In our study, we observed increased expression and protein, but no change in MMP13 activity (Fig. 5). This suggests that the IGF-I isoforms (or the E peptides) do not play a role in activation of the MMP cascade, and that the muscle may be poised to dramatically modify the ECM. Previous studies observed increased MMP7 after exposure to human EC (a homolog for rodent EB), which was independent of the IGF-I receptor (33). MMP7 is a matrilysin, and shares common targets of degradation with MMP13 (28). Changes in MMP7 expression were not observed in the microarray data, but these results suggest that matrix remodeling may be a generalized pathway regulated by the E peptides.
Gene targeting of MMP13 leads to defects in bone formation, as this protein is central to the endochondral ossification (21). The muscles from these mice are smaller (data not shown), but this may be an indirect effect from the striking bone phenotype. A direct role of MMP13 in muscle formation has yet to be determined, but if its matrix remodeling activities are similar in muscle as in other tissues, it may be important for the resolution of muscle damage.
In conclusion, we have demonstrated there are common and distinct actions of the IGF-I isoforms and mature IGF-I. These occur at the level of transcription and translation but also lead to clear differences in muscle growth, and potentially muscle function. The choice of isoform and the specification of their ultimate effects is an important consideration for the development of IGF-I-based therapies.
GRANTS
This study was supported by grants from the National Institutes of Health (R21-AR-056480) and the Parent Project for Muscular Dystrophy to E. R. Barton.
DISCLOSURES
No conflicts of interest (financial or otherwise) are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Zuozhen Tian for technical expertise and to Derek LeRoith for providing the MKR mouse for this study.
REFERENCES
- 1. Adamo ML, Neuenschwander S, LeRoith D, Roberts CT. Structure, expression, and regulation of the IGF-1 gene. Adv Exp Med Biol 343: 1–11, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol 84: 1716–1722, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Barton ER. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab 31: 791–797, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Barton ER. Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J Appl Physiol 100: 1778–1784, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA 95: 15603–15607, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol 115: 129–139, 1986 [DOI] [PubMed] [Google Scholar]
- 9. Chamberlain JS, Jaynes JB, Hauschka SD. Regulation of creatine kinase induction in differentiating mouse myoblasts. Mol Cell Biol 5: 484–492, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270: 12109–12116, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Conover CA, Baker BK, Bale LK, Clarkson JT, Liu F, Hintz RL. Human hepatoma cells synthesize and secrete insulin-like growth factor Ia prohormone under growth hormone control. Regul Pept 48: 1–8, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Dluzniewska J, Sarnowska A, Beresewicz M, Johnson I, Srai SK, Ramesh B, Goldspink G, Gorecki DC, Zablocka B. A strong neuroprotective effect of the autonomous C-terminal peptide of IGF-1 Ec (MGF) in brain ischemia. FASEB J 19: 1896–1898, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Dominov JA, Houlihan-Kawamoto CA, Swap CJ, Miller JB. Pro- and anti-apoptotic members of the Bcl-2 family in skeletal muscle: a distinct role for Bcl-2 in later stages of myogenesis. Dev Dyn 220: 18–26, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Donoghue M, Ernst H, Wentworth B, Nadal-Ginard B, Rosenthal N. A muscle-specific enhancer is located at the 3′ end of the myosin light-chain 1/3 gene locus. Genes Dev 2: 1779–1790, 1988 [DOI] [PubMed] [Google Scholar]
- 15. Duguay SJ, Milewski WM, Young BD, Nakayama K, Steiner DF. Processing of wild-type and mutant proinsulin-like growth factor-IA by subtilisin-related proprotein convertases. J Biol Chem 272: 6663–6670, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Dym H, Turner DC, Eppenberger HM, Yaffe D. Creatine kinase isoenzyme transition in actinomycin D-treated differentiating muscle cultures. Exp Cell Res 113: 15–21, 1978 [DOI] [PubMed] [Google Scholar]
- 17. Evans M, Morine K, Kulkarni C, Barton ER. Expression profiling reveals heightened apoptosis and supports fiber size economy in the murine muscles of mastication. Physiol Genomics 35: 86–95, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Fernandez AM, Dupont J, Farrar RP, Lee S, Stannard B, Le Roith D. Muscle-specific inactivation of the IGF-I receptor induces compensatory hyperplasia in skeletal muscle. J Clin Invest 109: 347–355, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gogly B, Groult N, Hornebeck W, Godeau G, Pellat B. Collagen zymography as a sensitive and specific technique for the determination of subpicogram levels of interstitial collagenase. Anal Biochem 255: 211–216, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Goldspink G, Yang SY. Effects of activity on growth factor expression. Int J Sport Nutr Exerc Metab 11, Suppl: S21–S27, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, Krane SM. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci USA 101: 17192–17197, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim DJ, Kataoka K, Sano S, Connolly K, Kiguchi K, DiGiovanni J. Targeted disruption of Bcl-xL in mouse keratinocytes inhibits both UVB- and chemically induced skin carcinogenesis. Mol Carcinog 48: 873–885, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim H, Barton E, Muja N, Yakar S, Pennisi P, Leroith D. Intact insulin and insulin-like growth factor-I receptor signaling is required for growth hormone effects on skeletal muscle growth and function in vivo. Endocrinology 146: 1772–1779, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Kornasio R, Riederer I, Butler-Browne G, Mouly V, Uni Z, Halevy O. Beta-hydroxy-beta-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta 1793: 755–763, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Kuo YH, Chen TT. Novel activities of pro-IGF-I E peptides: regulation of morphological differentiation and anchorage-independent growth in human neuroblastoma cells. Exp Cell Res 280: 75–89, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 280: 2737–2744, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Leeman MF, Curran S, Murray GI. The structure, regulation, and function of human matrix metalloproteinase-13. Crit Rev Biochem Mol Biol 37: 149–166, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Lewis MP, Machell JR, Hunt NP, Sinanan AC, Tippett HL. The extracellular matrix of muscle–implications for manipulation of the craniofacial musculature. Eur J Oral Sci 109: 209–221, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Lund PK. Insulin-like growth factors: gene structure and regulation. In: Handbook of Physiology. The Endocrine System. Cellular Endocrinology. Bethesda, MD: Am Physiol. Soc., 1998, p. 537–571 [Google Scholar]
- 30. Margue CM, Bernasconi M, Barr FG, Schafer BW. Transcriptional modulation of the anti-apoptotic protein BCL-XL by the paired box transcription factors PAX3 and PAX3/FKHR. Oncogene 19: 2921–2929, 2000 [DOI] [PubMed] [Google Scholar]
- 31. McKay BR, O'Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Co-expression of IGF-1 family members with myogenic regulatory factors following acute damaging muscle-lengthening contractions in humans. J Physiol 586: 5549–5560, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mikhailov VM, Kropotov AV, Zelenin AV, Krutilina RI, Kolesnikov VA, Zelenina IA, Baranov AN, Shtein GI, Ostapenko OV, Tomilin NV, Baranov VS. [The BCL-xL and ACR-1 genes promote differentiation and reduce apoptosis in muscle fibers of mdx mice]. Genetika 38: 1445–1450, 2002 [PubMed] [Google Scholar]
- 33. Mills P, Lafreniere JF, Benabdallah BF, El Fahime el M, Tremblay JP. A new pro-migratory activity on human myogenic precursor cells for a synthetic peptide within the E domain of the mechano growth factor. Exp Cell Res 313: 527–537, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267: 1506–1510, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27: 195–200, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Ostrovsky O, Eletto D, Makarewich C, Barton ER, Argon Y. Glucose regulated protein 94 is required for muscle differentiation through its control of the autocrine production of insulin-like growth factors. Biochim Biophys Acta 1803: 333–341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, Wannenes F, Battistini L, Rosenthal N, Molinaro M, Musaro A. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J 21: 1393–1402, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Pfeffer LA, Brisson BK, Lei H, Barton ER. The insulin-like growth factor-I E-peptides modulate cell entry of the mature IGF-I protein. Mol Biol Cell 20: 3810–3817, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, Olivetti G, Homcy CJ, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci USA 93: 8630–8635, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schertzer JD, Lynch GS. Comparative evaluation of IGF-I gene transfer and IGF-I protein administration for enhancing skeletal muscle regeneration after injury. Gene Ther 13: 1657–1664, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Shamblott MJ, Chen TT. Age-related and tissue-specific levels of five forms of insulin-like growth factor mRNA in a teleost. Mol Mar Biol Biotechnol 2: 351–361, 1993 [PubMed] [Google Scholar]
- 42. Shimatsu A, Rotwein P. Mosaic evolution of the insulin-like growth factors. Organization, sequence, and expression of the rat insulin-like growth factor I gene. J Biol Chem 262: 7894–7900, 1987 [PubMed] [Google Scholar]
- 43. Siegfried JM, Kasprzyk PG, Treston AM, Mulshine JL, Quinn KA, Cuttitta F. A mitogenic peptide amide encoded within the E peptide domain of the insulin-like growth factor IB prohormone. Proc Natl Acad Sci USA 89: 8107–8111, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol 586: 283–291, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tsai LK, Tsai MS, Ting CH, Li H. Multiple therapeutic effects of valproic acid in spinal muscular atrophy model mice. J Mol Med 86: 1243–1254, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wallis M. New insulin-like growth factor (IGF)-precursor sequences from mammalian genomes: the molecular evolution of IGFs and associated peptides in primates. Growth Horm IGF Res 19: 12–23, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Wilson HE, Westwood M, White A, Clayton PE. Monoclonal antibodies to the carboxy-terminal Ea sequence of pro-insulin-like growth factor-IA (proIGF-IA) recognize proIGF-IA secreted by IM9 B-lymphocytes. Growth Horm IGF Res 11: 10–17, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Wu N, Jansen ED, Davidson JM. Comparison of mouse matrix metalloproteinase 13 expression in free-electron laser and scalpel incisions during wound healing. J Invest Dermatol 121: 926–932, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Yang SY, Goldspink G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett 522: 156–160, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.