Abstract
Longitudinal in vivo proton magnetic resonance spectroscopy (1H-MRS) and immunohistochemistry were performed to investigate the tissue degeneration in traumatically injured rat spinal cord rostral and caudal to the lesion epicenter. On 1H-MRS significant decreases in N-acetyl aspartate (NAA) and total creatine (Cr) levels in the rostral, epicenter, and caudal segments were observed by 14 days, and levels remained depressed up to 56 days post-injury (PI). In contrast, the total choline (Cho) levels increased significantly in all three segments by 14 days PI, but recovered in the epicenter and caudal, but not the rostral region, at 56 days PI. Immunohistochemistry demonstrated neuronal cell death in the gray matter, and reactive astrocytes and axonal degeneration in the dorsal, lateral, and ventral white-matter columns. These results suggest delayed tissue degeneration in regions both rostrally and caudally from the epicenter in the injured spinal cord tissue. A rostral-caudal asymmetry in tissue recovery was seen both on MRI-observed hyperintense lesion volume and the Cho, but not NAA and Cr, levels at 56 days PI. These studies suggest that dynamic metabolic changes take place in regions away from the epicenter in injured spinal cord.
Key words: choline, histology, N-acetyl aspartate, proton magnetic resonance spectroscopy, rat, spinal cord injury
Introduction
Traumatic spinal cord injury (SCI) results in devastating loss of sensory and motor functions and adversely affects multiple organs within the body. The pathobiology of SCI is characterized by the primary mechanical insult followed by activation of a delayed secondary cascade of biochemical events that ultimately causes progressive tissue degeneration, including neuronal cell death, axonal degeneration, and cavitation in the spinal cord (Baptiste and Fehlings, 2006). The pathobiological changes of primary and secondary damage in SCI can be monitored by quantifying changes in metabolites such as N-acetyl aspartate (NAA), lactate, and pyruvate (Falconer et al., 1996).
In vivo proton magnetic resonance spectroscopy (1H-MRS) has been widely recognized as a valuable tool for noninvasive monitoring of brain biochemistry in vivo in animals and humans. The metabolites most amenable to study with 1H-MRS in the central nervous system (CNS) are NAA, total creatine (Cr; includes both phosphocreatine and creatine), and total choline (Cho; choline-containing compounds). Of these, NAA has attracted the greatest attention since it is considered to be a neuronal/axonal marker (Holly et al., 2009; Narayana, 2005). Cr is thought to be a marker of gliosis (Sajja et al., 2009), and Cho is considered to be an indicator of cellular turnover related to both membrane synthesis and degradation (Carpentier et al., 2006; Holly et al., 2009; Narayana, 2005).
In contrast to its extensive use in brain, relatively few in vivo 1H-MRS studies of spinal cords in animals have been reported (Balla and Faber 2007; Bilgen et al., 2001; Silver et al., 2001; Vink et al., 1989; Zelaya et al., 1996), and humans (Blamire et al., 2007; Cooke et al., 2004; Edden et al., 2007; Ge, 2006; Gomez-Anson et al., 2000; Henning et al., 2008; Holly et al., 2009; Kendi et al., 2004; Kim et al., 2004; Marliani et al., 2007). This can be attributed largely to the relatively small size of the spinal cord, magnetic susceptibility effects from the surrounding bony structures and/or hemorrhage in the case of acute injury, and the movement of the spinal cord during physiological cycles (cardiac and respiratory). These factors limit the ability to acquire MR spectra with adequate signal-to-noise ratio (SNR). To the best of our knowledge, the use of in vivo 1H-MRS for quantification of metabolites in spinal cord in SCI has not yet been reported. In an earlier study, the feasibility of acquiring high-quality MR spectra from a normal rat spinal cord with an implanted coil was demonstrated (Bilgen et al., 2001; Silver et al., 2001). In the present study, we measured the longitudinal changes in NAA, Cr, and Cho in injured rat spinal cord between 14 days and 56 days post-injury, and correlated the spectroscopic findings with immunohistochemistry.
Methods
Animals preparation
All surgical procedures and the subsequent care and treatment of the animals used in this study were in strict accordance with the National Institutes of Health (NIH) guidelines for animal care. These studies were approved by our institutional animal welfare committee.
These studies were performed on Sprague-Dawley rats weighing from 300–350 g. They were divided into two groups of six animals each: laminectomy controls (without injury to the cord) and injured. The spinal cord injury and RF coil implantation procedures were performed as previously described (Bilgen et al., 2001; Narayana et al., 2004). Briefly, the animals were anesthetized with 4% isoflurane and maintained under anesthesia with a mixture of 2% isoflurane, air, and oxygen, administered through a Harvard rodent ventilator (model 683; Harvard Apparatus, Holliston, MA) during the entire surgical procedure. A laminectomy was performed at the seventh thoracic vertebra (T7), and the T6 and T8 vertebral processes were clamped to stabilize the vertebral column. A 150-kDyn force was delivered to the exposed cord to produce a moderate level of injury using an Infinite Horizon Impactor (Precision Systems and Instrumentation, LLC, Lexington, KY). The control animals were subjected to laminectomy alone and RF coil implantation. The animals were allowed to recover in warmed cages and received subcutaneous injections of cephazone (15 mg/kg; Bulter Schein Animal Health) twice a day for 10 days, and buprenorphine (0.01 mg/kg; Hospira, Inc., Lake Forest, IL) twice a day for 5 days. The animals were also administered subcutaneous injections of saline twice daily for 5 days. The injured animals' bladders were manually expressed twice daily by the method of Crede until the return of spontaneous urination. Animals had free access to food and water.
Magnetic resonance imaging/spectroscopy measurements
Magnetic resonance imaging/spectroscopy (MRI/S) measurements were performed on days 14, 28, and 56 post-injury (PI). MR scans were not performed in the acute phase of injury because of concerns about high mortality. All MR studies were performed with a 7 Tesla Bruker scanner (70/30 USR; Bruker Biospec, Karlsruhe, Germany) equipped with a 116-mm shielded gradient coil. The animals were placed in supine position on a acrylic glass bed with a 35 × 40-mm coil that was inductively coupled to the implanted radio frequency (RF) coil. For MR studies, the animals were anesthetized with an induction dose of 4% isoflurane, and were then intubated and mechanically ventilated with 2–2.5% isoflurane, 30% oxygen, and 67.5–68% air, through a rodent ventilator (model 683; Harvard Apparatus) for the duration of the scan (approximately 3 h). The respiratory rate and rectal temperature were monitored throughout the experiment with a physiologic monitoring unit (model 1025; SA Instruments, Inc., Stony Brook, NY). A pulse oximeter (model 8600V; Nonin Medical Inc., Plymouth, MN) was used to monitor heart rate and oxygen saturation levels. For the duration of the experiment, the animal's body temperature was maintained at 36.5°C with a heating system (model 11007B; SA Instruments).
Following the acquisition of tripilot scan (for locating the spinal cord), high-resolution dual spin echo images were acquired using rapid acquisition with relaxation enhancement (RARE) sequence (acquisition parameters: repetition time [TR] = 3159 msec; echo times [TE1/TE2] = 21/64 msec; field-of-view (FOV) = 26 × 26 mm; slice thickness = 1.0/0.5 mm [axial/sagittal]; acquisition matrix = 256 × 256).
Point-resolved spectroscopy (PRESS) sequence was used to acquire localized 1H spectra from segments 3 mm rostral (+3 mm from the epicenter), at the lesion epicenter (the laminectomy site in control animals; 0 mm), and 3 mm caudal (–3 mm from the epicenter), with voxel sizes of 2 × 2 × 3 mm (dorsal-ventral × left-right × caudal-rostral; Fig. 1A). The acquisition parameters were TR = 4000 msec, TE = 20 msec, spectral width = 10,000 Hz, 4 k data points, and 128 averages. The total MRS acquisition time for each region was about 9 min. The water signal was suppressed by variable power RF pulses with optimized relaxation delays (VAPOR). A short TE and moderately long TR were used to reduce the effects of relaxation times on the estimated metabolite concentrations. Pulse oximeter triggering was used for the MRS acquisition to reduce artifacts arising from movement of the spinal cord and cerebrospinal fluid pulsation. The automated shimming routine provided by the scanner manufacturer, in our hands, did not yield consistent results; therefore magnetic field shimming was performed manually (line width of 15–25 Hz).
FIG. 1.
The locations of voxels selected for in vivo proton magnetic resonance spectroscopy (1H-MRS) measurement (white open boxes) in the sagittal (A) and axial (B) orientations. The two dark circular regions at the right (in A) or bottom (in B) of the images represent the edges of the copper wire of the implanted radio frequency coil.
The free induction decay was multiplied by an 8-Hz exponential line-broadening window function, Fourier transformed, manually phased, and corrected for baseline before quantitative measurements of peak areas using the standard routines provided in the Bruker software TOPSPIN. Integrated peak areas were measured and normalized to that of the unsuppressed water resonance from the rostral segment, which appeared normal on the RARE images.
Magnetic resonance imaging lesion volume analysis
The high-resolution RARE images were analyzed to determine the lesion volumes using Image-Pro Plus 5.1 software (Media Cybernetics, Inc., Silver Spring, MD). Intensity thresholds for both hypointense and hyperintense lesions were determined by comparison with the normal uninjured spinal cord tissue. The thresholds were applied to the elliptical region of interest (ROI), encompassing the entire spinal cord (Fig. 2). The hypointense and hyperintense lesion areas were calculated for each slice, and the total volume of three slices in each segment was determined.
FIG. 2.
Axial T2-weighted (repetition time/echo time [TR/TE] = 3159/64 msec) images acquired from the spinal cord of an injured rat on days 14, 28, and 56 post-injury (PI). Hypointense and hyperintense areas used in lesion volume measurements are shown in the image slice at the epicenter on day 14 PI. The ellipse shown in the first image slice indicates the location of the region of interest selected for in vivo tissue atrophy and lesion volume analysis (D, day).
Histology
Following the terminal MRI scans on day 56 PI, the animals were transcardially perfused with saline, followed by 4% paraformaldehyde in phosphate-buffered saline (PBS). The spinal cords were removed, post-fixed overnight in 4% paraformaldehyde, and then immersed in 30% sucrose-PBS (0.1 M PBS) for 2–3 days at 4°C. The spinal cords were divided into epicenter, rostral, and caudal segments, each 3 mm long. The segments were sectioned coronally at a thickness of 30 μm using a cryostat (model CM1800; Leica Microsystems, Inc., Bannockburn, IL), and the sections were stored at −20°C in tissue-storing media.
The spinal cord sections were processed as free floating and were incubated in the following antibodies: neuronal nuclei (NeuN, 1:1000 MAB377; Millipore, Billerica, MA), glial fibrillary acidic protein (GFAP, 1:1000 Z0334; Dako North America, Inc., Carpenteria, CA), myelin basic protein (MBP, 1:100 MAB381; Millipore), and neurofilament-heavy protein (NF-H, 1:1000 AB1991; Millipore). The primary antibody was diluted with blocking solution (0.1 M PBS containing 5% goat serum and 0.3% Triton X-100). For controls, only secondary antibodies were applied to determine the antibody specificity. Appropriate secondary antibody was used at a dilution of 1:500 in 0.1 M PBS containing 5% goat serum and 0.3% Triton X-100. The following Alexafluor dye-conjugated secondary antibodies were used: goat anti-mouse IgG Alexa Fluor 488 (Invitrogen, Carlsbad, CA) and goat anti-rabbit IgG (H + L) Alex Fluor 568 (Invitrogen). Tissue sections were viewed and captured using a Spot Flex digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI) attached to a Leica RX1500 upright microscope, and the images were collected using the Spot software. The operator acquiring the images was blinded to group.
Histological assessment
Quantitative analysis was performed using ImagePro Plus software. Free-floating spinal cord sections (n = 8 sections/animal) from both rostral and caudal segments of all animals (n = 6 animals/group) were used for quantification of the percentage areas or fluorescent intensities of NF-H, MBP, and GFAP, and the numbers of NeuN-positive neuronal nuclei in the defined ROI. Using the predefined threshold levels from control spinal cord sections, the fluorescent intensities were determined in both groups. The epicenter segment was not analyzed due to the large amount of tissue damage. The nuclei were quantified with ImagePro and verified by manually counting neuronal nuclei. The operator performing the analysis was blinded to group.
Statistical analysis
All data are presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to compare the relative metabolite peak areas and lesion volume measured at the different time points, followed by a Dunnett's multiple comparison post-hoc test. The Mann-Whitney rank-sum test was used to determine differences in the percent expression of NF-H, MBP, and GFAP in the ROI. The differences in the total numbers of NeuN-positive neuronal nuclei between control and injured animals were determined by two-tailed unpaired Student's t-tests, based on the assumption that this measurement was normally distributed. A p value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS 10.0 software (SPSS Inc., Chicago, IL).
Results
Magnetic resonance imaging/spectroscopy
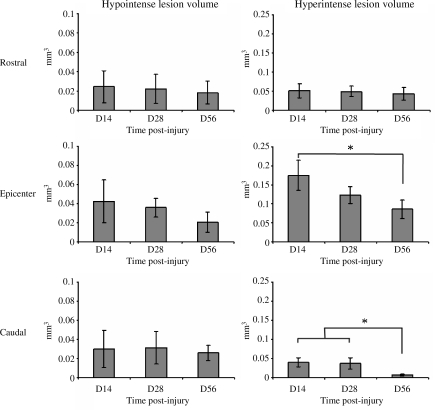
Hypointense and hyperintense regions extended both rostral and caudal to the epicenter by 14 days PI (Fig. 2). The hyperintense lesion volume was reduced over the course of the study, and significant differences in the lesion volume were observed in the epicenter and caudal segments between 14 and 56 days PI, while the change seen in the rostral segment was much less pronounced, as shown in Figure 3. In contrast, the volume of the hypointense lesion did not show any significant change with time in any of the segments (Fig. 3).
FIG. 3.
Temporal changes in the hypointense and hyperintense lesion volumes in rostral (upper row), epicenter (middle row), and caudal (bottom row) spinal cord segments in the injured rats (D, day; error bars indicate standard deviations; *p < 0.05).
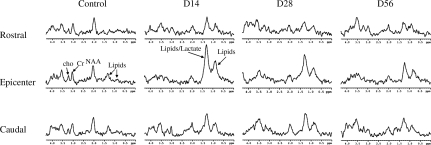
A typical set of in vivo 1H-MR spectra acquired from the rostral, epicenter, and caudal segments of laminectomy control and injured rats at 14, 28, and 56 days PI is shown in Figure 4. Significant reductions in the NAA and Cr peak areas relative to controls was observed in all three sections on days 14, 28, and 56 PI. The resonance signals at 0.85 ppm (lipids) and 1.26 ppm (lipids/lactate) in the rostral, epicenter, and caudal segments in injured rats were observed to be more pronounced relative to control rats on days 14, 28, and 56 PI.
FIG. 4.
In vivo proton magnetic resonance (1H-MR) spectra acquired from the rostral (upper row), epicenter (middle row), and caudal (bottom row) spinal cord segments from a control rat and an injured rat, on days 14, 28, and 56 post-injury. The spectra were acquired with repetition time = 4000 msec and echo time = 20 msec (D, day; NAA, N-acetyl aspartate; Cr, total creatine; Cho, total choline).
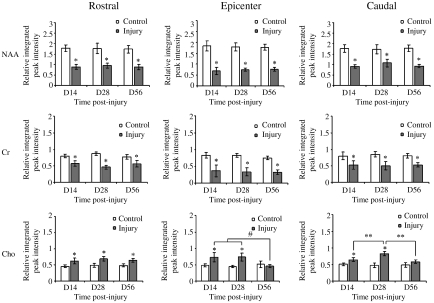
Figure 5 shows the temporal changes in the relative peak areas (relative to water) of NAA, Cr, and Cho in the rostral, epicenter, and caudal segments. The relative NAA peak area in the control rats did not show any statistically significant changes with time. However, in injured rats, by 14 days PI there were marked decreases in the relative NAA peak area in the rostral, epicenter, and caudal segments, by about 50%, 62%, and 49%, respectively. Even at 56 days PI, no significant recovery in the NAA levels in these three segments was observed. The relative Cr peak area showed similarly significant decreases by 14 days PI in the rostral, epicenter, and caudal segments (about 27%, 56%, and 34%, respectively), and remained relatively constant up to 56 days PI. In contrast to NAA and Cr, the relative Cho peak area increased significantly, about 37%, 50%, and 28%, respectively, in rostral, epicenter, and caudal segments by 14 days PI. The Cho levels recovered gradually afterwards, and were no longer statistically significantly different from controls in epicenter and caudal segments. However, statistically significant differences in the Cho levels relative to controls were still observed in the rostral segment at 56 days PI.
FIG. 5.
Relative integrated peak intensities of NAA, Cr, and Cho from the rostral (left column), epicenter (middle column), and caudal (right column) spinal cord segments in control rats (n = 6) and injured rats (n = 6), measured on days 14, 28, and 56 post-surgery (D, day; NAA, N-acetyl aspartate; Cr, total creatine; Cho, total choline; *p < 0.05 compared to control rats; #p < 0.05 compared to D56 by one-way analysis of variance [ANOVA] followed by Dunnett's test; **p < 0.05 compared to D28 by one-way ANOVA followed by Dunnett's test; error bars represent standard deviations).
Histology
In order to understand the pathological basis underlying the in vivo 1H-MRS-observed changes in NAA, Cr, and Cho levels, we used immunohistology to label axons, myelin, astrocytes, and neurons. As an example, Figure 6 shows the NF-H-, MBP-, GFAP-, and NeuN-stained histological sections of spinal cord from the rostral segment of a control rat at 56 days post-surgery. The corresponding stains from rostral and caudal sections of an injured cord on day 56 PI are shown in Figures 7 and 8, respectively. A significant decrease in the amount of neurofilament and myelin in the rostral and caudal segments was detected in injured animals (Fig. 9). These decreases were particularly marked in the dorsal, lateral, and ventral white-matter regions. A significant decrease in the number of NeuN-positive cells was observed in the rostral (p < 0.05) and caudal (p < 0.001) segments in injured animals. The GFAP immunoreactivity indicated no statistically significant differences between injured and control animals in either the rostral or caudal regions (p = 0.175 and p = 0.337, respectively).
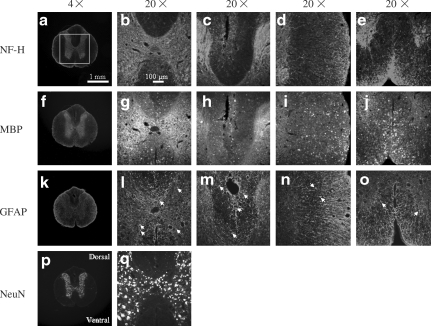
FIG. 6.
NF-H-, MBP-, GFAP- and NeuN-stained histological images of spinal cord sections obtained from the rostral segment of a control rat at 56 days post-surgery. (a, f, k, and p) Images made at 4 × original magnification. The images made at 20 × original magnification show the gray matter (b, g, l, and q), dorsal (c, h, and m), lateral (d, i, and n), and ventral (e, j, and o) regions of interest (ROI). The white open square in (a) shows the ROI selected for measurements of the percent areas of NF-H, MBP, and GFAP expression, and the numbers of NeuN-positive neuronal nuclei. “Dorsal” and “Ventral” indicate the orientation of the spinal cord section (NF-H, neurofilament-heavy protein; MBP, myelin basic protein; GFAP, glial fibrillary acidic protein; NeuN, neuronal nuclei; scale bar = 1 mm for 4 × images and 100 μm for 20 × images).
FIG. 7.
NF-H-, MBP-, GFAP- and NeuN-stained histological images of spinal cord sections obtained from the rostral segment of an injured rat at 56 days post-surgery. (a, f, k, and p) Images made at 4 × original magnification. The images made at 20 × original magnification show the gray matter (b, g, l, and q), dorsal (c, h, and m), lateral (d, i, and n), and ventral (e, j, and o) regions of interest (ROI). The white open square in (a) shows the ROI selected for measurements of the percent areas of NF-H, MBP, and GFAP expression, and the numbers of NeuN-positive neuronal nuclei. Axonal degeneration in dorsal (c and h), lateral (d and i), and ventral (e and j) white matter columns is clearly visible as a lack of stained NF-H protein and MBP. “Dorsal” and “Ventral” indicate the orientation of the spinal cord section (arrows represent reactive astrocytes; NF-H, neurofilament-heavy protein; MBP, myelin basic protein; GFAP, glial fibrillary acidic protein; NeuN, neuronal nuclei; scale bar = 1 mm for 4 × images and 100 μm for 20 × images).
FIG. 8.
NF-H-, MBP-, GFAP- and NeuN-stained histological images of spinal cord sections obtained from the caudal segment of an injured rat at 56 days post-surgery. (a, f, k, and p) Images made at 4 × original magnification. The images made at 20 × original magnification show the gray matter (b, g, l, and q), dorsal (c, h, and m), lateral (d, i, and n), and ventral (e, j, and o) regions of interest (ROI). The white open square in (a) shows the ROI selected for measurements of the percent areas of NF-H, MBP, and GFAP expression, and the numbers of NeuN-positive neuronal nuclei. Axonal degeneration in dorsal (c and h), lateral (d and i), and ventral (e and j) white matter columns is clearly visible as a lack of stained NF-H protein and MBP. “Dorsal” and “Ventral” indicate the orientation of the spinal cord section (arrows represent reactive astrocytes; NF-H, neurofilament-heavy protein; MBP, myelin basic protein; GFAP, glial fibrillary acidic protein; NeuN, neuronal nuclei; scale bar = 1 mm for 4 × images and 100 μm for 20 × images).
FIG. 9.
Percent areas of NF-H, MBP, and GFAP expression, and total number of NeuN-positive cells of eight sections in the rostral (A) and caudal (B) segments of control and injured rats (-R, rostral; -C, caudal; error bars indicate standard deviations; *p < 0.05; NF-H, neurofilament-heavy protein; MBP, myelin basic protein; GFAP, glial fibrillary acidic protein; NeuN, neuronal nuclei).
Atrophy
The injured spinal cord atrophies with time (Deng et al., 2007). To evaluate spinal cord atrophy, we performed volume analysis on both the high-resolution RARE images (in vivo) and histological sections (ex vivo). The total volume of spinal cord tissue based on the ROI analysis of the RARE images in each segment was recorded for in vivo tissue atrophy evaluation (Table 1). As shown in Table 1, by 56 days PI, there was significant volume reduction in the rostral, epicenter, and caudal segments compared to control levels, by about 17% (10.893 ± 1.403 versus 13.127 ± 0.682, p < 0.05), 25% (10.040 ± 1.885 versus 13.423 ± 0.909, p < 0.05), and 17% (11.119 ± 1.336 versus 13.475 ± 0.772, p < 0.05), respectively.
Table 1.
In Vivo MRI-Measured Volumes of Injured and Control Spinal Cords at Rostral, Epicenter, and Caudal Segments at Different Time Points Post-Injury
| |
Volumes (mean ± SD) of different spinal cord segments (mm3) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
Rostral |
Epicenter |
Caudal |
||||||
| Post-injury (days) | Control | Injured | p | Control | Injured | p | Control | Injured | p |
| 14 | 13.00 ± 1.09 | 12.44 ± 0.76 | 0.447 | 13.26 ± 1.00 | 12.91 ± 1.21 | 0.670 | 13.50 ± 0.88 | 12.93 ± 1.16 | 0.469 |
| 28 | 13.10 ± 0.91 | 11.86 ± 1.20 | 0.128 | 13.54 ± 1.02 | 11.25 ± 1.34 | 0.041 | 13.49 ± 0.89 | 11.79 ± 0.98 | 0.037 |
| 56 | 13.13 ± 0.68 | 10.89 ± 1.40 | 0.042 | 13.42 ± 0.91 | 10.04 ± 1.89 | 0.028 | 13.48 ± 0.77 | 11.12 ± 1.34 | 0.030 |
The p values indicate the level of statistical significance in the differences between the control and injured cords; p < 0.05 are indicated in bold type.
SD, standard deviation; MRI, magnetic resonance imaging.
For ex vivo evaluation of tissue atrophy, the average ROI sizes defined in histological sections were determined for control and injured animals and are summarized in Table 2. As shown in Table 2, in ex vivo, statistically significant reductions of tissue volume were detected in the rostral and caudal segments compared to control animals at 56 days PI, by about 25% (1.973 ± 0.357 versus 2.637 ± 0.237, p = 0.001) and 32% (1.857 ± 0.270 versus 2.732 ± 0.348, p < 0.001), respectively.
Table 2.
Ex vivo Histology-Based Volumes in Injured and Control Spinal Cords at the Rostral and Caudal Segments at 56 Days Post-Injury
| |
Rostral (mm2) |
Caudal (mm2) |
||||
|---|---|---|---|---|---|---|
| Stain | Control | Injured | p | Control | Injured | p |
| NF-H | 2.57 ± 0.20 | 1.88 ± 0.35 | 0.003 | 2.70 ± 0.32 | 1.86 ± 0.29 | <0.001 |
| MBP | 2.56 ± 0.20 | 1.91 ± 0.37 | 0.005 | 2.69 ± 0.32 | 1.86 ± 0.30 | <0.001 |
| GFAP | 2.64 ± 0.24 | 1.97 ± 0.36 | 0.001 | 2.73 ± 0.35 | 1.86 ± 0.27 | <0.001 |
| NeuN | 2.60 ± 0.35 | 1.96 ± 0.33 | 0.002 | 2.72 ± 0.33 | 1.86 ± 0.28 | <0.001 |
The p values indicate the level of statistical significance in the differences between the control and injured cords; p < 0.05 are indicated in bold type.
NF-H, neurofilament-heavy protein; MBP, myelin basic protein; GFAP, glial fibrillary acidic protein; NeuN, neuronal nuclei.
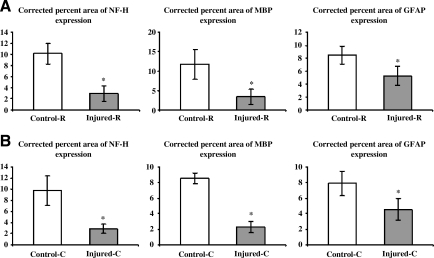
As can be seen from Tables 1 and 2, there was a mismatch between the results for in vivo and ex vivo analyses in the tissue atrophy in the rostral and caudal segments. This mismatch could be due to the partial loss of non-organized material such as cell debris and macrophages mixed with previous hemorrhage that filled the cyst during the preparation of the histological sections (Weber et al., 2006). To minimize this tissue shrinkage during histology, we determined the corrected percent areas of NF-H, MBP, and GFAP based on the assumption that spinal cord tissue shrinkage occurred linearly in the transverse direction (perpendicular to the spinal cord axis). At 56 days PI, the corrected percent areas of NF-H and MBP expression were further reduced compared to the uncorrected values. The corrected GFAP expression decreased significantly compared to control levels in both rostral and caudal segments (p = 0.009 and p = 0.004, respectively), as illustrated in Figure 10.
FIG. 10.
Corrected percent areas of NF-H, MBP, and GFAP expression in the rostral (A) and caudal (B) segments of control and injured rats. Significance levels were set at p < 0.05 (-R, rostral; -C, caudal; error bars indicate standard deviations; NF-H, neurofilament-heavy protein; MBP, myelin basic protein; GFAP, glial fibrillary acidic protein).
Discussion
In this study, we investigated tissue degeneration in traumatically injured rat spinal cord in rostral, epicenter, and caudal regions using in vivo MRI/S. MRS is perhaps the only technique that can provide important information about neuronal and axonal integrity. It has a high degree of relevance in SCI and the subsequent neurodegeneration that is known to occur. In the current study, MRS results were correlated with immunohistochemistry for a rational interpretation of the MRS- and MRI-observed changes. We believe that these are the first in vivo longitudinal studies of 1H-MRS of injured spinal cord in rats over a period of 56 days PI.
Metabolic changes and their relations to neuronal and axonal degeneration after spinal cord injury
N-acetyl aspartate, a free amino acid, is almost exclusively located in neurons and axons, and is the most prominent signal observed in 1H-MRS of the CNS. Decreases in NAA and the NAA/Cr ratio have been shown to be closely correlated with neuronal or axonal dysfunction/loss in cervical spondylotic myelopathy (Holly et al., 2009) and multiple sclerosis (Blamire et al., 2007; Mader et al., 2000; Narayana, 2005). Our MRS data showed that NAA levels decreased by 14 days, and remained relatively constant up to 56 days after injury, in rostral, epicenter, and caudal spinal cord segments. This NAA reduction is consistent with previous results that found that in a similar animal spinal cord injury model NAA levels in rostral and epicenter regions declined at 7 days PI as measured by gas chromatography-mass spectrometry (Falconer et al., 1996). Moreover, in our study, the results of immunohistochemistry clearly showed that neuronal loss in gray matter was accompanied by a loss of phosphorylated neurofilament heavy chain and myelin basic protein in dorsal, lateral, and ventral white-matter regions, which indicated axonal loss and demyelination in these regions. As pointed out in a number of reports, reduced NAA can also reflect neuronal mitochondrial dysfunction (Demougeot et al., 2001, 2004; Moffett et al., 2007; Narayana, 2005; Signoretti et al., 2008, 2001). These findings indicate that the NAA reductions seen in this study most likely reflect frank neuronal or axonal loss and possibly metabolic dysfunction. These results appear to indicate relatively little axonal/neuronal injury in the regions distant from the epicenter by day 56 PI.
Interestingly, compared to controls, NAA levels remained around 30% in the epicenter segment in injured animals where tissue, especially gray matter, was almost completely damaged or lost. It could be that the residual NAA represents molecules trapped in the cell debris of dead neurons (Sager et al., 2000), and/or residual axons in the MRS voxels examined in this study.
The longitudinal changes in the Cr signal at 3.02 ppm followed a similar time course as those observed for NAA. The Cr levels in rostral, epicenter, and caudal segments showed significant decreases by 14 days PI, and remained relatively constant at all subsequent time points. Creatine and phosphocreatine, both of which contribute to the Cr pool, are contained in both neurons and glial cells, and the level of Cr has been shown to be higher in the latter (Urenjak et al., 1993). The total concentration of creatine and phosphocreatine remains relatively stable in vivo, and therefore their peak is often used as an internal concentration standard (Malisza et al., 1998). However, recent studies have suggested that the Cr level may be altered in some disease states (Bitsch et al., 1999; Higuchi et al., 1996; Konaka et al., 2003; Pilatus et al., 2009; Simoes et al., 2008; van Walderveen et al., 1999). Using MRI/S with biopsy correlations, Bitsch and associates found that the Cr level was elevated in normal-appearing white matter in patients with multiple sclerosis (MS), which has been attributed to glial proliferation since fibrillary gliosis with no evident neuronal/axonal damage or MR-detectable lesions were observed in these regions (Bitsch et al., 1999). Interestingly, in an animal model of transient cerebral ischemia, Konaka and associates found a good linear correlation between the Cr level and the density of reactive astrocytes, even with consistent neuronal loss in the hippocampal CA1 sector during reperfusion (Konaka et al., 2003). These studies suggest that the Cr level might be an indicator of total cell density, especially astrocytic density. The 30–60% decreases of the Cr signal observed in the three segments by 56 days PI in our study may be mainly the result of loss of the cell population, for example neuronal loss in the gray matter. Although GFAP-positive reactive astrocytes were observed in regions surrounding the cavity in gray matter and dorsal, lateral, and ventral white matter in the relatively intact spinal cord sections (Figs. 7 and 8), the percent area of GFAP expression declined after correcting for the reduced volume due to tissue shrinkage compared to control levels. It is therefore likely that the low astrocyte population due to tissue shrinkage also contributes to the reduction of Cr levels seen in the three spinal cord segments.
In the current study, the total Cho levels in the rostral, epicenter, and caudal segments were found to increase by about 37%, 50%, and 28%, respectively, by 14 days after injury. The Cho levels recovered gradually afterwards, and were no longer statistically significantly different from controls in the epicenter and caudal segments. The recovery of the Cho level in the rostral segment was nonetheless less pronounced, and a significant increase was still observed compared to control levels at 56 days PI. The Cho resonance at 3.02 ppm represents a number of mobile Cho compounds (R-[CH3]3), including phosphorylcholine and glycerophosphorylcholine, as well as free choline and acetylcholine (<5%) (Moore and Galloway, 2002). Thus, the Cho peak is generally considered to be a potential biomarker for cell membrane phospholipid metabolism (Moore and Galloway, 2002). Elevated Cho or Cho/Cr ratios were found in various studies, and were attributed to changes in myelin, with or without associated inflammation, in pre-lesional normal-appearing white matter (Narayana et al., 1998; Tartaglia et al., 2002), active demyelination and/or inflammation (Arnold et al., 1990, 1992; Larsson et al., 1991; Narayana et al., 1998), and remyelination (Narayana, 2005; Sajja et al., 2009) in acute plaques in MS patients, and membrane degradation/synthesis and glial proliferation in patients with brain tumors or brain trauma (Garnett et al., 2000; McBride et al., 1995); these were also seen in a rat model of traumatic brain injury (Schuhmann et al., 2003). The increase in Cho seen in this study could be due to cell membrane breakdown and/or demyelination, mainly in the white matter region, since our histological data demonstrated myelin loss as evidenced by a lack of MBP staining in dorsal, lateral, and ventral white-matter columns. Furthermore, we observed a correlation between total Cho levels and hyperintense lesion volume as measured on T2-weighted images, which represents edema and demyelination in the chronic phase of spinal cord injury (Narayana et al., 2004). Alternatively, since GFAP-positive reactive astrocytes were also observed in this study, the increase in Cho levels could also be associated with inflammation, similar to that found in MS (Arnold et al., 1990, 1992; Larsson et al., 1991; Narayana et al., 1998).
As described above, both the total Cho levels and hyperintense lesion volume results indicate a rostral-caudal asymmetry in SCI. The recovery in the Cho levels and hyperintense lesion volume were more marked in the caudal compared to the rostral segment, which is in agreement with findings of previous studies (Aimone et al., 2004; Deo et al., 2006; Narayana et al., 2004). These studies suggest that the better recovery seen in the caudal section could be related to angiogenesis, and thus enhanced endothelial cell survival or recovery of atrophied neurons within the lesion site and caudal section. However, in this study we did not find significant recovery in NAA or Cr levels, which were related to neurons and cell density, respectively, in all three segments. This may suggest that total Cho is a sensitive indicator of tissue recovery in animal models of traumatic SCI.
We did not investigate the changes in lactate (resonance at 1.33 ppm) and lipid signals at 1.26 and 0.85 ppm because it is not possible to separate lactate and lipid signals at 1.26 ppm in short echo time spectra. Although we tried to acquire MR spectra with long echo time (e.g., 136 msec), the low SNR at long echo time did not allow for reliable data analysis. Furthermore, in spite of the care with which the spectroscopy voxels were selected, the 0.85 ppm and 1.26 ppm peaks may be due to contamination from the fatty tissue surrounding the spinal cord.
Changes in MRI lesion volume and spinal cord atrophy after spinal cord injury
The T2-weighted images exhibited hypointense and hyperintense signal areas in the injured cord. It has been suggested that depending on the phase of injury (acute, subacute, or chronic), the hyperintense areas may represent edema and demyelination, and the hypointense areas may represent hemorrhage, necrosis, gliosis, and cavitation (Narayana et al., 2004; Weirich et al., 1990). The reduced hyperintense lesion volume observed in the epicenter and caudal segments between 14 and 56 days PI may indicate resolution of edema and/or remyelination, suggesting partial tissue recovery in the epicenter and caudal, but not rostral segment, by 56 days PI. In contrast, the volume of the hypointense lesions did not vary with time in any of the three segments. The relatively constant hypointense lesion volume seen at all time points may be due to confounding effects such as hemorrhage, necrosis, gliosis, and cavitation in those regions. In addition, significant spinal cord tissue atrophy was observed in rostral, epicenter, and caudal segments by 56 days PI. It is likely that the removal of damaged neurons and axonal degenerating debris by microglia, and repair by reactive astrocytes, are the main reasons for tissue shrinkage and glial scar formation (Fawcett and Asher, 1999; Konaka et al., 2003).
The limited SNR in MRS of the spinal cord may hinder detection of subtle changes in metabolite levels. Unlike in brain tissue, where the majority of the studies have been done, the small size and shape of the spinal cord prevents enlarging the spectroscopic voxel size. This is further aggravated by the loss of tissue near the epicenter of the injury. Despite these limitations, we were able to acquire spectra of adequate quality, as judged by the standard deviation of ∼10% in the pooled metabolite levels from six animals. It is possible to further improve the spectral quality by using higher magnetic fields. For example, Balla and Faber (Balla and Faber, 2007) reported high-quality MRS of normal spinal cord at 17.6 T from a 2-mm3 voxel with a 45-min acquisition time. In our studies, which were performed at 7 T, the acquisition time for each voxel was about 9 min. The SNR could have been improved by increasing the acquisition time; however, this is not practical in serial in vivo studies.
In these studies, we observed small lipid peaks at 0.85 ppm and 1.26 ppm in the control animals from the epicenter and caudal segments, but not from the rostral segments. The lipid peaks may be due to contamination by the non-spinal cord tissue surrounding the spinal cord, even though extreme care was taken in selecting the ROI. The absence of such contamination in the rostral segment suggests that the ROI in this area was fully contained within the spinal cord tissue.
In summary, we have demonstrated serial changes of metabolites as measured by in vivo 1H-MR spectroscopy, not only in the epicenter, but also in regions rostral and caudal to the injury site in experimental SCI. Our results demonstrate that neuronal and axonal degeneration occurs in areas both rostral and caudal to the epicenter in SCI. However, by day 56 PI, the neuronal/axonal loss appeared to have stabilized. A rostral-caudal asymmetry in tissue recovery was also observed.
Acknowledgments
This work is supported by NIH grant NS045624 awarded to P.A.N. We thank Tessy Chacko, B.S., for assistance with animal care, and Chirag Patel, M.S.E., for assistance with histology.
Author Disclosure Statement
No competing financial interests exist.
References
- Aimone J.B. Leasure J.L. Perreau V.M. Thallmair M. Spatial and temporal gene expression profiling of the contused rat spinal cord. Exp. Neurol. 2004;189:204–221. doi: 10.1016/j.expneurol.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Arnold D.L. Matthews P.M. Francis G. Antel J. Proton magnetic resonance spectroscopy of human brain in vivo in the evaluation of multiple sclerosis: assessment of the load of disease. Magn. Reson. Med. 1990;14:154–159. doi: 10.1002/mrm.1910140115. [DOI] [PubMed] [Google Scholar]
- Arnold D.L. Matthews P.M. Francis G.S. O'Connor J. Antel J.P. Proton magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann. Neurol. 1992;31:235–241. doi: 10.1002/ana.410310302. [DOI] [PubMed] [Google Scholar]
- Balla D.Z. Faber C. In vivo intermolecular zero-quantum coherence MR spectroscopy in the rat spinal cord at 17.6 T: a feasibility study. MAGMA. 2007;20:183–191. doi: 10.1007/s10334-007-0081-3. [DOI] [PubMed] [Google Scholar]
- Baptiste D.C. Fehlings M.G. Pharmacological approaches to repair the injured spinal cord. J. Neurotrauma. 2006;23:318–334. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- Bilgen M. Elshafiey I. Narayana P.A. In vivo magnetic resonance microscopy of rat spinal cord at 7 T using implantable RF coils. Magn. Reson. Med. 2001;46:1250–1253. doi: 10.1002/mrm.1325. [DOI] [PubMed] [Google Scholar]
- Bitsch A. Bruhn H. Vougioukas V. Stringaris A. Lassmann H. Frahm J. Bruck W. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am. J. Neuroradiol. 1999;20:1619–1627. [PMC free article] [PubMed] [Google Scholar]
- Blamire A.M. Cader S. Lee M. Palace J. Matthews P.M. Axonal damage in the spinal cord of multiple sclerosis patients detected by magnetic resonance spectroscopy. Magn. Reson. Med. 2007;58:880–885. doi: 10.1002/mrm.21382. [DOI] [PubMed] [Google Scholar]
- Carpentier A. Galanaud D. Puybasset L. Muller J.C. Lescot T. Boch A.L. Riedl V. Cornu P. Coriat P. Dormont D. van Effenterre R. Early morphologic and spectroscopic magnetic resonance in severe traumatic brain injuries can detect “invisible brain stem damage” and predict “vegetative states”. J. Neurotrauma. 2006;23:674–685. doi: 10.1089/neu.2006.23.674. [DOI] [PubMed] [Google Scholar]
- Cooke F.J. Blamire A.M. Manners D.N. Styles P. Rajagopalan B. Quantitative proton magnetic resonance spectroscopy of the cervical spinal cord. Magn. Reson. Med. 2004;51:1122–1128. doi: 10.1002/mrm.20084. [DOI] [PubMed] [Google Scholar]
- Demougeot C. Garnier P. Mossiat C. Bertrand N. Giroud M. Beley A. Marie C. N-Acetylaspartate, a marker of both cellular dysfunction and neuronal loss: its relevance to studies of acute brain injury. J. Neurochem. 2001;77:408–415. doi: 10.1046/j.1471-4159.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- Demougeot C. Marie C. Giroud M. Beley A. N-acetylaspartate: a literature review of animal research on brain ischaemia. J. Neurochem. 2004;90:776–783. doi: 10.1111/j.1471-4159.2004.02583.x. [DOI] [PubMed] [Google Scholar]
- Deng X. Ramu J. Narayana P.A. Spinal cord atrophy in injured rodents: high-resolution MRI. Magn. Reson. Med. 2007;57:620–624. doi: 10.1002/mrm.21163. [DOI] [PubMed] [Google Scholar]
- Deo A.A. Grill R.J. Hasan K.M. Narayana P.A. In vivo serial diffusion tensor imaging of experimental spinal cord injury. J. Neurosci. Res. 2006;83:801–810. doi: 10.1002/jnr.20783. [DOI] [PubMed] [Google Scholar]
- Edden R.A. Bonekamp D. Smith M.A. Dubey P. Barker P.B. Proton MR spectroscopic imaging of the medulla and cervical spinal cord. J. Magn. Reson. Imaging. 2007;26:1101–1105. doi: 10.1002/jmri.21008. [DOI] [PubMed] [Google Scholar]
- Falconer J.C. Liu S.J. Abbe R.A. Narayana P.A. Time dependence of N-acetyl-aspartate, lactate, and pyruvate concentrations following spinal cord injury. J. Neurochem. 1996;66:717–722. doi: 10.1046/j.1471-4159.1996.66020717.x. [DOI] [PubMed] [Google Scholar]
- Fawcett J.W. Asher R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Garnett M.R. Blamire A.M. Corkill R.G. Cadoux-Hudson T.A. Rajagopalan B. Styles P. Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain. 2000;123:2046–2054. doi: 10.1093/brain/123.10.2046. [DOI] [PubMed] [Google Scholar]
- Ge Y. Multiple sclerosis: the role of MR imaging. AJNR Am. J. Neuroradiol. 2006;27:1165–1176. [PMC free article] [PubMed] [Google Scholar]
- Gomez-Anson B. MacManus D.G. Parker G.J. Davie C.A. Barker G.J. Moseley I.F. McDonald W.I. Miller D.H. In vivo 1H-magnetic resonance spectroscopy of the spinal cord in humans. Neuroradiology. 2000;42:515–517. doi: 10.1007/s002340000323. [DOI] [PubMed] [Google Scholar]
- Henning A. Schar M. Kollias S.S. Boesiger P. Dydak U. Quantitative magnetic resonance spectroscopy in the entire human cervical spinal cord and beyond at 3T. Magn. Reson. Med. 2008;59:1250–1258. doi: 10.1002/mrm.21578. [DOI] [PubMed] [Google Scholar]
- Higuchi T. Fernandez E.J. Maudsley A.A. Shimizu H. Weiner M.W. Weinstein P.R. Mapping of lactate and N-acetyl-L-aspartate predicts infarction during acute focal ischemia: in vivo 1H magnetic resonance spectroscopy in rats. Neurosurgery. 1996;38:121–129. doi: 10.1097/00006123-199601000-00030. ; discussion 129–130. [DOI] [PubMed] [Google Scholar]
- Holly L.T. Freitas B. McArthur D.L. Salamon N. Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J. Neurosurg. Spine. 2009;10:194–200. doi: 10.3171/2008.12.SPINE08367. [DOI] [PubMed] [Google Scholar]
- Kendi A.T. Tan F.U. Kendi M. Yilmaz S. Huvaj S. Tellioglu S. MR spectroscopy of cervical spinal cord in patients with multiple sclerosis. Neuroradiology. 2004;46:764–769. doi: 10.1007/s00234-004-1231-1. [DOI] [PubMed] [Google Scholar]
- Kim Y.G. Choi G.H. Kim D.H. Kim Y.D. Kang Y.K. Kim J.K. In vivo proton magnetic resonance spectroscopy of human spinal mass lesions. J. Spinal Disord. Tech. 2004;17:405–411. doi: 10.1097/01.bsd.0000124762.36865.9f. [DOI] [PubMed] [Google Scholar]
- Konaka K. Ueda H. Li J.Y. Matsumoto M. Sakoda S. Yanagihara T. N-acetylaspartate to total creatine ratio in the hippocampal CA1 sector after transient cerebral ischemia in gerbils: influence of neuronal elements, reactive gliosis, and tissue atrophy. J. Cereb. Blood Flow Metab. 2003;23:700–708. doi: 10.1097/01.WCB.0000071888.63724.56. [DOI] [PubMed] [Google Scholar]
- Larsson H.B. Christiansen P. Jensen M. Frederiksen J. Heltberg A. Olesen J. Henriksen O. Localized in vivo proton spectroscopy in the brain of patients with multiple sclerosis. Magn. Reson. Med. 1991;22:23–31. doi: 10.1002/mrm.1910220104. [DOI] [PubMed] [Google Scholar]
- Mader I. Roser W. Kappos L. Hagberg G. Seelig J. Radue E.W. Steinbrich W. Serial proton MR spectroscopy of contrast-enhancing multiple sclerosis plaques: absolute metabolic values over 2 years during a clinical pharmacological study. AJNR Am. J. Neuroradiol. 2000;21:1220–1227. [PMC free article] [PubMed] [Google Scholar]
- Malisza K.L. Kozlowski P. Peeling J. A review of in vivo 1H magnetic resonance spectroscopy of cerebral ischemia in rats. Biochem. Cell Biol. 1998;76:487–496. doi: 10.1139/bcb-76-2-3-487. [DOI] [PubMed] [Google Scholar]
- Marliani A.F. Clementi V. Albini-Riccioli L. Agati R. Leonardi M. Quantitative proton magnetic resonance spectroscopy of the human cervical spinal cord at 3 Tesla. Magn. Reson. Med. 2007;57:160–163. doi: 10.1002/mrm.21113. [DOI] [PubMed] [Google Scholar]
- McBride D.Q. Miller B.L. Nikas D.L. Buchthal S. Chang L. Chiang F. Booth R.A. Analysis of brain tumors using 1H magnetic resonance spectroscopy. Surg. Neurol. 1995;44:137–144. doi: 10.1016/0090-3019(95)00139-5. [DOI] [PubMed] [Google Scholar]
- Moffett J.R. Ross B. Arun P. Madhavarao C.N. Namboodiri A.M. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog. Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G.J. Galloway M.P. Magnetic resonance spectroscopy: neurochemistry and treatment effects in affective disorders. Psychopharmacol. Bull. 2002;36:5–23. [PubMed] [Google Scholar]
- Narayana P.A. Doyle T.J. Lai D. Wolinsky J.S. Serial proton magnetic resonance spectroscopic imaging, contrast-enhanced magnetic resonance imaging, and quantitative lesion volumetry in multiple sclerosis. Ann. Neurol. 1998;43:56–71. doi: 10.1002/ana.410430112. [DOI] [PubMed] [Google Scholar]
- Narayana P.A. Grill R.J. Chacko T. Vang R. Endogenous recovery of injured spinal cord: longitudinal in vivo magnetic resonance imaging. J. Neurosci. Res. 2004;78:749–759. doi: 10.1002/jnr.20275. [DOI] [PubMed] [Google Scholar]
- Narayana P.A. Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. J. Neuroimaging. 2005;15(4 Suppl):46S–57S. doi: 10.1177/1051228405284200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilatus U. Lais C. Rochmont Adu M. Kratzsch T. Frolich L. Maurer K. Zanella F.E. Lanfermann H. Pantel J. Conversion to dementia in mild cognitive impairment is associated with decline of N-acetylaspartate and creatine as revealed by magnetic resonance spectroscopy. Psychiatry Res. 2009;173:1–7. doi: 10.1016/j.pscychresns.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Sager T.N. Hansen A.J. Laursen H. Correlation between N-acetylaspartate levels and histopathologic changes in cortical infarcts of mice after middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2000;20:780–788. doi: 10.1097/00004647-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Sajja B.R. Wolinsky J.S. Narayana P.A. Proton magnetic resonance spectroscopy in multiple sclerosis. Neuroimaging Clin. N. Am. 2009;19:45–58. doi: 10.1016/j.nic.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann M.U. Stiller D. Skardelly M. Bernarding J. Klinge P.M. Samii A. Samii M. Brinker T. Metabolic changes in the vicinity of brain contusions: a proton magnetic resonance spectroscopy and histology study. J. Neurotrauma. 2003;20:725–743. doi: 10.1089/089771503767869962. [DOI] [PubMed] [Google Scholar]
- Signoretti S. Marmarou A. Aygok G.A. Fatouros P.P. Portella G. Bullock R.M. Assessment of mitochondrial impairment in traumatic brain injury using high-resolution proton magnetic resonance spectroscopy. J. Neurosurg. 2008;108:42–52. doi: 10.3171/JNS/2008/108/01/0042. [DOI] [PubMed] [Google Scholar]
- Signoretti S. Marmarou A. Tavazzi B. Lazzarino G. Beaumont A. Vagnozzi R. N-Acetylaspartate reduction as a measure of injury severity and mitochondrial dysfunction following diffuse traumatic brain injury. J. Neurotrauma. 2001;18:977–991. doi: 10.1089/08977150152693683. [DOI] [PubMed] [Google Scholar]
- Silver X. Ni W.X. Mercer E.V. Beck B.L. Bossart E.L. Inglis B. Mareci T.H. In vivo 1H magnetic resonance imaging and spectroscopy of the rat spinal cord using an inductively-coupled chronically implanted RF coil. Magn. Reson. Med. 2001;46:1216–1222. doi: 10.1002/mrm.1319. [DOI] [PubMed] [Google Scholar]
- Simoes R.V. Martinez-Aranda A. Martin B. Cerdan S. Sierra A. Arus C. Preliminary characterization of an experimental breast cancer cells brain metastasis mouse model by MRI/MRS. MAGMA. 2008;21:237–249. doi: 10.1007/s10334-008-0114-6. [DOI] [PubMed] [Google Scholar]
- Tartaglia M.C. Narayanan S. De Stefano N. Arnaoutelis R. Antel S.B. Francis S.J. Santos A.C. Lapierre Y. Arnold D.L. Choline is increased in pre-lesional normal appearing white matter in multiple sclerosis. J. Neurol. 2002;249:1382–1390. doi: 10.1007/s00415-002-0846-6. [DOI] [PubMed] [Google Scholar]
- Urenjak J. Williams S.R. Gadian D.G. Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J. Neurosci. 1993;13:981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Walderveen M.A. Barkhof F. Pouwels P.J. van Schijndel R.A. Polman C.H. Castelijns J.A. Neuronal damage in T1-hypointense multiple sclerosis lesions demonstrated in vivo using proton magnetic resonance spectroscopy. Ann. Neurol. 1999;46:79–87. doi: 10.1002/1531-8249(199907)46:1<79::aid-ana12>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- Vink R. Noble L.J. Knoblach S.M. Bendall M.R. Faden A.I. Metabolic changes in rabbit spinal cord after trauma: magnetic resonance spectroscopy studies. Ann. Neurol. 1989;25:26–31. doi: 10.1002/ana.410250105. [DOI] [PubMed] [Google Scholar]
- Weber T. Vroemen M. Behr V. Neuberger T. Jakob P. Haase A. Schuierer G. Bogdahn U. Faber C. Weidner N. In vivo high-resolution MR imaging of neuropathologic changes in the injured rat spinal cord. AJNR Am. J. Neuroradiol. 2006;27:598–604. [PMC free article] [PubMed] [Google Scholar]
- Weirich S.D. Cotler H.B. Narayana P.A. Hazle J.D. Jackson E.F. Coupe K.J. McDonald C.L. Langford L.A. Harris J.H., Jr. Histopathologic correlation of magnetic resonance imaging signal patterns in a spinal cord injury model. Spine (Phila. Pa. 1976) 1990;15:630–638. doi: 10.1097/00007632-199007000-00004. [DOI] [PubMed] [Google Scholar]
- Zelaya F.O. Chalk J.B. Mullins P. Brereton I.M. Doddrell D.M. Localized 1H NMR spectroscopy of rat spinal cord in vivo. Magn. Reson. Med. 1996;35:443–448. doi: 10.1002/mrm.1910350401. [DOI] [PubMed] [Google Scholar]