Abstract
Mossy fiber activity can evoke Ca2+ release from internal stores in CA3 neurons, but the physiological conditions under which this occurs and the mechanisms underlying the release are not understood. Using rat hippocampal slices we report here that short trains of mossy fiber stimulation activate group I metabotropic glutamate receptors (mGluRs) on CA3 pyramidal neurons and elicit waves of Ca2+ release from inositol 1,4,5-trisphosphate (IP3) sensitive internal stores that propagate from stratum lucidum to the soma and in some cases distally out the dendrites. Activation of mGluR1,5 receptors by an agonist trans-azetidine-2,4-dicarboxylic acid (tADA) applied to stratum lucidum was also sufficient to induce waves of Ca2+ release. This release was blocked by internal heparin, but not by dantrolene, suggesting the involvement of IP3 rather than ryanodine receptors in not only the initial release but also in the maintenance of the propagating waves. Release could be facilitated by Ca2+ influx through voltage-gated Ca2+ channels, which is consistent with the known Ca2+ sensitivity of IP3 receptors.
These results provide insight into the mechanisms and conditions of Ca2+ release in CA3 neurons and demonstrate the powerful influence mossy fiber input can have on these neurons.
Keywords: hippocampus; inositol 1,4,5-trisphosphate receptors; Ca2+ imaging; Ca2+ waves; synaptic plasticity; stratum lucidum
Elevation of the intracellular Ca2+ concentration ([Ca2+]i) in CA3 pyramidal neurons is required for the initiation and modulation of numerous cellular processes including various forms of synaptic plasticity such as long-term potentiation (LTP) (Williams and Johnston, 1989; Zalutsky and Nicoll, 1990; Kapur et al., 1998a,b; Yeckel et al., 1999). Increases in [Ca2+]i can occur in these neurons due to influx through multiple types of voltage-gated Ca2+ channels (VGCCs) (Fisher et al., 1990; Mogul and Fox, 1991; Elliott et al., 1995; Avery and Johnston, 1996), entry through N-methyl-D-aspartate (NMDA) receptor-activated channels (Zalutsky and Nicoll, 1990; Yeckel et al., 1999), or release from internal Ca2+ stores (Pozzo-Miller et al., 1995, 1996; Yeckel et al., 1999). Although the different subtypes of VGCCs present on CA3 pyramidal neurons have been investigated, Ca2+ release from internal stores has not been well characterized. Previous studies have shown that release of Ca2+ from stores can occur in CA3 pyramidal neurons when mossy fibers are stimulated at high frequencies in the presence of ionotropic glutamate receptor antagonists (Pozzo-Miller et al., 1996; Yeckel et al., 1999). In this paper we report that release can occur in CA3 neurons under more physiological conditions – without glutamate receptor antagonists present in the bath and with moderate synaptic stimulation – and we also investigated the mechanisms underlying this release.
EXPERIMENTAL PROCEDURES
Hippocampal slices (400 μm) were prepared from Sprague–Dawley rats (19–25 days, Harlan) as described previously (Kapur et al., 1998a,b; Yeckel et al., 1999). All experimental procedures conformed to the NIH guidelines for the ethical use of animals, and all efforts were made to minimize the number of animals used and their discomfort. In some experiments, after deep anesthesia was achieved (using a mixture of ketamine, xylazine, and acepromazine), animals were placed in a −20°C environment (~5 min) before the brain was removed for dissection. The bathing solution was maintained at 29–31°C and contained (in mM): 124 NaCl, 2.5 KCl, 25 NaHCO3, 4 MgCl2, 5 CaCl2, and 10 dextrose, bubbled with 95% O2–5% CO2. NMDA receptor antagonists 50 μM (±)-2-amino-5-phosphono-pentanoic acid and (+)-MK-801 hydrogen maleate (20 μM), plus GABAA receptor antagonists (−)-bicuculline methiodide (10–20 μM) and picrotoxin (10 μM) were always present in the bathing solution during recording. Where specified, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10–40 μM), ± -α-methyl-4-carboxyphenylglycine (MCPG; 500 μM), naloxone (10 μM), naltrexone (5 μM), and nimodipine (10 μM) were bath-applied. In MCPG experiments, the maximal effect was obtained within 10–20 min of bath application, after which the drug was washed out (10–30 min). trans-Azetidine-2,4-dicarboxylic acid (tADA; 25–35 mM) was pressure-applied through a glass pipette (outer diameter ~5 μm) using a picospritzer. Pressure pulses (5–40 psi, 5–50 ms) were applied singly or in bursts (up to 100 pulses at 20–100 Hz). In some experiments, heparin or dantrolene were included in the recording pipette. Bicuculline and picrotoxin were from Sigma (St. Louis, MO, USA), while all other drugs were from Research Biochemicals (Natick, MA, USA).
Whole-cell recordings were obtained from visually identified CA3 pyramidal neurons while in bridge or discontinuous voltage-clamp modes using a SEC 05L amplifier (Adams&List Associates, Westbury, NY, USA). The SEC 05L amplifier has the advantage over more traditional whole-cell clamp amplifiers in allowing the actual membrane potential of the cell to be monitored during the voltage clamp. Changes in the membrane potential of the cell during synaptic stimulation can thus be measured directly with this method. The recording electrode solution contained (in mM) 120 K-gluconate, 20 KCl, 10 HEPES, 2 MgCl2, 4 ATP (disodium salt), 0.3 GTP (Tris salt), 7 phosphocreatine (pH 7.3 KOH) and 100 μM fura-2. Methods for mossy fiber stimulation and Ca2+ fluorescence imaging have been previously described (Kapur et al., 1998a,b; Yeckel et al., 1999). Changes in [Ca2+]i were quantified by calculating ΔF/F, where F is the fluorescence intensity before stimulation (after subtracting autofluorescence) and ΔF is the change in F during neuronal activity (corrected for bleaching). Fura-2 bleaching was determined by measuring the change in F during a run without stimulation, and autofluorescence was taken from an area of the slice adjacent to the recorded cell (see Jaffe et al., 1992). Sequential frame rate was one frame every 10–20 ms and pixels were binned in a 10 by 10 array. Statistical significance was tested using Student’s t-test at P <0.05. All data are presented as mean ± S.E.M.
RESULTS
Mossy fiber-evoked Ca2+ release from internal stores
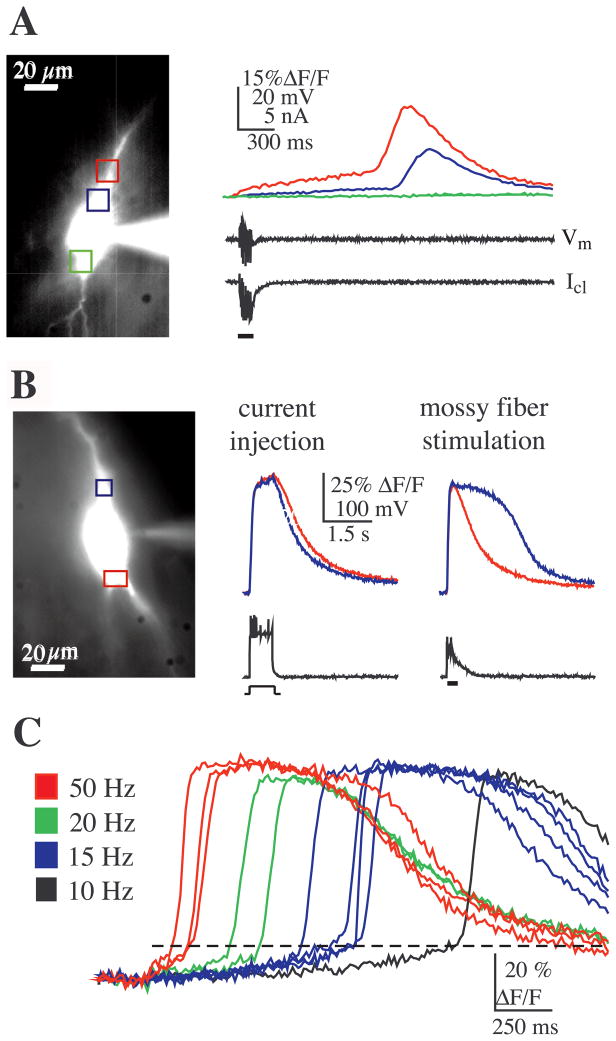
Stimulation of mossy fibers at low intensity and moderate to high frequencies (10–100 Hz) elicited large [Ca2+] elevations in the soma and proximal dendrites of CA3 pyramidal neurons that occurred in the absence of action potential firing and at latencies beyond the duration of the synaptic response. Discontinuous voltage-clamp recordings from the soma of these neurons showed that these [Ca2+] rises were not accompanied by changes in transmembrane current or voltage indicating that they were not due to Ca2+ influx through VGCCs (Fig. 1A). Consistent with previous findings showing mossy fiber-evoked release of Ca2+ from internal stores (Pozzo-Miller et al., 1996; Yeckel et al., 1999), the latency to the rise in Ca2+ (in some cases more than 1 s following the end of synaptic activity, cf. Fig. 1A), the kinetics of the rise in [Ca2+], and the fact that release occurred independently of transmembrane current, strongly suggested that Ca2+ was released from internal stores under these conditions.
Fig. 1.
Bursts of mossy fiber stimulation elicit release of Ca2+ from internal stores. (A) A brief burst of mossy fiber stimulation (six pulses at 50 Hz) evoked a large, long latency rise in {Ca2+]i (top panel) (in all figures the colored boxes correspond to the site on the neuron where the correspondingly colored traces were recorded). The temporal mismatch between the synaptic response (bar) and the Ca2+ response, as well as the absence of transmembrane current flow (voltage-clamp current, Icl) and membrane potential change (Vm), suggested release of Ca2+ from internal stores and not through VGCCs. (Note: Vm represents a direct measure of the membrane potential, because the whole-cell voltage clamp was made via a discontinuous voltage-clamp amplifier (see Experimental procedures). Previous studies using dual recordings from the soma have indicated that such an amplifier provides an accurate monitor of the true membrane potential in the soma during voltage clamp (Kapur et al., 1998a,b). While perfect voltage control cannot be assured in the red region, any escape from voltage control would have been detected as transmembrane current and/or a change in the discontinuous measure of Vm.) The rise in Ca2+ occurred first in the soma/proximal apical dendrite (in the region of synaptic input, red box) and was totally absent from the mid to basal somal region (green box). Note that the delay in the Ca2+ signals between the red and blue regions suggests a propagated wave of [Ca2+] (see text). (B) Synaptic excitation was required for release. In bridge mode, current injection sufficient for action potential initiation resulted in a Ca2+ response with a time course that correlated in time with membrane depolarization and action potential firing. The rise in postsynaptic Ca2+ was uniform throughout the soma, consistent with Ca2+ influx through VGCCs. In contrast, a brief burst of mossy fiber stimulation (seven pulses at 100 Hz) elicited a Ca2+ signal in the apical soma/proximal dendrite (blue) with a time course that did not correlate with the membrane potential. (C) Increasing the frequency of mossy fiber stimulation from 10 to 50 Hz decreased the latency to release (in all neuron images, the apical direction is upward).
To determine whether mossy fiber synaptic input was required for release of Ca2+ from internal stores or whether intrinsic activation of the cell was sufficient for eliciting release, we compared the spatial and temporal properties of the [Ca2+]i transients evoked by the two different conditions: current injection suprathreshold for action potential generation elicited a transient rise in [Ca2+]i that correlated with changes in membrane potential and was initiated in the basal end of the soma, whereas brief trains of synaptic stimulation evoked a longer duration rise in [Ca2+]i in which an initial rise in [Ca2+]i correlated with action potential firing and was localized to the basal soma region, while a longer latency rise in [Ca2+]i that did not correlate with membrane potential was initiated in the apical somatic/dendritic region (Fig. 1B). Rises in [Ca2+]i that occurred simultaneously with action potential firing are consistent with influx of Ca2+ through VGCCs and rises in [Ca2+]i that occurred well after changes in membrane potential are best explained by release of Ca2+ from internal stores. This difference in time course for [Ca2+]i, evoked by either current injection or mossy fiber stimulation, was seen in all cells tested (n = 6) suggesting that bursts of synaptic stimulation are required for internal release of Ca2+.
A threshold amount of mossy fiber activation appeared to be necessary for the release of Ca2+, because release never appeared to be triggered with single stimuli, regardless of the stimulus intensity (data not shown, n = 39). Furthermore, a burst of stimulation that was previously subthreshold for release could be made supra-threshold by increasing either the number of pulses in the burst (n = 11), or by increasing the frequency of stimulation (n = 5). Although previous studies have described release of Ca2+ in CA3 pyramidal neurons that was evoked by long trains (100 Hz for 1s) of mossy fiber stimulation in the presence of glutamate receptor antagonists (Pozzo-Miller et al., 1996; Yeckel et al., 1999), we found that even brief bursts of stimulation (as short as five stimuli at 20 Hz) in the absence of these antagonists could trigger release. We also found that when we increased the frequency of stimulation, the latency to release of internal Ca2+ decreased (Fig. 1C; n = 6/6), suggesting that the firing frequency of granule cells has some temporal control over internal release in CA3 pyramidal neurons.
Calcium waves
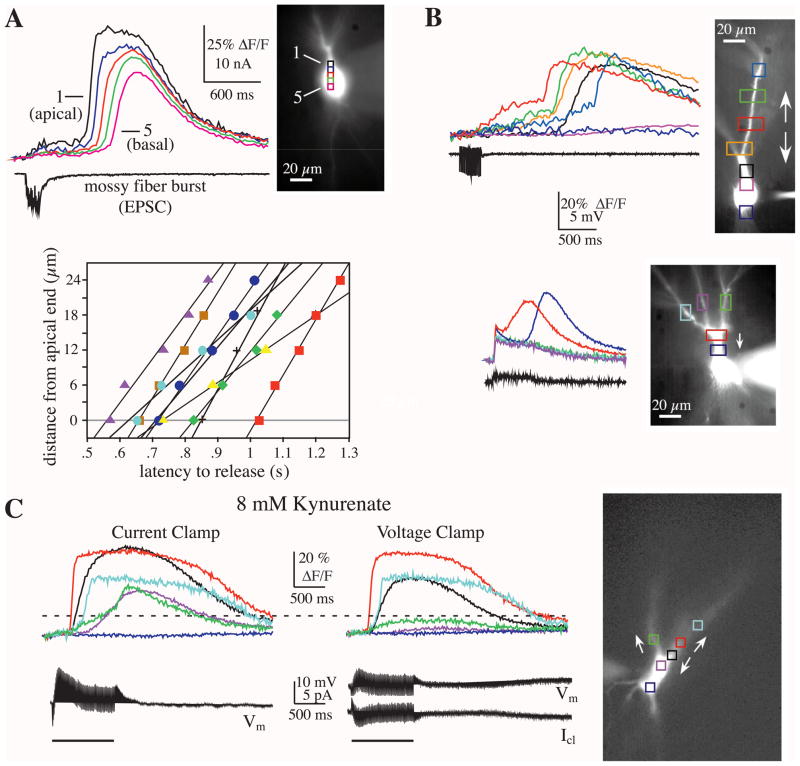
Brief bursts of mossy fiber stimulation (5–20 stimuli at 20 Hz) typically triggered Ca2+ release between the apical end of the soma and the proximal apical dendrite, but not in the mid to basal end of the soma (n = 14; see Fig. 2A for example). Increasing either the number of stimulation pulses or stimulation frequency resulted in a rise in [Ca2+] throughout the soma (n = 6). When release was observed in the soma, the wave of [Ca2+]i elevation always progressed from the apical to the basal compartments of the soma (conduction velocity, 71 ± 21 μm/s; n = 9) (Fig. 2A). Although it was more difficult to detect propagation into the apical dendrites due to the branching of thin dendrites out of the focal plane, in some cases we found that waves of [Ca2+] backpropagated into the apical dendrites (Fig. 2B, C) (Pozzo-Miller et al., 1996; Jaffe and Brown, 1997).
Fig. 2.
Wave propagation of Ca2+ release. (A) Stronger mossy fiber bursts (here, 15 stimuli at 100 Hz) evoked Ca2+ waves that were initiated in the apical soma/proximal dendrite (black box) and propagated in the apical to basal direction. Based on a number of factors, the observed Ca2+ waves represent the propagation of internal release along the neuron (see text). The propagation velocity for individual neurons is plotted below (each symbol represents a different cell). The average velocity for all cells was 71 ± 21 μm/s (n = 9). (B) In some cases, mossy fiber burst stimulation evoked Ca2+ waves that propagated bidirectionally (top panel; see arrows). In other cases, the Ca2+ waves appeared to propagate in one direction (bottom panel; in this case propagation was limited to the soma; the smaller, shorter duration signal in the dendrites was likely due to Ca2+ flux in response to backpropagating sodium action potentials). In both examples, the electrical traces show the voltage recorded while in voltage clamp (five pulses at 40 Hz and 20 pulses at 20 Hz, respectively). (C) Under some conditions, Ca2+ waves were observed to propagate bidirectionally and inter-dendritically. In this example, a low dose of the weakly competitive glutamate antagonist kynurenate was added to the media in order to dampen excitability and gain additional voltage control while in voltage clamp. In current clamp, a long train of stimulation (100 pulses at 100 Hz) elicited the release of Ca2+ from one dendritic branch that propagated up the dendrite, down the dendrite into proximal dendritic trunk and apical soma, and to an adjacent daughter dendrite via the dendritic trunk. In voltage clamp, the soma region was sufficiently clamped to prevent propagation of the Ca2+ wave and also blocked propagation to the adjacent dendrite.
This wave of [Ca2+] elevation likely represents a sequence of release events and for a number of reasons cannot be due to simple diffusion of Ca2+ from the dendrites. First, there was an increasing latency but abrupt onset to the rise in [Ca2+] at increasing distances from site of origin. In contrast, the rise time for the passive diffusion of [Ca2+] would be expected to slow considerably with increasing distance from the origin (Murthy et al., 2000). Second, the propagation time of the Ca2+ waves was linear with distance (Fig. 2A). The velocity of a passive wave of diffusion would instead be expected to be non-linear and decline with increasing distance from the site of origin (Jack et al., 1975). Third, in some cells [Ca2+] propagation was not observed despite having a relatively large Ca2+ signal (both in amplitude and duration) at the apical end of the soma. For example, under some conditions it was possible to selectively block propagation into the soma or a dendritic branch by applying a voltage clamp at the soma (Fig. 2C). Also, in some cases the wave would propagate into one dendritic branch and not another (Fig. 2B). These results suggest that the initiation site for release is located near the proximal dendrites adjacent to active mossy fiber synapses and that release of Ca2+ can propagate bidirectionally, at least for short distances.
Dependence on metabotropic glutamate receptors (mGluRs)
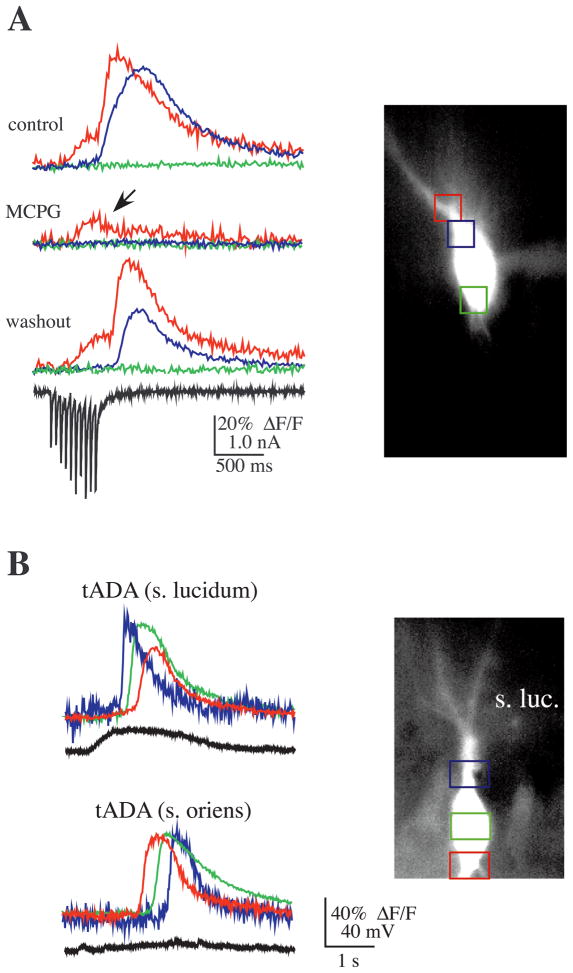
mGluRs have been implicated in the release of Ca2+ from inositol 1,4,5-trisphosphate (IP3) sensitive stores in a number of systems (Berridge, 1993; Abdul-Ghani et al., 1996; Berridge, 1998; Finch and Augustine, 1998; Takechi et al., 1998; Nakamura et al., 1999). Consistent with a functional role for group I mGluRs in mossy fiber synaptic transmission, group I mGluRs have been shown to be located on CA3 dendritic spines, and electron microscopic studies have shown immunolabeling for mGluR1 in stratum lucidum on the thorny excrescences of CA3 pyramidal dendrites on the periphery of postsynaptic densities (Luján et al., 1996). The presence of mGluR1,5 receptors and the requirement for bursts of mossy fiber input to elicit release of Ca2+ from stores suggested the possibility that the mGluR–IP3 pathway might be involved. To test this hypothesis, the mGluR antagonist MCPG (500 μM) was added to the bath. MCPG significantly suppressed a rise in postsynaptic Ca2+ in nine of 10 cells tested (overall peak Ca2+ in MCPG was 33.7 ± 13% of control; n = 10) (Fig. 3A).
Fig. 3.
mGluR activation is required for mossy fiber-evoked Ca2+ release. (A) A typical experiment showing that the addition of the mGluR1,5 antagonist MCPG reversibly blocked mossy fiber-evoked release of Ca2+ from internal stores (20 pulses at 20 Hz). MCPG did not appear to affect a rise in Ca2+ mediated through VGCCs (arrow) during the stimulation. In nine of 10 cases MCPG significantly suppressed mossy fiber-evoked Ca2+ release (peak Ca2+ in MCPG was 33.7 ± 13% of control; n = 10). (B) In the absence of synaptic stimulation, pressure application of the mGluR1,5 agonist tADA elicited Ca2+ release. The Ca2+ wave that spread throughout the soma was initiated at the site closest to the tADA electrode (stratum lucidum, upper panel; stratum oriens, lower panel). As can be seen in the electrical trace measured during application of tADA to stratum lucidum, tADA depolarized the neuron; in contrast, application to stratum oriens did not cause a similar depolarization, obviating the possibility that the membrane depolarization that sometimes occurred during tADA application elicited the rise in Ca2+ through activation of VGCCs. In addition, comparable depolarization by current injection did not appear to cause a significant rise in Ca2+, either by release from stores or by influx through VGCCs (data not shown).
To test further whether activation of postsynaptic metabotropic receptors leads to the release of Ca2+ from stores, we pressure-applied the mGluR1,5 metabotropic agonist, tADA, to the vicinity of the proximal apical and basal dendrites of CA3 pyramidal neurons (mossy fiber axons were not stimulated in these experiments). Brief pulses of tADA evoked a wave of Ca2+ release in the soma (n = 5; Fig. 3B). The somatic Ca2+ wave started near the tADA electrode tip in every case and propagated away from the tip; propagation was not unidirectional because moving the pipette tip to another site resulted in propagation to the original site (Fig. 3B, lower panel). Consistent with previous findings in which application of mGluR1,5 agonists to stratum lucidum elicited a slow inward current (Heuss et al., 1999), tADA often evoked a slow depolarization in the CA3 neurons. This slow depolarization, however, cannot account for the observed rise in [Ca2+]i because, first, it was not always present (compare the top and bottom set of traces in Fig. 3B), second, the rise in [Ca2+]i occurred abruptly and was delayed from the onset of the depolarization, and, third, action potentials are usually required to trigger significant rises in [Ca2+]i in the somata of hippocampal pyramidal neurons (Jaffe et al., 1992). Therefore, it appears that activation of mGluR1 and/or mGluR5 can evoke Ca2+ release in CA3 neurons, consistent with the hypothesis that mossy fiber bursts activate mGluR(s) in stratum lucidum, which in turn initiate Ca2+ release in CA3 pyramidal neurons.
Role of VGCCs in calcium release
The onset of internal Ca2+ release (the point at which an abrupt rise in [Ca2+] was observed) consistently occurred after a slow, small rise in Ca2+ (cf. Fig. 1C). Analysis of this transition point in Ca2+ kinetics was performed by quantifying the point at which the second derivative of ΔF/F was maximal. Variation of this measure was minimal when computed for repeated episodes of release in a given neuron. The variability (i.e. standard deviation) measure was ~3.0% ΔF/F (n = 14 cells, range 0.6–5.9) versus the average peak amplitude of ~60.0% ΔF/F, consistent with the possibility that the transition point in the rate of [Ca2+] rise represents a threshold point before internal release is triggered.
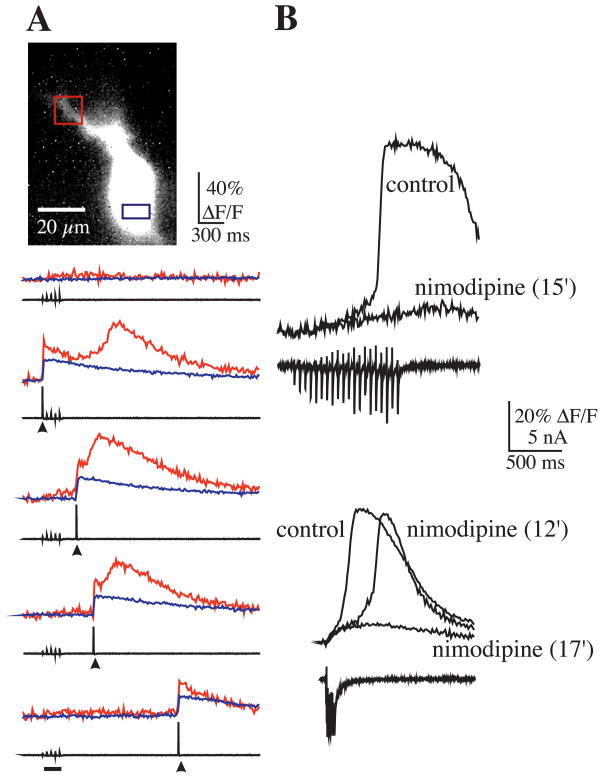
The presence of a threshold for internal Ca2+ release suggested that the rise in [Ca2+]i that precedes internal release might be produced by other mechanisms, such as influx through voltage-gated channels, and might influence mGluR-mediated release from internal stores. Such a mechanism has been described previously in CA1 neurons (Nakamura et al., 1999). To test this hypothesis, brief bursts of mossy fiber stimulation, subthreshold for eliciting release, were paired with a large (60–80 mV), brief (5 ms), step depolarization that resulted in the loss of voltage clamp and the generation of a single action potential. Pairing of the action potential with subthreshold stimuli resulted in the release of Ca2+ locally in the apical region of the soma or base of the apical dendrite (Fig. 4A). This was observed in all cells (n = 7). In two other cells, while release at the apical soma/dendrite was observed in the absence of a spike, release at the basal end of soma was dependent on spiking. The time period during which the pairing resulted in release was not constant across cells, possibly due to variable levels of mGluR activation in the different cells. For example, time intervals between spike and synaptic stimulation that elicited release ranged from −300 ms (spike preceding start of mossy fiber burst) to +600 ms (spike beyond end of the mossy fiber burst). Therefore, these data suggest that Ca2+ influx through VGCC can act synergistically with mGluR-activated mechanisms to induce Ca2+ release.
Fig. 4.
Voltage and Ca2+ dependence of Ca2+ release. (A) Sodium action potentials enable synaptically mediated Ca2+ release. Weak synaptic stimulation was subthreshold for evoking Ca2+ release (five pulses at 20 Hz; stimulation duration indicated by bar). Pairing subthreshold, weak synaptic stimulation with a single action potential (arrow head) within a specific time window (middle three traces) evoked release in the proximal apical dendrite (red traces) but not in the basal soma region (blue traces). When pairing between synaptic stimulation and action potential initiation was sufficiently delayed, only a short latency rise was observed during the action potential and likely resulted from Ca2+ influx through VGCCs. (B) Nimodipine blocked Ca2+ release induced with bursts of mossy fiber stimulation in half the neurons tested (n = 6). Shown here are two examples using different stimulation parameters (top example, 20 pulses at 20 Hz; bottom example, seven pulses at 50 Hz).
To determine whether L-type channels contributed to the synergistic mechanism involved in Ca2+ release, the L-type channel blocker nimodipine was added to the bath. In some cases, nimodipine (10 μM) blocked Ca2+ release evoked with mossy fiber stimulation (n = 3) (Fig. 4B), but had no effect on other cells (n = 3). Moreover, in cells in which nimodipine did block release, we found that release could be restored with stronger (i.e. higher frequency or longer duration) stimulation. It is thus likely that other types of VGCCs, which are known to be present in these neurons (Fisher et al., 1990; Mogul and Fox, 1991), also contribute to the Ca2+ influx that helps to trigger Ca2+ release.
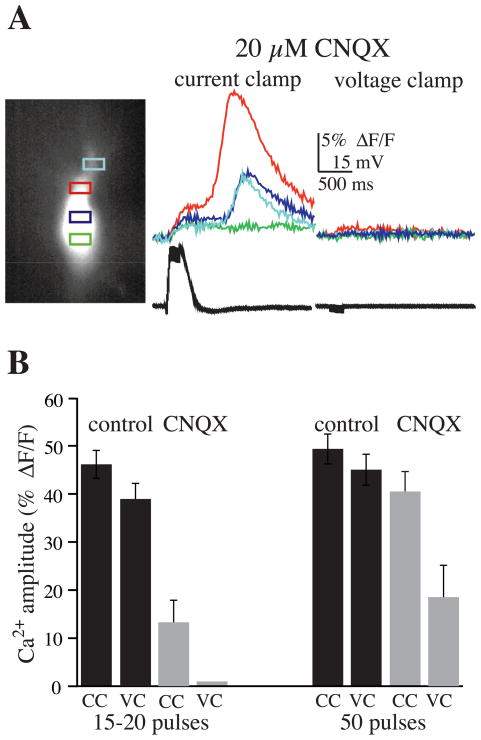
To further examine this possibility, we attempted to gain additional control over the cell membrane potential during bursts of stimulation by including the ionotropic glutamate antagonist CNQX (10–40 μM). By suppressing fast synaptic transmission (and subsequent depolarization), while clamping cells to ~−75 mV, we hoped to eliminate all Ca2+ influx through VGCCs. First, we evaluated release in the presence of CNQX while recording responses in current-clamp mode. In this series of experiments, CNQX suppressed release (control, 46 ± 2.9% ΔF/F; CNQX, 13.3 ± 4.6% ΔF/F; n = 9; P <0.001) evoked by brief bursts of stimulation (20 pulses at 100 Hz) (Fig. 5). Although CNQX blocked synaptic responses to single stimulation pulses, during bursts of stimulation a plateau depolarization (10–30 mV) was always observed. Associated with this plateau potential was a small rise in [Ca2+]i. When the membrane potential was clamped to ~−75 mV with CNQX present, there was little voltage escape during the brief bursts, and a rise in [Ca2+]i was totally blocked (0% ΔF/F). These findings were significantly different from the rise in [Ca2+]i observed in cells that were not voltage-clamped in the presence of CNQX (P <0.005). When longer trains of stimulation (50 pulses) were given, CNQX had no effect on release in current-clamp mode (control 45.1 ± 3.0%; CNQX 40.6 ± 4.0% ΔF/F; n = 9; P >0.1), but significantly suppressed release in voltage clamp (18.6 ± 6.3% ΔF/F; P <0.005) (Fig. 5).
Fig. 5.
Suppressing both fast synaptic transmission and VGCCs prevents internal Ca2+ release evoked by brief bursts. (A) In this experiment CNQX (20 μM) alone did not block Ca2+ release evoked by bursts of mossy fiber stimulation (20 pulses at 100 Hz) while in current-clamp mode. When the cell was voltage-clamped to ~−75 mV (still in the presence of CNQX), however, there was little voltage escape (see voltage trace), and consequently, internal Ca2+ release was completely blocked, providing further support for the hypothesis that a threshold rise in cytosolic [Ca2+] is necessary for internal release. (B) Summary graph showing that CNQX (20 μM) suppressed Ca2+ release with brief bursts of stimulation (<20 pulses), and totally blocked release when cells were voltage-clamped to rest (n = 9). When long trains of stimulation (50 pulses) were given, however, CNQX had little effect on internal release when in current-clamp mode (CC), and only suppressed release when in voltage-clamp mode (VC) (n = 9).
Mechanisms of internal calcium release
The implication of mGluRs in the release mechanism, and previous work suggesting the involvement of IP3 in mossy fiber-evoked release when fast synaptic transmission was blocked (Pozzo-Miller et al., 1996), led us to test whether IP3 receptors were required for release under these conditions. The presence of a constant [Ca2+] threshold for release suggested that Ca2+-induced Ca2+ release might also be involved, although this threshold could reflect the Ca2+ dependence of release from IP3 sensitive stores. To assess the contribution of postsynaptic IP3 and ryanodine receptors to release, the antagonists heparin (1 mM) or dantrolene (20 μM to 1 mM) were included in the recording pipette, respectively. Heparin blocked Ca2+ release even with very strong synaptic stimuli (100 stimuli at 100 Hz) (n = 5/5, Fig. 6A). In three cells, [Ca2+]i increased with a time course corresponding to the depolarization caused by synaptic input, whereas in the other two cells no [Ca2+]i elevation was observed with synaptic stimulation. Action potentials evoked while in either current or voltage clamp (the latter due to voltage escape during clamp) elicited typical Ca2+ responses (Fig. 6A), although the number of action potentials elicited with depolarizing pulses in current clamp appeared to be fewer than normal (data not shown). Thus, activation of the IP3 receptor pathway, subsequent to mGluR activation, appears to be involved in the release of Ca2+ in CA3 pyramidal neurons.
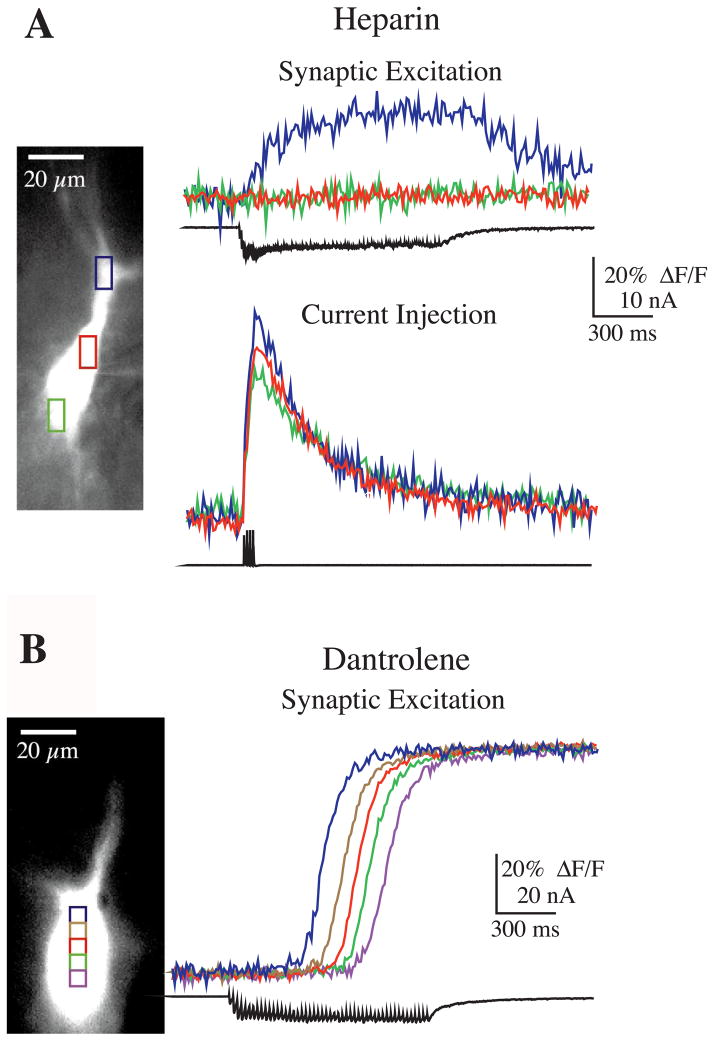
Fig. 6.
Role of IP3 and ryanodine receptors in Ca2+ release. (A) Intracellular heparin (1 mM) blocked Ca2+ release. (Top panel) The rise in Ca2+ in the proximal apical dendrite (blue) occurred simultaneously with synaptic stimulation (100 pulses at 100 Hz), and most notably, not beyond the stimulation period, consistent with influx through VGCCs and not release from internal stores (i.e. there was not a long latency response). (Bottom panel) In the same cell, current injection sufficient for evoking action potentials elicited a rise in Ca2+ influx through VGCCs (four action potentials were evoked at 75 Hz; escape voltage shown in bottom trace). (B) The ryanodine antagonist dantrolene (500 μM) did not block mossy fiber-evoked Ca2+ release or the propagation of a Ca2+ wave (50 pulses at 50 Hz).
Dantrolene, a ryanodine receptor antagonist, did not block release with brief or long bursts of mossy fiber stimulation (n = 7), nor did it block the propagation of Ca2+ waves from apical to basal ends of the soma (n = 3; Fig. 6B). Furthermore, in three cells loaded with dantrolene, action potentials still enabled release when paired with burst stimuli subthreshold for evoking release, supporting the hypothesis that the Ca2+ threshold for release reflects the Ca2+ dependence of IP3 receptors, and not simply calcium-induced calcium release via ryanodine receptor activation. Although our data show that ryanodine receptors do not appear to contribute to release or propagation of Ca2+ waves near the soma and proximal apical dendrites, it is still possible that they are involved in these processes in the dendrites and spines where they are present at higher densities (Fotuhi et al., 1993; Sharp et al., 1993).
Opioid receptor antagonists do not prevent internal release of calcium
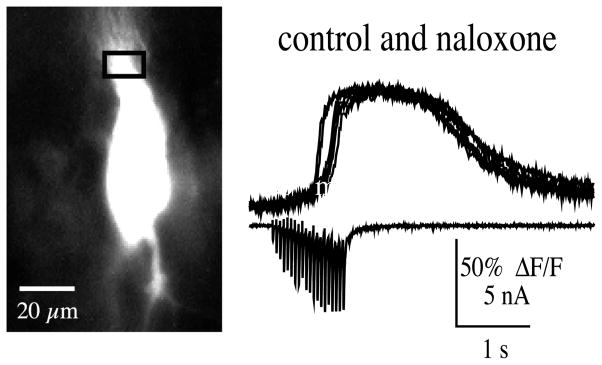
Because opioid peptides are released with high frequency stimulation of mossy fibers (Wagner et al., 1990; Caudle et al., 1991), the effects of the opioid receptor antagonists naltrexone (5 μM) and naloxone (10 μM) on internal Ca2+ release were tested (Fig. 7). In all experiments, opioid antagonists did not appear to block Ca2+ release from CA3 pyramidal neurons (n = 7). In some cases the experiment was done in current clamp and Ca2+ release determined by the presence of a secondary rise of the Ca2+ signal (that is, following the primary rise in Ca2+ via influx through VGCCs) evoked by mossy fiber bursts of 25–100 pulses at 100 Hz (n = 3). In other experiments, Ca2+ release was isolated in voltage clamp and milder mossy fiber bursts (20 stimuli at 20 Hz) were used (n = 4) (Fig. 7). In one case, naloxone blocked release with 20 Hz stimulation, the apparent block, however, was overcome when stimulus frequency was increased to 50 Hz.
Fig. 7.
Naloxone (10 μM) did not block mossy fiber-evoked Ca2+ release (20 pulses at 20 Hz). Optical traces recorded before and up to 30 min in naloxone (at 4 min intervals) are overlaid. Bottom trace shows the excitatory postsynaptic currents during the burst.
DISCUSSION
The results presented here show that relatively brief bursts of mossy fiber activation can produce large increases in postsynaptic [Ca2+] through release from internal stores. These findings support previous studies in which long trains of mossy fiber stimulation in the presence of ionotropic glutamate blockers were shown to evoke Ca2+ release that was prevented by depleting Ca2+ stores with either ryanodine or thapsigargin (Yeckel et al., 1999; Pozzo-Miller et al., 1996). As with these previous reports, we also found that release depended on group I mGluR activation, which in turn triggers the IP3 second messenger pathway and subsequent binding to IP3 receptors. In addition, we show that Ca2+ influx through VGCCs contributes to this release process. Our results are most readily explained by a model in which IP3 receptors require both IP3 and Ca2+ for opening. This synergistic action of IP3 and Ca2+ has been well documented in several preparations, including isolated IP3 receptors in lipid bilayers (Ehrlich et al., 1994; Kaftan et al., 1997), microsomal vesicles (Finch et al., 1991), CA1 pyramidal neurons (Nakamura et al., 1999), and cerebellar Purkinje cells (for review, see Simpson et al., 1995). In our experiments, the synergistic action of IP3 and Ca2+ was evident from the apparent [Ca2+]i threshold for release, the sensitivity of release to heparin and mGluR antagonist, the facilitatory effect of action potentials on release, and the sensitivity of release to nimodipine. While 20 μM CNQX reduced the magnitude of the Ca2+ release evoked with brief trains, it was not blocked under these conditions. Furthermore, during the stimulation train there was still a substantial depolarization that produced a small rise in [Ca2+]i. If a voltage clamp to the soma was effective in reducing this plateau depolarization, and the resulting rise in [Ca2+]i, then the release of Ca2+ from internal stores could be prevented for brief but not long trains of stimulation. These data thus support a role for both IP3 and Ca2+ in triggering Ca2+ release. The lack of effect of intracellular dantrolene suggests that ryanodine receptors do not play a required role in either the release or the waves of release that were observed to propagate towards the soma. Therefore, the waves must be sustained by IP3 receptors and the released Ca2+ although ryanodine receptors could still play some supporting role.
These findings may help to explain some of the inconsistent results that have been reported in conjunction with the induction of LTP at this synapse (Castillo et al., 1994; Ito and Sugiyama, 1991; Jaffe and Johnston, 1990; Langdon et al., 1998; Urban and Barrionuevo, 1996). For example, even in the absence of a large voltage-gated Ca2+ influx, increases in cytosolic Ca2+ can still be achieved by strongly activating the mossy fibers leading to a release of Ca2+ from internal stores (Yeckel et al., 1999). The ineffectiveness of postsynaptic hyper-polarization protocols or nimodipine in blocking mossy fiber LTP when long trains are used for its induction (Zalutsky and Nicoll, 1990) might be explained on this basis. With brief bursts of mossy fiber activity, postsynaptic depolarization and action potentials may play a larger role in eliciting increases in cytosolic Ca2+ due to the synergism between voltage-gated Ca2+ influx and mGluR activation. The exact physiological conditions under which Ca2+, mGluRs, and IP3 receptors operate at this synapse, however, remain to be determined.
Acknowledgments
We thank Rick Gray, Mahmud Haque, and Rob Gereau for help with aspects of this project. This work was supported by NIH Grants MH44754, MH48432, and NS37444, and the Hankamer Foundation.
Abbreviations
- [Ca2+]i
internal calcium ion concentration
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- HEPES
N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)
- IP3
inositol 1,4,5-trisphosphate
- LTP
long-term potentiation
- MCPG
α-methyl-4-carboxyphenylglycine
- mGluR
metabotropic glutamate receptor
- NMDA
N-methyl-D-aspartate
- tADA
trans-azetidine-2,4-dicarboxylic acid
- VGCC
voltage-gated calcium channel
References
- Abdul-Ghani MA, Valiante TA, Carlen PL, Pennefather PS. Metabotropic glutamate receptors coupled to IP3 production mediate inhibition of IAHP in rat dentate granule neurons. J Neurophysiol. 1996;76:2691–2700. doi: 10.1152/jn.1996.76.4.2691. [DOI] [PubMed] [Google Scholar]
- Avery RB, Johnston D. Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J Neurosci. 1996;16:5567–5582. doi: 10.1523/JNEUROSCI.16-18-05567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12:261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Caudle RM, Wagner JJ, Chavkin C. Endogenous opioids released from perforant path modulate norepinephrine actions and inhibitory postsynaptic potentials in guinea pig CA3 pyramidal cells. J Pharmacol Exp Ther. 1991;258:18–26. [PubMed] [Google Scholar]
- Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+-release channels. Trends Pharmacol Sci. 1994;15:145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Elliott EM, Malouf AT, Catterall WA. Role of calcium channel subtypes in calcium transients in hippocampal CA3 neurons. J Neurosci. 1995;15:6433–6444. doi: 10.1523/JNEUROSCI.15-10-06433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Fisher RE, Gray R, Johnston D. Properties and distribution of single voltage-gated calcium channels in adult hippocampal neurons. J Neurophysiol. 1990;64:91–104. doi: 10.1152/jn.1990.64.1.91. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Sharp AH, Glatt CE, Hwang PM, von Krosigk M, Snyder SH, Dawson TM. Differential localization of phospho-inositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci. 1993;13:2001–2012. doi: 10.1523/JNEUROSCI.13-05-02001.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuss C, Scanziani M, Gahwiler BH, Gerber U. G-protein-independent signaling mediated by metabotropic glutamate receptors. Nat Neurosci. 1999;2:1070–1077. doi: 10.1038/15996. [DOI] [PubMed] [Google Scholar]
- Ito I, Sugiyama H. Roles of glutamate receptors in long-term potentiation at hippocampal mossy fiber synapses. NeuroReport. 1991;2:333–336. doi: 10.1097/00001756-199106000-00008. [DOI] [PubMed] [Google Scholar]
- Jack JJB, Noble D, Tsien RW. Electric Current Flow in Excitable Cells. London: Oxford University Press; 1975. p. 36. [Google Scholar]
- Jaffe DB, Brown TH. Calcium dynamics in thorny excrescences of CA3 pyramidal neurons. J Neurophysiol. 1997;78:10–18. doi: 10.1152/jn.1997.78.1.10. [DOI] [PubMed] [Google Scholar]
- Jaffe D, Johnston D. The induction of long-term potentiation at hippocampal mossy fibers follows a Hebbian rule. J Neurophysiol. 1990;64:948–960. doi: 10.1152/jn.1990.64.3.948. [DOI] [PubMed] [Google Scholar]
- Jaffe DB, Johnston D, Lasser-Ross N, Lisman JE, Miyakawa H, Ross WN. The spread of Na+ spikes determines the pattern of dendritic Ca2+ entry into hippocampal neurons. Nature. 1992;357:244–246. doi: 10.1038/357244a0. [DOI] [PubMed] [Google Scholar]
- Kaftan EJ, Ehrlich BE, Watras J. Inositol 1,4,5-trisphosphate (InsP3) and calcium interact to increase the dynamic range of InsP3 receptor-dependent calcium signaling. J Gen Physiol. 1997;110:529–538. doi: 10.1085/jgp.110.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A, Yeckel MF, Gray R, Johnston D. L-type calcium channels are required for one form of hippocampal mossy fiber LTP. J Neurophysiol. 1998a;79:2181–2190. doi: 10.1152/jn.1998.79.4.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A, Yeckel MF, Johnston D. Postsynaptic factors that contribute to hippocampal mossy fiber LTP. Soc Neurosci Abstr. 1998b;24:9. [Google Scholar]
- Langdon RB, Johnson JW, Barrionuevo G. Posttetanic potentiation and presynaptically induced long-term potentiation at the mossy fiber synapse in rat hippocampus. J Neurobiol. 1995;26:370–385. doi: 10.1002/neu.480260309. [DOI] [PubMed] [Google Scholar]
- Luján R, Nusser Z, Roberts JDB, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Mogul DJ, Fox AP. Evidence for multiple types of Ca2+ channels in acutely isolated hippocampal CA3 neurones of the guineapig. J Physiol (Lond) 1991;433:259–281. doi: 10.1113/jphysiol.1991.sp018425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF. Dynamics of dendritic calcium transients evoked by quantal release at excitatory hippocampal synapses. Proc Natl Acad Sci USA. 2000;97:901–906. doi: 10.1073/pnas.97.2.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Barbara JG, Nakamura K, Ross WN. Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron. 1999;24:727–737. doi: 10.1016/s0896-6273(00)81125-3. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Petrozzino JJ, Connor JA. G protein-coupled receptors mediate a fast excitatory postsynaptic current in CA3 pyramidal neurons in hippocampal slices. J Neurosci. 1995;15:8320–8330. doi: 10.1523/JNEUROSCI.15-12-08320.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Petrozzino JJ, Golarai G, Connor JA. Ca2+ release from intracellular stores induced by afferent stimulation of CA3 pyramidal neurons in hippocampal slices. J Neurophysiol. 1996;76:554–562. doi: 10.1152/jn.1996.76.1.554. [DOI] [PubMed] [Google Scholar]
- Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PB, Challiss J, Nahorski SR. Neuronal Ca2+ stores: activation and function. Trends Neurosci. 1995;18:299–306. doi: 10.1016/0166-2236(95)93919-o. [DOI] [PubMed] [Google Scholar]
- Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396:757–760. doi: 10.1038/25547. [DOI] [PubMed] [Google Scholar]
- Urban NN, Barrionuevo G. Induction of hebbian and non-hebbian mossy fiber long-term potentiation by distinct patterns of high-frequency stimulation. J Neurosci. 1996;16:4293–4299. doi: 10.1523/JNEUROSCI.16-13-04293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JJ, Caudle RM, Chavkin C. Stimulation of endogenous opioid release displaces mu receptor binding in rat hippocampus. Neuroscience. 1990;37:45–53. doi: 10.1016/0306-4522(90)90190-f. [DOI] [PubMed] [Google Scholar]
- Williams SH, Johnston D. Long-term potentiation of hippocampal mossy fiber synapses is blocked by postsynaptic injection of calcium chelators. Neuron. 1989;3:583–588. doi: 10.1016/0896-6273(89)90268-7. [DOI] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]