Abstract
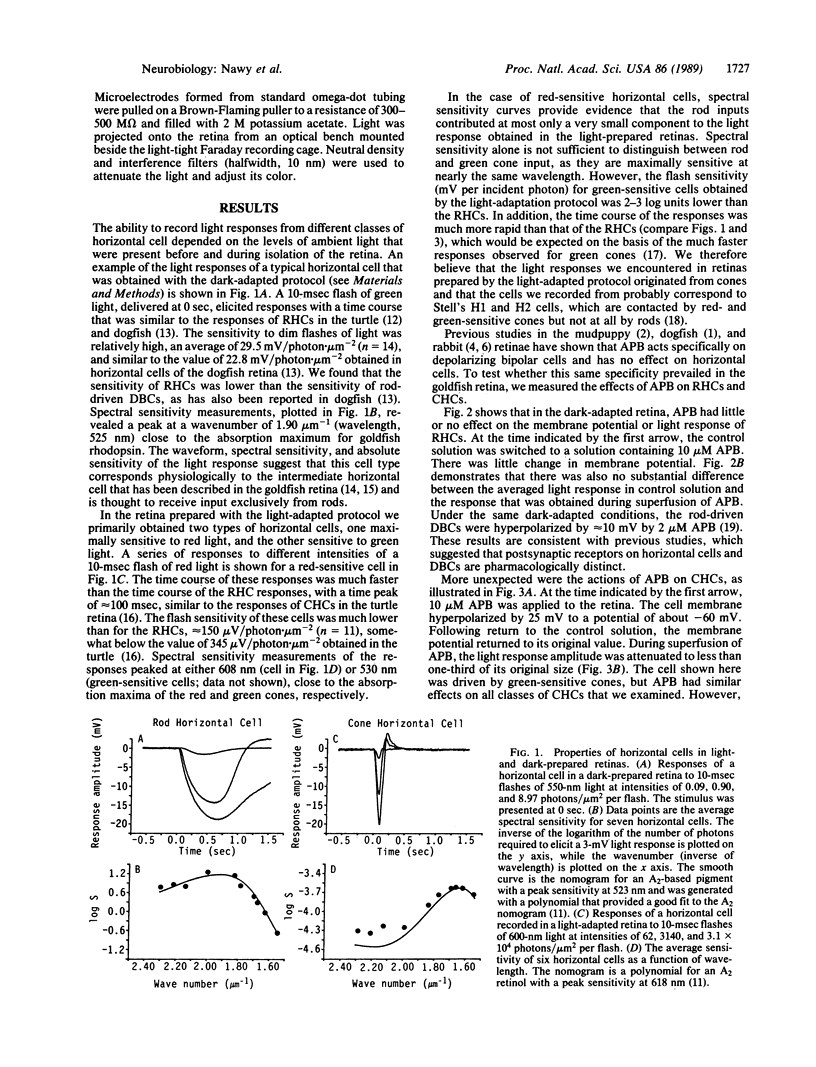
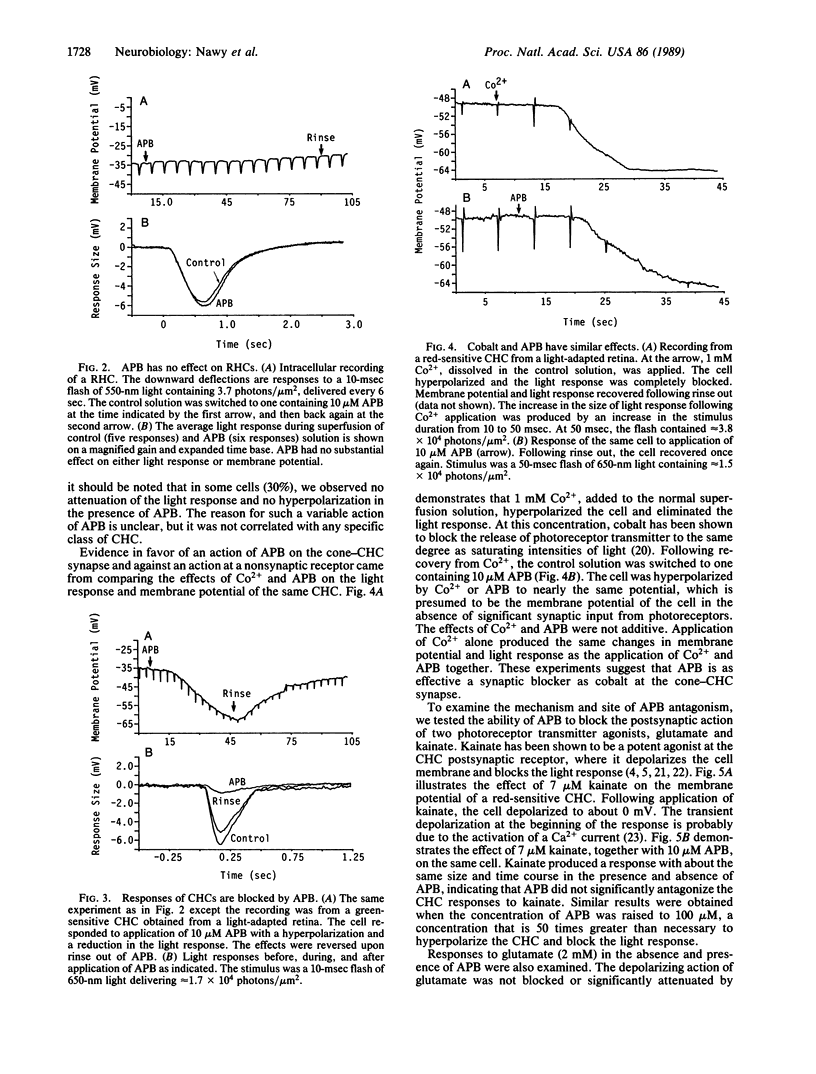
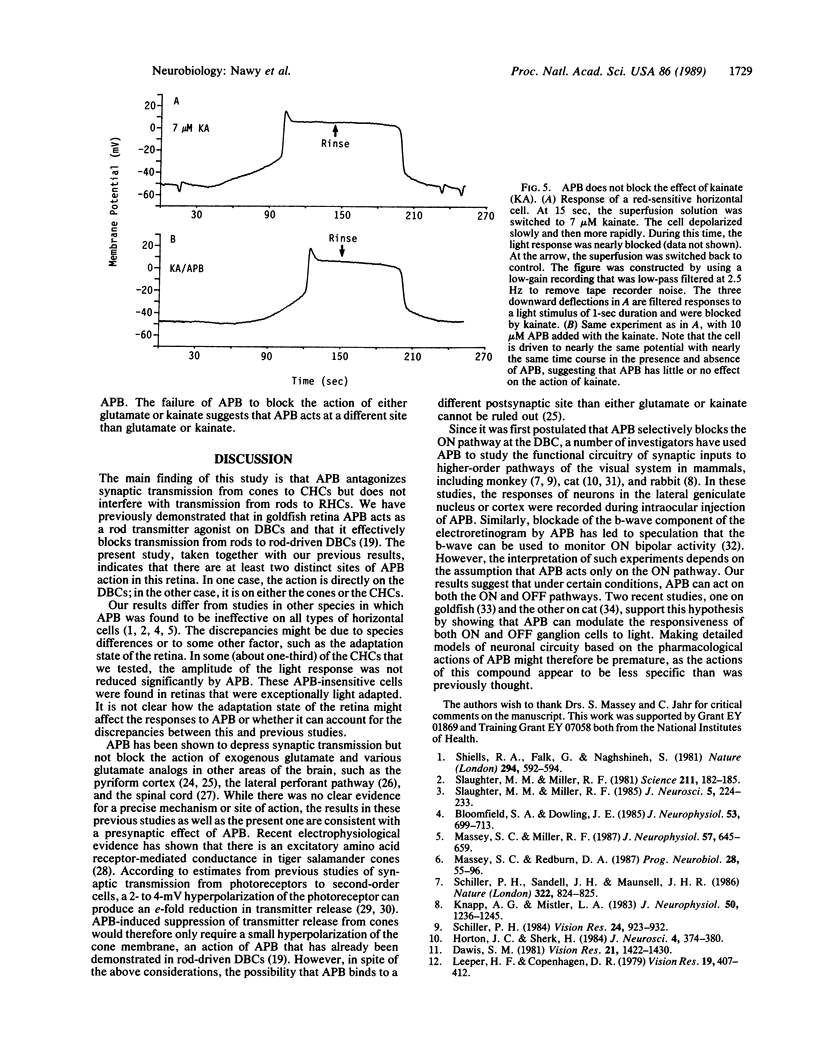
In the retina, the glutamate analog 2-amino-4-phosphonobutyrate (APB) distinguishes a class of glutamate receptors that is thought to be found only on depolarizing bipolar cells (DBCs). We now report that APB is a potent antagonist of cone-driven horizontal cells in the goldfish retina. APB hyperpolarized the membrane to the same potential as cobalt Ringer's and blocked the light responses. APB acted specifically on the cone pathway, as it had no effect on rod-driven horizontal cells. The lowest effective APB concentration for antagonistic action on the horizontal cells (approximately 2 microM) was similar to the concentration for agonist action on DBCs. APB was not able to block the actions of exogenous glutamate or kainate on horizontal cells. We propose that the action of APB on the cone-horizontal cell synapse is mediated at a site that is distinct from the glutamate and kainate binding site. Therefore, APB is most probably acting at a different locus on the synaptic glutamatergic receptors of the horizontal cells or at presynaptic receptors located on the cones themselves.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Falk G. Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. J Physiol. 1980 Mar;300:115–150. doi: 10.1113/jphysiol.1980.sp013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgum J. H., Copenhagen D. R. Synaptic transfer of rod signals to horizontal and bipolar cells in the retina of the toad (Bufo marinus). J Physiol. 1988 Feb;396:225–245. doi: 10.1113/jphysiol.1988.sp016960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield S. A., Dowling J. E. Roles of aspartate and glutamate in synaptic transmission in rabbit retina. I. Outer plexiform layer. J Neurophysiol. 1985 Mar;53(3):699–713. doi: 10.1152/jn.1985.53.3.699. [DOI] [PubMed] [Google Scholar]
- Bolz J., Wässle H., Thier P. Pharmacological modulation of on and off ganglion cells in the cat retina. Neuroscience. 1984 Jul;12(3):875–885. doi: 10.1016/0306-4522(84)90176-3. [DOI] [PubMed] [Google Scholar]
- Dawis S. M. Polynomial expressions of pigment nomograms. Vision Res. 1981;21(9):1427–1430. doi: 10.1016/0042-6989(81)90250-9. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T., Madison D., Lynch G. Synaptic transmission is required for initiation of long-term potentiation. Brain Res. 1978 Jul 14;150(2):413–417. doi: 10.1016/0006-8993(78)90293-7. [DOI] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Jones A. W., Smith D. A., Watkins J. C. The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br J Pharmacol. 1982 Jan;75(1):65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori N., Auker C. R., Braitman D. J., Carpenter D. O. Pharmacologic sensitivity of amino acid responses and synaptic activation of in vitro prepyriform neurons. J Neurophysiol. 1982 Dec;48(6):1289–1301. doi: 10.1152/jn.1982.48.6.1289. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Sherk H. Receptive field properties in the cat's lateral geniculate nucleus in the absence of on-center retinal input. J Neurosci. 1984 Feb;4(2):374–380. doi: 10.1523/JNEUROSCI.04-02-00374.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Shimazaki H. Synaptic transmission from photoreceptors to bipolar and horizontal cells in the carp retina. Cold Spring Harb Symp Quant Biol. 1976;40:537–546. doi: 10.1101/sqb.1976.040.01.050. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Yamada M. S-potentials in the dark-adapted retina of the carp. J Physiol. 1972 Dec;227(1):261–273. doi: 10.1113/jphysiol.1972.sp010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp A. G., Mistler L. A. Response properties of cells in rabbit's lateral geniculate nucleus during reversible blockade of retinal on-center channel. J Neurophysiol. 1983 Nov;50(5):1236–1245. doi: 10.1152/jn.1983.50.5.1236. [DOI] [PubMed] [Google Scholar]
- Knapp A. G., Schiller P. H. The contribution of on-bipolar cells to the electroretinogram of rabbits and monkeys. A study using 2-amino-4-phosphonobutyrate (APB). Vision Res. 1984;24(12):1841–1846. doi: 10.1016/0042-6989(84)90016-6. [DOI] [PubMed] [Google Scholar]
- Leeper H. F., Copenhagen D. R. Mixed rod-cone responses in horizontal cells of snapping turtle retina. Vision Res. 1979;19(4):407–412. doi: 10.1016/0042-6989(79)90105-6. [DOI] [PubMed] [Google Scholar]
- Massey S. C., Miller R. F. Excitatory amino acid receptors of rod- and cone-driven horizontal cells in the rabbit retina. J Neurophysiol. 1987 Mar;57(3):645–659. doi: 10.1152/jn.1987.57.3.645. [DOI] [PubMed] [Google Scholar]
- Massey S. C., Redburn D. A. Transmitter circuits in the vertebrate retina. Prog Neurobiol. 1987;28(1):55–96. doi: 10.1016/0301-0082(87)90005-0. [DOI] [PubMed] [Google Scholar]
- Murakami M., Takahashi K. Calcium action potential and its use for measurement of reversal potentials of horizontal cell responses in carp retina. J Physiol. 1987 May;386:165–180. doi: 10.1113/jphysiol.1987.sp016528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S., Copenhagen D. R. Multiple classes of glutamate receptor on depolarizing bipolar cells in retina. Nature. 1987 Jan 1;325(6099):56–58. doi: 10.1038/325056a0. [DOI] [PubMed] [Google Scholar]
- Rowe J. S., Ruddock K. H. Depolarization of retinal horizontal cells by excitatory amino acid neurotransmitter agonists. Neurosci Lett. 1982 Jun 30;30(3):257–262. doi: 10.1016/0304-3940(82)90409-8. [DOI] [PubMed] [Google Scholar]
- Sarantis M., Everett K., Attwell D. A presynaptic action of glutamate at the cone output synapse. Nature. 1988 Mar 31;332(6163):451–453. doi: 10.1038/332451a0. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Sandell J. H., Maunsell J. H. Functions of the ON and OFF channels of the visual system. 1986 Aug 28-Sep 3Nature. 322(6082):824–825. doi: 10.1038/322824a0. [DOI] [PubMed] [Google Scholar]
- Schiller P. H. The connections of the retinal on and off pathways to the lateral geniculate nucleus of the monkey. Vision Res. 1984;24(9):923–932. doi: 10.1016/0042-6989(84)90067-1. [DOI] [PubMed] [Google Scholar]
- Schnapf J. L., Copenhagen D. R. Differences in the kinetics of rod and cone synaptic transmission. Nature. 1982 Apr 29;296(5860):862–864. doi: 10.1038/296862a0. [DOI] [PubMed] [Google Scholar]
- Shiells R. A., Falk G., Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981 Dec 10;294(5841):592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981 Jan 9;211(4478):182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. Characterization of an extended glutamate receptor of the on bipolar neuron in the vertebrate retina. J Neurosci. 1985 Jan;5(1):224–233. doi: 10.1523/JNEUROSCI.05-01-00224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci. 1983 Aug;3(8):1701–1711. doi: 10.1523/JNEUROSCI.03-08-01701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell W. K., Lightfoot D. O. Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol. 1975 Feb 15;159(4):473–502. doi: 10.1002/cne.901590404. [DOI] [PubMed] [Google Scholar]
- Stell W. K. The structure and relationships of horizontal cells and photoreceptor-bipolar synaptic complexes in goldfish retina. Am J Anat. 1967 Sep;121(2):401–423. doi: 10.1002/aja.1001210213. [DOI] [PubMed] [Google Scholar]
- Thibos L. N., Werblin F. S. The response properties of the steady antagonistic surround in the mudpuppy retina. J Physiol. 1978 May;278:79–99. doi: 10.1113/jphysiol.1978.sp012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Mullen J. M., Hori N., Carpenter D. O. Receptors for excitatory amino acids on neurons in rat pyriform cortex. J Neurophysiol. 1986 Jun;55(6):1283–1294. doi: 10.1152/jn.1986.55.6.1283. [DOI] [PubMed] [Google Scholar]


