Abstract
Amber is of great paleontological importance because it preserves a diverse array of organisms and associated remains from different habitats in and close to the amber-producing forests. Therefore, the discovery of amber inclusions is important not only for tracing the evolutionary history of lineages with otherwise poor fossil records, but also for elucidating the composition, diversity, and ecology of terrestrial paleoecosystems. Here, we report a unique find of African amber with inclusions, from the Cretaceous of Ethiopia. Ancient arthropods belonging to the ants, wasps, thrips, zorapterans, and spiders are the earliest African records of these ecologically important groups and constitute significant discoveries providing insight into the temporal and geographical origins of these lineages. Together with diverse microscopic inclusions, these findings reveal the interactions of plants, fungi and arthropods during an epoch of major change in terrestrial ecosystems, which was caused by the initial radiation of the angiosperms. Because of its age, paleogeographic location and the exceptional preservation of the inclusions, this fossil resin broadens our understanding of the ecology of Cretaceous woodlands.
Keywords: Arachnida, Ethiopia, Hexapoda, microorganisms, paleoecology
Ambers contain very important terrestrial taphocenoses. Arthropods and other invertebrates, small reptiles, feathers, mammal hairs, plants, and various microbes from different habitats in and close to the amber forests are commonly preserved (1–6). Ambers and other fossil tree resins are found in hundreds of Old and New World localities (7), with particular abundance in the Cretaceous and the Eocene to Miocene. Until now, most amber deposits have been discovered on the former northern supercontinent Laurasia (SI Appendix, Fig. S1). With the exception of the Cretaceous Lebanese and Jordanian ambers (8, 9) and the Eocene Indian and Miocene Amazonian ambers (10, 11), no fossiliferous amber deposit was known from the southern continents that formerly formed Gondwana.
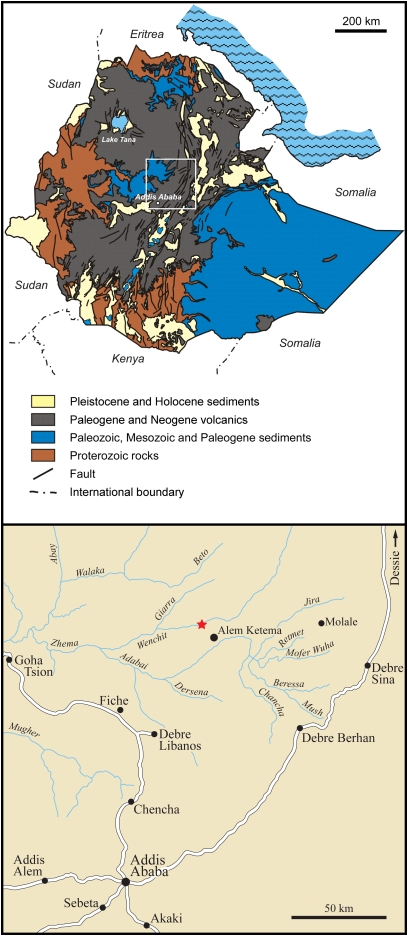
Here, we report a unique fossiliferous African amber. The amber pieces were discovered within the Debre Libanos Sandstone Unit from the northwestern Plateau of Ethiopia (Fig. 1). Because this geologic unit lacks index fossils (12, 13), we used independent age-relevant information from the amber itself, its inclusions, and the sporomorphs of the amber-bearing layer to date the amber. Combined analysis of these physicochemical and biological parameters revealed a Late Cenomanian age (≈93–95 million years old) for the amber (SI Appendix).
Fig. 1.
Location of the Ethiopian amber deposit. The white rectangle on the geologic map indicates the area shown in detail. The red asterisk designates the locality of the outcrop (10°08'45'' N latitude, 38°57'56'' E longitude). Area map redrawn from Assefa (12).
This fossil resin provides a unique window into a mid-Cretaceous African woodland ecosystem and helps to elucidate the trophic relationships within this environment. Diverse inclusions of arthropods, microorganisms, and plant remains reveal manifold interactions of plants, fungi, and animals during the early phase of angiosperm evolution.
Results and Discussion
Amber Characteristics.
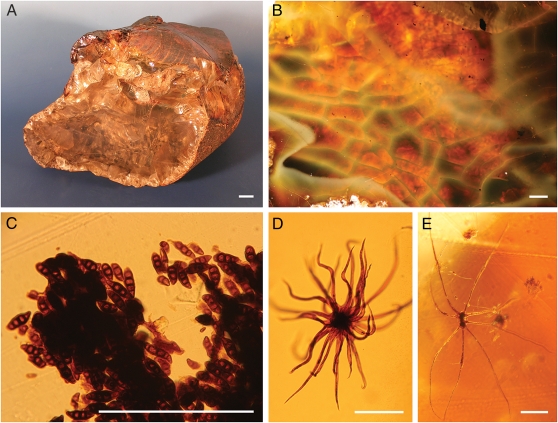
The amber pieces are colorful and translucent (Fig. 2 A and B). Chemical analysis of the Ethiopian amber indicates that it is a Class Ic amber. This result suggests Cheirolepidiaceae did not produce the resin, despite the presence of pollen grains of this Mesozoic conifer family in the amber-bearing sediment. Infrared spectroscopy shows that this amber is unique compared with all other fossil resins studied to date. Although we do not have conclusive information regarding its botanical source, the resin could have conceivably been produced by a previously unknown Cretaceous gymnosperm or possibly even by a mid- to late-Cretaceous angiosperm (SI Appendix).
Fig. 2.
Ethiopian amber and its microinclusions. (A) Large translucent piece of amber of ≈1150 g (NHMW, N3881). (B) Color of amber pieces (MB. Pb. 2009/205). (C) Curvularia-like fungal spores in an insect fecal pellet (MB. Pb. 2009/201). (D) Stellate hair resembling those of modern tree ferns of the family Cyatheaceae (NHMW, N6964). (E) Stellate hair resembling those of the modern epiphytic filmy fern family Hymenophyllaceae (MB.Pb. 2009/206). (Scale bars: A, 1 cm, B, 1 mm; C–E, 100 μm.)
Arthropods.
Arthropod inclusions are abundant in this amber, with 30 fossil specimens preserved in nine of the studied pieces. These fossils cover an impressive diversity, including the arachnid orders Acari and Araneae, and at least 13 families of Hexapoda in the orders Collembola, Psocoptera, Hemiptera, Thysanoptera, Zoraptera, Lepidoptera, Coleoptera, Diptera, and Hymenoptera (SI Appendix, Table S1).
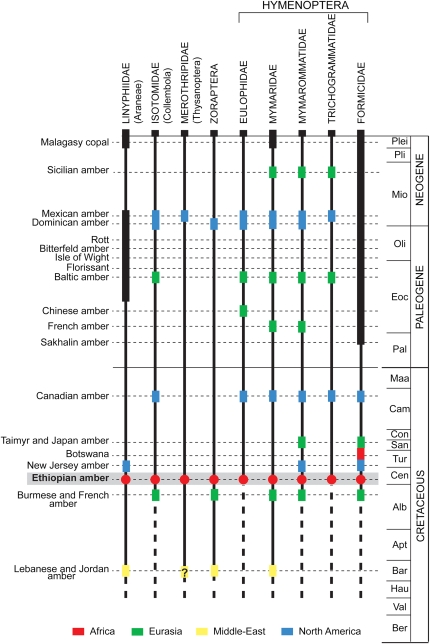
There is a large gap in the Mesozoic terrestrial arthropod fossil record from the African continent. Compression fossils are found in only one insect-bearing locality in the Triassic, one in the Jurassic, and five in the Cretaceous (14). Thus, the Ethiopian amber assemblage is of major significance for understanding the biogeography and evolutionary history of African biota. Preliminary identification of the inclusions has revealed noteworthy discoveries: Most specimens represent a unique fossil record of their group from Africa, and some are among the oldest records in the world (Figs. 3 and 4, and SI Appendix, Fig. S10).
Fig. 3.
Assorted arthropods fossilized in Cretaceous Ethiopian amber. (A) A worker ant (Hymenoptera, Formicidae; NHMW, N6976). (B) A male sheet-web weaver spider (Araneae, Linyphiidae; MB. A 1664). (C) A thrips (Thysanoptera, Merothripidae; NHMW, N6974a). (D) A springtail (Collembola, Isotomidae; NHMW, N6969). (E) A Zoraptera (NHMW, N6991). (F) A wasp of the Eulophidae (Hymenoptera; NHMW, N6966a). (G) A fairy wasp (Hymenoptera, Mymaridae; NHMW, N6970). (H) A false fairy wasp (Hymenoptera, Mymarommatidae; NHMW, N6965a). (I) A representative of the Trichogrammatidae (Hymenoptera; MB. I 5654). (Scale bars: 500 μm.)
Fig. 4.
Fossil record of significant arthropods found in Ethiopian amber.
The most outstanding discovery is a complete, well-preserved although enrolled, wingless female ant (Formicidae; Fig. 3A). Visible characters preclude affinities with the extinct Sphecomyrminae, which is the only subfamily recorded for contemporaneous and older ants in mid-Cretaceous Burmese and French amber (15, 16). Regardless of the subfamily, this discovery is significant because it is one of the oldest records of an ant and the earliest from Gondwana. It has been suggested that ants arose in Laurasia during the Early Cretaceous (16–18), but the present discovery challenges this hypothesis. Ants evolved concurrent with the rise of angiosperms but apparently remained scarce until radiating into the world’s most diverse and ecologically dominant eusocial organisms during the Paleogene (19). The discovery will aid in resolving the phylogeny and timescale of ant lineages.
Other significant inclusions comprise a male spider that likely belongs in the Linyphiidae (Fig. 3B), a family of small spiders that weave sheet-webs (20). This specimen is the second-oldest linyphiid discovered to date, the oldest being from Hauterivian–lower Aptian Lebanese amber (21), and only the third fossil spider species to be described from the African continent (22). Extant linyphiids are globally distributed but most diverse and common in mesic and hydric regions of the Northern Hemisphere (23). A thrips (Fig. 3C) belonging to the Merothripidae is only the second Cretaceous merothripid found to date, the other one being a dubious specimen from Lebanese amber (24), and only the third known fossil from this group. The merothripids are a small but worldwide family, with most species residing in the Neotropics and in North America; these tiny insects feed on fungal mycelia on the forest floor or under the bark of decaying trees. The only identifiable springtail (Fig. 3D) belongs to the Isotomidae, which is known from one older record in Burmese amber and a few younger Cretaceous and Cenozoic ambers (25). Also identified is a zorapteran (Fig. 3E), an order of minute, gregarious insects that are exceedingly rare in the fossil record, with the oldest examples from Lower Cretaceous Jordanian and Burmese ambers (9, 26). This order exhibits remarkable morphological stability, because most Cretaceous representatives belong to the extant genus Zorotypus. The fossil and extant distribution of this genus in virtually all tropical areas indicates a great antiquity for the group. We found various wasps of the cosmopolitan families Eulophidae, Mymaridae, Mymarommatidae, and Trichogrammatidae (Fig. 3 F–I and SI Appendix, Fig. S10 J and K), which are among the earliest fossils of these small wasps that parasitize eggs and larvae of microlepidoptera, beetles, various other insects, arachnids, and nematodes. Lepidoptera are evidenced by the presence of microscopic scales (SI Appendix, Fig. S10F). Cretaceous ambers mostly fossilized microlepidopterans such as Micropterigidae, but the preservation of scales alone do not allow for a familial determination. Finally, one leg and a partial head of a beetle were found fossilized in the amber (SI Appendix, Fig. S10 H and I), but it was not possible to assign those fragments to any beetle lineage.
Microorganisms.
Microinclusions of bacteria and fungi occur in almost every studied amber piece, and a few nematodes were also found (Fig. 2C and SI Appendix, Fig. S11). Rod-shaped bacteria are seen as chains of cells attached to detritus and decayed arthropods (SI Appendix, Fig. S11 A–C).
Thousands of septate, mostly four-celled and slightly curved fungal conidia, which are related to the extant anamorphic ascomycete genus Curvularia, are enclosed in the amber (Fig. 2C and SI Appendix, Fig. S11F). Extant species of this genus are common parasites of vascular plants. The fossil spores are attached to surfaces of successive resin flows in the amber pieces, indicating that they dropped down onto the liquid resin. The plethora of conidia and the occurrence of the related conidiophores in the amber (SI Appendix, Fig. S11E) suggest that this Cretaceous Curvularia-like species sporulated plentifully at the site of the resin-bearing trees.
The Ethiopian amber also provides evidence of fungivory in the Mesozoic. Many Curvularia-like conidia and possible remnants of the perithecia of its teleomorph were found inside insect fecal pellets of up to 800 × 250 μm (Fig. 2C and SI Appendix, Fig. S11 G–I); these pellets are too large to have been produced by springtails and mites trapped close to the fungi in the amber pieces. Instead, beetles or their larvae are the most probable grazers of this Cretaceous fungus.
Epiphytic fungi with globular, dark-colored hyphae are also enclosed (SI Appendix, Fig. S11 J–L). They belong to the sooty molds, an ecological group of saprophytic fungi that are mostly assignable to the Capnodiales (Ascomycota), which produce colonies on the surface of living plants. Sooty molds typically obtain their nutrients from the excretions of aphids, scale insects, and other producers of honeydew, or from plant exudates. The discovery of mid-Cretaceous epiphytic sooty molds is an example of the morphological stability or stasis of taxa that, once adapted to particular microhabitats, preserve their morphological features over some 100 million years.
Plant Remains.
Remnants of plants are preserved in the form of spores from homosporous ferns and lycophytes, pollen grains likely belonging to the gymnosperm family Podocarpaceae and leaf cuticles that are probably remnants from some early diverging angiosperm families such as the Lauraceae, Chloranthaceae, or Proteaceae (SI Appendix, Fig. S12 A–C). Some of the more remarkable minute plant remnants are abundant stellate hairs (Fig. 2 D and E and SI Appendix, Fig. S12 D–F). Comparison of these hairs to modern plants excludes any relation to angiosperms and instead suggests ferns as the likely source, e.g., the tree fern family Cyatheaceae and the mostly epiphytic filmy fern family Hymenophyllaceae. As in the fossils, the stellate hairs of extant representatives of these fern families are composed of flat rays with margins that curve upward when drying and later drop from the fronds.
Paleoecology.
The onset and evolutionary success of angiosperms led to a reorganization of terrestrial ecosystems in the middle Cretaceous (27, 28). Coevolutionary events of plants and animals provided a basis for radiation and speciation. Archaic gymnosperms declined and modern gymnosperm and fern families diversified in the shadow of the angiosperms. As a result, there was tremendous macroscopic change in terrestrial ecosystems that influenced many groups of insects, fungi, and liverworts, and probably also amphibians and mammals (28–31). In the Cenomanian, angiosperms were no longer restricted to early succession stages but became dominant in woodlands worldwide. In this time of change, the Ethiopian amber forest represents an ecosystem in which both conifers, such as members of the extinct Cheirolepidiaceae and the extant Podocarpaceae families, and early angiosperms co-occurred. The sporomorphs of the amber-bearing sediment show that other conifers, different angiosperms and diverse fungi, mosses, lycophytes, and ferns were also abundant in this amber forest (SI Appendix, Fig. S9 and Fig. S12). An epiphytic community comprising ferns and fungi developed. Ascomycetes played a role not only as decomposers but also as parasites and served as a food source for insects. Although angiosperms were present in the Ethiopian amber forest, no insects were found that would have pollinated their early flowers. Many hexapods trapped by the tree resin occupy other functional niches in extant forests, such as small aerial parasitoids (wasps) and fungivores or detritivores (springtails, merothripid, psocopteran, zorapteran) living among the leaf litter or under tree bark. Predators are represented only by the ant, which was probably foraging on the soil and on plants, and the linyphiid spider, whose extant relatives typically construct webs close to the ground in leaf litter, although some build webs in bushes or other vegetation.
The Ethiopian amber is of great importance for improving our knowledge of the evolutionary history of terrestrial arthropods, plants, and fungi during the initial radiation of angiosperms in the Cretaceous. Additional discoveries of unique amber deposits will aid in closing the large gap in the Mesozoic record of terrestrial arthropods from the African continent.
Materials and Methods
Locality.
The unique amber deposit was discovered near the town of Alem Ketema in the eastern part of the northwestern Plateau of Ethiopia (Fig. 1). The amber pieces occur in a siltstone within the Debre Libanos Sandstone Unit, exposed along the slopes of the Wenchit River valley (SI Appendix, Fig. S2). A few hundred pieces of amber have been recovered so far; we had access to 62 of them for investigation. Most of the pieces are 1–5 cm in size, but single pieces of up to 25 cm were also found (Fig. 2A and SI Appendix, Fig. S3 A and B).
Preparation and Microscopy.
The pieces of amber were ground and polished manually with a series of wet silicon carbide abrasive papers [grit from FEPA P 600–4000 (25.8 μm to 5 μm particle size), firm Struers] to remove the weathered opaque surface and to minimize light scattering for the investigation. The original amber pieces were sometimes separated to isolate inclusions for investigation. The inclusions were studied by using incident-light microscopes (Carl Zeiss Stemi 2000 and Olympus SZH-ILLD) and transmitted-light microscopes (Carl Zeiss AxioScope A1 and Olympus BX50) equipped with Canon 450D and Olympus Color View IIIu digital cameras. In some instances, incident and transmitted light was used simultaneously. To better illustrate the three-dimensional inclusions, some photomicrographs were combined by using the software package HeliconFocus 4.45. Fig. 3 C–I and SI Appendix, Fig. S9 A, C, D and H–N, Fig. S10 A and C–L, Fig. S11 B, D, and E, and Fig. S12 A–C and E, were obtained from several optical sections.
Repository.
The amber pieces are deposited in the Museum für Naturkunde zu Berlin, (MB. Pb. 2009/200 to MB. Pb. 2009/211, MB Pb. 2009/313 to MB Pb. 2009/341, MB. I 5654 to MB. I 5657, and MB. A 1664), in the Department of Mineralogy and Petrography, Naturhistorisches Museum Wien, (NHMW, N3881 and NHMW, N6964 to NHMW, N6991) and in the American Museum of Natural History, New York (three pieces, sine numero). Please note that some of the original 62 amber pieces were divided to investigate the inclusions; each obtained fragment now has a separate collection number.
Supplementary Material
Acknowledgments
We thank S. and H. Kaiser (Maria Enzersdorf, Austria) for providing amber pieces for this study, A. Beran (Vienna), A. Chilin (Padua, Italy), A. Giaretta (Padua, Italy), V. M. F. Hammer (Vienna), S. Salzmann (Berlin), E. Stenzel (Berlin), and M. Weber (Vienna) for technical support and B. Kosmowska-Ceranowicz (Warsaw), C. Pott (Stockholm), and E. Schrank (Berlin) for discussion. This is contribution number 35 from the Courant Research Centre Geobiology that is funded by the German Initiative of Excellence, and a contribution to the project AMBRACE (BLAN07-1-184190) of the French National Research Agency.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000948107/DCSupplemental.
References
- 1.Grimaldi DA. Amber: Window to the Past. New York: Abrams; 1996. [Google Scholar]
- 2.Weitschat W, Wichard W. Atlas of Plants and Animals in Baltic Amber. Munich: Pfeil; 2002. [Google Scholar]
- 3.Schmidt AR, Ragazzi E, Coppellotti O, Roghi G. A microworld in Triassic amber. Nature. 2006;444:835. doi: 10.1038/444835a. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt AR, Dörfelt H, Perrichot V. Carnivorous fungi from Cretaceous amber. Science. 2007;318:1743. doi: 10.1126/science.1149947. [DOI] [PubMed] [Google Scholar]
- 5.Girard V, et al. Evidence for marine microfossils from amber. Proc Natl Acad Sci USA. 2008;105:17426–17429. doi: 10.1073/pnas.0804980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrichot V, Girard V. A unique piece of amber and the complexity of ancient forest ecosystems. Palaios. 2009;24:137–139. [Google Scholar]
- 7.Martínez-Delclòs X, Briggs DEG, Peñalver E. Taphonomy of insects in carbonates and amber. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;203:19–64. [Google Scholar]
- 8.Poinar GO, Jr, Milki R. Lebanese Amber. The Oldest Insect Ecosystem in Fossilized Resin. Corvallis: Oregon State Univ Press; 2001. [Google Scholar]
- 9.Kaddumi HF. Amber of Jordan. Amman: Eternal River Museum of Natural History; 2007. [Google Scholar]
- 10.Alimohammadian H, Sahni A, Patnaik R, Rana RS, Singh H. First record of an exceptionally diverse and well preserved amber-embedded biota from Lower Eocene (∼52 Ma) lignites, Vastan, Gujarat. Curr Sci. 2005;89:1328–1330. [Google Scholar]
- 11.Antoine P-O, et al. Amber from western Amazonia reveals Neotropical diversity during the middle Miocene. Proc Natl Acad Sci USA. 2006;103:13595–13600. doi: 10.1073/pnas.0605801103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assefa G. Lithostratigraphy and environment of deposition of the Late Jurassic – Early Cretaceous sequence of the central part of the Northwestern Plateau, Ethiopia. Neues Jahrb Geol P-A. 1991;182:255–284. [Google Scholar]
- 13.Russo A, Assefa G, Atnafu B. Sedimentary evolution of the Abay River (Blue Nil) Basin, Ethiopia. Neues Jahrb Geol P-M. 1994;5:291–308. [Google Scholar]
- 14.Schlüter T. Fossil insects in Gondwana – localities and palaeodiversity trends. Acta Zool Cracov. 2003;46(Suppl):345–371. [Google Scholar]
- 15.Engel MS, Grimaldi DA. Primitive new ants in Cretaceous amber from Myanmar, New Jersey, and Canada (Hymenoptera, Formicidae) Am Mus Novit. 2005;3485:1–23. [Google Scholar]
- 16.Perrichot V, Lacau S, Néraudeau D, Nel A. Fossil evidence for the early ant evolution. Naturwissenschaften. 2008;95:85–90. doi: 10.1007/s00114-007-0301-8. [DOI] [PubMed] [Google Scholar]
- 17.Grimaldi DA, Agosti D. A formicine in New Jersey Cretaceous amber (Hymenoptera: Formicidae) and early evolution of the ants. Proc Natl Acad Sci USA. 2000;97:13678–13683. doi: 10.1073/pnas.240452097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson EO, Hölldobler B. The rise of the ants: A phylogenetic and ecological explanation. Proc Natl Acad Sci USA. 2005;102:7411–7414. doi: 10.1073/pnas.0502264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreau CS, Bell CD, Vila R, Archibald SB, Pierce NE. Phylogeny of the ants: Diversification in the age of angiosperms. Science. 2006;312:101–104. doi: 10.1126/science.1124891. [DOI] [PubMed] [Google Scholar]
- 20.Platnick NI. 2009. The World Spider Catalog, version 10.0. Available at http://research.amnh.org/entomology/spiders/catalog/index.html (Am Mus Nat Hist New York). Accessed September 9, 2009. [Google Scholar]
- 21.Penney D, Selden PA. The oldest linyphiid spider, in Lower Cretaceous Lebanese amber (Araneae, Linyphiidae, Linyphiinae) J Arachnol. 2002;30:487–493. [Google Scholar]
- 22.Selden PA, Anderson HM, Anderson JM. A review of the fossil record of spiders (Araneae) with special reference to Africa, and description of a new specimen from the Triassic Molteno Formation of South Africa. Afr Invertebr. 2009;50:105–116. [Google Scholar]
- 23.Draney ML, Buckle DJ. In: Spiders of North America: An Identification Manual. Ubick DP, Paquin P, Cushing PE, Roth V, editors. California: Am Arachnol Soc; 2005. pp. 124–161. [Google Scholar]
- 24.Bhatti JS. A revised classification of Thysanoptera. Abstract Volume, the Workshop on Advances in Insect Taxonomy in India and the Orient (Association for the study of Oriental Insects, New Dehli) 1979. pp. 46–48. [Google Scholar]
- 25.Christiansen K, Nascimbene PC. Collembola (Arthropoda, Hexapoda) from the mid Cretaceous of Myanmar (Burma) Cretac Res. 2006;27:318–363. [Google Scholar]
- 26.Engel MS, Grimaldi DA. The first Mesozoic Zoraptera. Am Mus Novit. 2002;3362:1–20. [Google Scholar]
- 27.Dilcher DL. Towards a new synthesis: Major evolutionary trends in the angiosperm evolution. Proc Natl Acad Sci USA. 2000;97:7030–7036. doi: 10.1073/pnas.97.13.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, et al. Rosid radiation and the rapid rise of angiosperm-dominated forests. Proc Natl Acad Sci USA. 2009;106:3853–3858. doi: 10.1073/pnas.0813376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crane PR. In: The Origin of Angiosperms and their Biological Consequences. Friis EM, Chaloner WG, Crane, PR, editors. Cambridge: Cambridge Univ Press; 1987. pp. 107–144. [Google Scholar]
- 30.Schneider H, et al. Ferns diversified in the shadow of angiosperms. Nature. 2004;428:553–557. doi: 10.1038/nature02361. [DOI] [PubMed] [Google Scholar]
- 31.Heinrichs J, Hentschel J, Wilson R, Feldberg K, Schneider H. Evolution of leafy liverworts (Jungermanniales, Marchantiophyta): Estimating divergence times from chloroplast DNA sequences using penalized likelyhood with integrated fossil evidence. Taxon. 2007;56:31–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.