Abstract
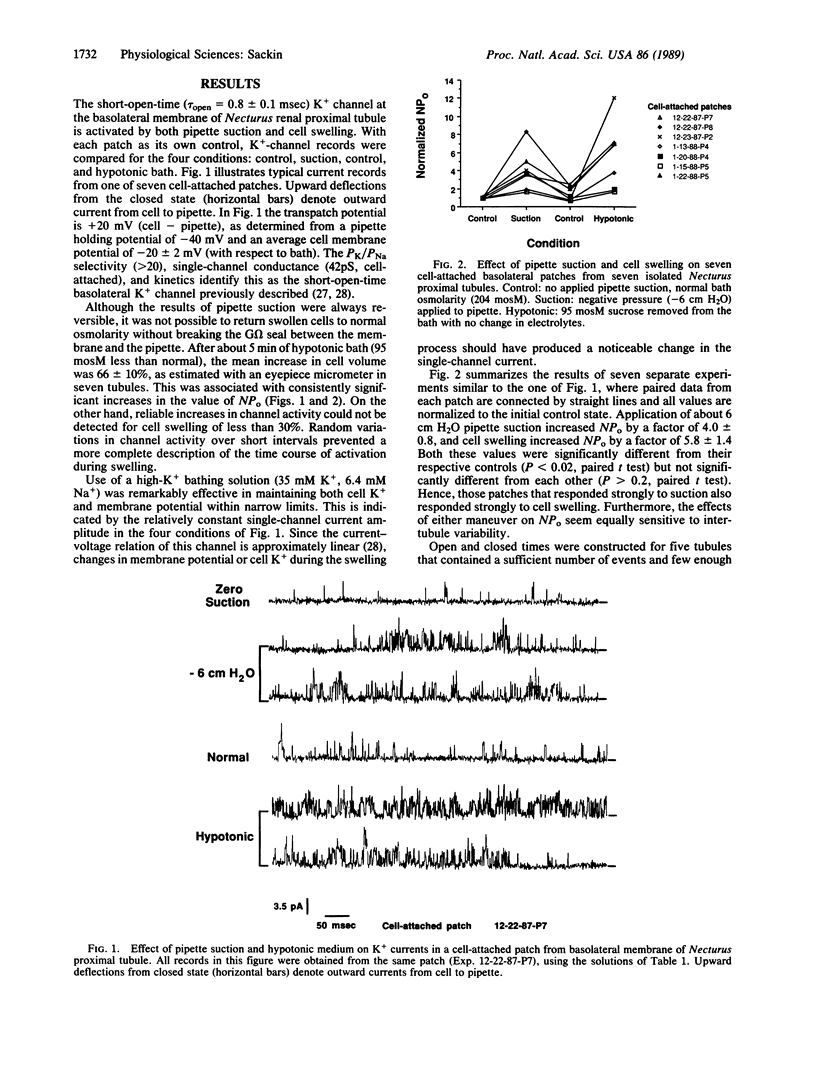
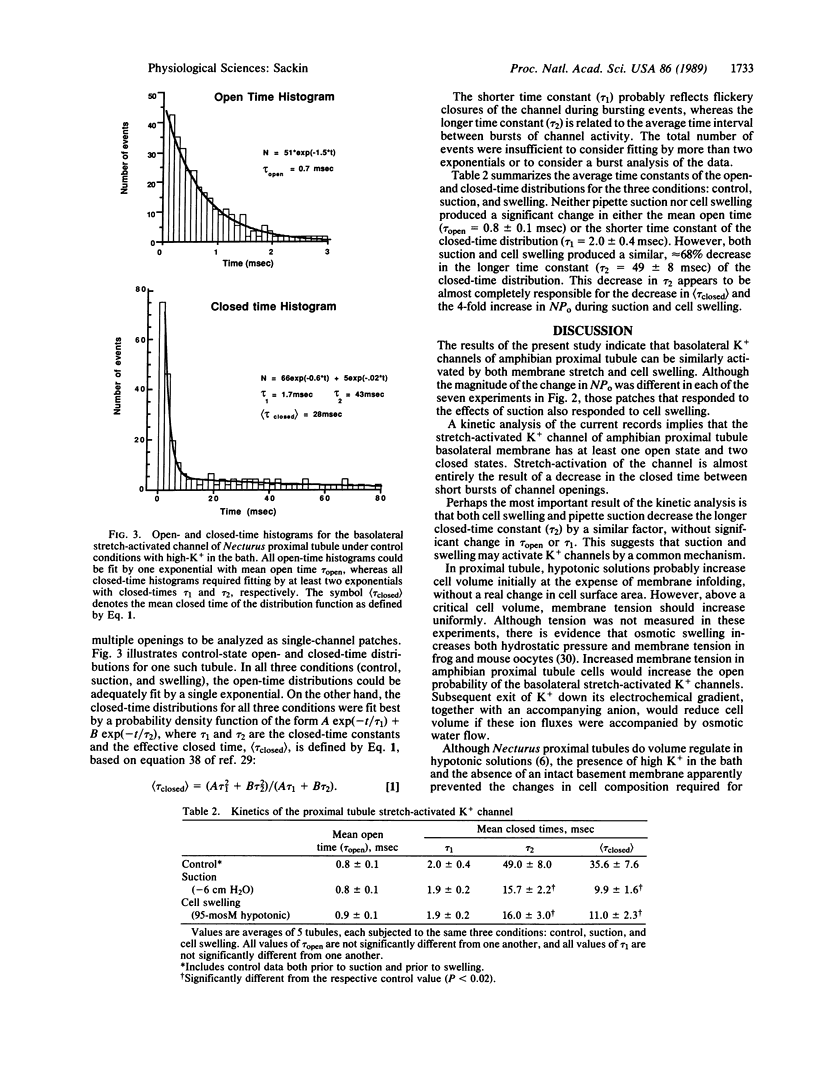
The role of K+ channels in cell osmoregulation was investigated by using the patch-clamp technique. In cell-attached patches from Necturus proximal tubule, the short-open-time K+ channel at the basolateral membrane could be stretch-activated by pipette suction, where a negative pressure of 6 cm H2O (588.6 Pa) was sufficient to increase the open probability of the channel by a factor of 4.0 +/- 0.8 (n = 7 tubules). A 50% reduction in bath osmolarity increased cell volume by 66 +/- 10% and increased the K+-channel open probability by a factor of 5.8 +/- 1.4 (n = 7) in the same cell-attached patches that were activated by pipette suction. A kinetic analysis indicates one open state and at least two closed states for this epithelial K+ channel. Both suction and swelling shorten the longest time constant of the closed-time distribution by a factor of 3, without significant effect on either the mean open time or the shorter closed-state time constant. The similar effect of suction and swelling is consistent with the hypothesis that stretch-activated K+ channels mediate the increase in macroscopic K+ conductance that occurs during osmoregulation of amphibian proximal tubules. Calculations based on a simple model indicate that small increments in cell volume could produce statistically significant increases in K+-channel activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987 Nov 5;330(6143):66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- Cooper K. E., Tang J. M., Rae J. L., Eisenberg R. S. A cation channel in frog lens epithelia responsive to pressure and calcium. J Membr Biol. 1986;93(3):259–269. doi: 10.1007/BF01871180. [DOI] [PubMed] [Google Scholar]
- Davis C. W., Finn A. L. Sodium transport inhibition by amiloride reduces basolateral membrane potassium conductance in tight epithelia. Science. 1982 Apr 30;216(4545):525–527. doi: 10.1126/science.7071599. [DOI] [PubMed] [Google Scholar]
- Dellasega M., Grantham J. J. Regulation of renal tubule cell volume in hypotonic media. Am J Physiol. 1973 Jun;224(6):1288–1294. doi: 10.1152/ajplegacy.1973.224.6.1288. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Waugh R., Melnik L. Elastic area compressibility modulus of red cell membrane. Biophys J. 1976 Jun;16(6):585–595. doi: 10.1016/S0006-3495(76)85713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett J. K., Spring K. R. Involvement of calcium and cytoskeleton in gallbladder epithelial cell volume regulation. Am J Physiol. 1985 Jan;248(1 Pt 1):C27–C36. doi: 10.1152/ajpcell.1985.248.1.C27. [DOI] [PubMed] [Google Scholar]
- Gagnon J., Ouimet D., Nguyen H., Laprade R., Le Grimellec C., Carrière S., Cardinal J. Cell volume regulation in the proximal convoluted tubule. Am J Physiol. 1982 Oct;243(4):F408–F415. doi: 10.1152/ajprenal.1982.243.4.F408. [DOI] [PubMed] [Google Scholar]
- Germann W. J., Ernst S. A., Dawson D. C. Resting and osmotically induced basolateral K conductances in turtle colon. J Gen Physiol. 1986 Aug;88(2):253–274. doi: 10.1085/jgp.88.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J. J., Lowe C. M., Dellasega M., Cole B. R. Effect of hypotonic medium on K and Na content of proximal renal tubules. Am J Physiol. 1977 Jan;232(1):F42–F49. doi: 10.1152/ajprenal.1977.232.1.F42. [DOI] [PubMed] [Google Scholar]
- Grasset E., Gunter-Smith P., Schultz S. G. Effects of Na-coupled alanine transport on intracellular K activities and the K conductance of the basolateral membranes of Necturus small intestine. J Membr Biol. 1983;71(1-2):89–94. doi: 10.1007/BF01870677. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Dupre A., Rothstein A. Volume regulation by human lymphocytes. Role of calcium. J Gen Physiol. 1982 May;79(5):849–868. doi: 10.1085/jgp.79.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A., Sarkadi B., Gelfand E. W. Responses of lymphocytes to anisotonic media: volume-regulating behavior. Am J Physiol. 1984 Mar;246(3 Pt 1):C204–C215. doi: 10.1152/ajpcell.1984.246.3.C204. [DOI] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. K. Role of separate K+ and Cl- channels and of Na+/Cl- cotransport in volume regulation in Ehrlich cells. Fed Proc. 1985 Jun;44(9):2513–2519. [PubMed] [Google Scholar]
- Hudson R. L., Schultz S. G. Sodium-coupled glycine uptake by Ehrlich ascites tumor cells results in an increase in cell volume and plasma membrane channel activities. Proc Natl Acad Sci U S A. 1988 Jan;85(1):279–283. doi: 10.1073/pnas.85.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk K. L., DiBona D. R., Schafer J. A. Regulatory volume decrease in perfused proximal nephron: evidence for a dumping of cell K+. Am J Physiol. 1987 May;252(5 Pt 2):F933–F942. doi: 10.1152/ajprenal.1987.252.5.F933. [DOI] [PubMed] [Google Scholar]
- Kregenow F. M. Osmoregulatory salt transporting mechanisms: control of cell volume in anisotonic media. Annu Rev Physiol. 1981;43:493–505. doi: 10.1146/annurev.ph.43.030181.002425. [DOI] [PubMed] [Google Scholar]
- Larson M., Spring K. R. Volume regulation by Necturus gallbladder: basolateral KCl exit. J Membr Biol. 1984;81(3):219–232. doi: 10.1007/BF01868715. [DOI] [PubMed] [Google Scholar]
- Lau K. R., Hudson R. L., Schultz S. G. Cell swelling increases a barium-inhibitable potassium conductance in the basolateral membrane of Necturus small intestine. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3591–3594. doi: 10.1073/pnas.81.11.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes A. G., Guggino W. B. Volume regulation in the early proximal tubule of the Necturus kidney. J Membr Biol. 1987;97(2):117–125. doi: 10.1007/BF01869418. [DOI] [PubMed] [Google Scholar]
- Martinac B., Buechner M., Delcour A. H., Adler J., Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner G., Oberleithner H., Lang F. The effect of phenylalanine on the electrical properties of proximal tubule cells in the frog kidney. Pflugers Arch. 1985 May;404(2):138–144. doi: 10.1007/BF00585409. [DOI] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Richards N. W., Dawson D. C. Single potassium channels blocked by lidocaine and quinidine in isolated turtle colon epithelial cells. Am J Physiol. 1986 Jul;251(1 Pt 1):C85–C89. doi: 10.1152/ajpcell.1986.251.1.C85. [DOI] [PubMed] [Google Scholar]
- Romero P. J. Is the Ca2+-sensitive K+ channel under metabolic control in human red cells? Biochim Biophys Acta. 1978 Feb 2;507(1):178–181. doi: 10.1016/0005-2736(78)90385-1. [DOI] [PubMed] [Google Scholar]
- Sachs F. Baroreceptor mechanisms at the cellular level. Fed Proc. 1987 Jan;46(1):12–16. [PubMed] [Google Scholar]
- Sackin H., Palmer L. G. Basolateral potassium channels in renal proximal tubule. Am J Physiol. 1987 Sep;253(3 Pt 2):F476–F487. doi: 10.1152/ajprenal.1987.253.3.F476. [DOI] [PubMed] [Google Scholar]
- Sackin H. Stretch-activated potassium channels in renal proximal tubule. Am J Physiol. 1987 Dec;253(6 Pt 2):F1253–F1262. doi: 10.1152/ajprenal.1987.253.6.F1253. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Mack E., Rothstein A. Ionic events during the volume response of human peripheral blood lymphocytes to hypotonic media. I. Distinctions between volume-activated Cl- and K+ conductance pathways. J Gen Physiol. 1984 Apr;83(4):497–512. doi: 10.1085/jgp.83.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by "flush-through". Am J Physiol. 1981 Dec;241(6):F579–F590. doi: 10.1152/ajprenal.1981.241.6.F579. [DOI] [PubMed] [Google Scholar]
- Ubl J., Murer H., Kolb H. A. Ion channels activated by osmotic and mechanical stress in membranes of opossum kidney cells. J Membr Biol. 1988 Sep;104(3):223–232. doi: 10.1007/BF01872324. [DOI] [PubMed] [Google Scholar]
- Welling P. A., Linshaw M. A., Sullivan L. P. Effect of barium on cell volume regulation in rabbit proximal straight tubules. Am J Physiol. 1985 Jul;249(1 Pt 2):F20–F27. doi: 10.1152/ajprenal.1985.249.1.F20. [DOI] [PubMed] [Google Scholar]
- Wong S. M., Chase H. S., Jr Role of intracellular calcium in cellular volume regulation. Am J Physiol. 1986 Jun;250(6 Pt 1):C841–C852. doi: 10.1152/ajpcell.1986.250.6.C841. [DOI] [PubMed] [Google Scholar]


