Abstract
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease more prevalent in people of African and Asian origin than Caucasian origin. FcγRIIb is an inhibitory Fc receptor with a critical role in immune regulation. Mouse data suggest that FcγRIIb deficiency increases susceptibility to autoimmune disease but protects against infection. We show that a SNP in human FCGR2B that abrogates receptor function is strongly associated with susceptibility to SLE in both Caucasians and Southeast Asians. The minor allele of this SNP is more common in Southeast Asians and Africans, populations from areas where malaria is endemic, than in Caucasians. We show that homozygosity for the minor allele is associated with substantial protection against severe malaria in an East African population (odds ratio = 0.56; P = 7.1 × 10−5). This protective effect against malaria may contribute to the higher frequency of this SNP and hence, SLE in Africans and Southeast Asians.
Keywords: genetic association study, autoimmunity, infectious disease, selection, bacterial septicaemia
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease. The incidence of disease is estimated to be between 12 and 64 cases per 100,000 individuals with at least a two- to four-fold higher incidence in non-Caucasians compared with Caucasians (1). The high sibling risk ratio (λs) of 29 indicates a strong genetic contribution to disease susceptibility (2), and five recent genome-wide association studies have identified and replicated several loci as well as confirmed association at a number of those previously implicated (reviewed in ref. 3). Histologically, the disease is characterized by deposition of immune complexes containing both Ig and complement components. Any organ may be affected, but those most commonly involved are skin, joints, and kidney (4).
FCGR2B encodes Fc gamma receptor IIb (FcγRIIb), an IgG receptor expressed on immune cells including B cells, dendritic cells, macrophages, and plasma cells. When coligated by immune complexes to activatory Fc receptors on myeloid-lineage cells or the B cell receptor on B cells, FcγRIIb signals through an immunoreceptor tyrosine-based inhibitory motif (ITIM) to suppress downstream events such as cellular proliferation, phagocytosis, and inflammatory cytokine release (5). Manipulation of this receptor in mouse models emphasizes the importance of FcγRIIb in immune regulation. FcγRIIb-deficient mice are prone to inducible and spontaneous autoimmune disease with a phenotype that resembles human SLE (5, 6). Restoring FcγRIIb expression in lupus-prone mice prevents autoimmunity (7), and even subtle B cell-specific overexpression is sufficient to alleviate SLE (8).
Polymorphisms in FCGR2B and its regulatory regions are found in mouse and man. A mouse promoter haplotype associated with reduced expression of FcγRIIb is present in all polygenic mouse models of SLE (9, 10). In humans, FCGR gene duplication and low levels of linkage disequilibrium have led to poor coverage of the region on the platforms used for genome-wide association studies. Candidate gene association studies have, however, linked a polymorphism in FCGR2B (rs1050501) with susceptibility to SLE. The minor allele of this SNP codes for a threonine instead of isoleucine at position 232 in the transmembrane domain of FcγRIIb. In vitro studies have shown that the threonine form of the receptor (FcγRIIbT232) is excluded from lipid rafts, and thus, it is unable to interact with activatory receptors and exert an inhibitory effect on cellular function (11, 12).
Although previous small studies have reported an association of FcγRIIbT232 with SLE in Asians (13–16), no such association had been shown in two studies in Caucasians (17, 18). We found this surprising, because the functional effect of the polymorphism in vitro is seen in B cells and monocyte-derived macrophages from Caucasian individuals (11). The minor allele frequency (MAF) of rs1050501 is subject to considerable ethnic variation, being lower in Caucasians (0.10) (19) than East Africans (0.25) (19) or Southeast Asians (0.22–0.25) (13–16). This low allele frequency meant that previous studies in Caucasians were underpowered to detect the odds ratio (OR) observed in Southeast Asians (power < 21%). We have, therefore, performed a larger study in Caucasians as well as the largest study performed to date in Southeast Asians, and we meta-analyzed all available data.
The higher MAF in people of Southeast Asian and African descent, populations from areas where malaria is endemic, raises the possibility that decreased FcγRIIb function may provide a survival advantage against this parasitic infection (19). Malaria causes 1–3 million deaths annually, predominantly in children, and it has exerted considerable selective pressure on the human genome (20). The decreased inhibitory function caused by FcγRIIbT232 results in increased B cell and myeloid cell activation. Although this might predispose to SLE, a more active immune system may be beneficial in response to infection. FcγRIIb-deficient mice are resistant to the manifestations of severe disease after infection with Plasmodium chabaudi chabaudi, a rodent malaria causing disease with some similarities to human infection with Plasmodium falciparum (19). Similarly, in humans, FcγRIIbT232 increases phagocytosis of P. falciparum by monocyte-derived macrophages in vitro (19). In this study, we genotyped rs1050501 in children with mild and severe malaria to determine if FcγRIIbT232 was associated with protection against malaria, because this may explain the increased MAF in populations from malarial areas.
Results
SLE and FcγRIIbT232.
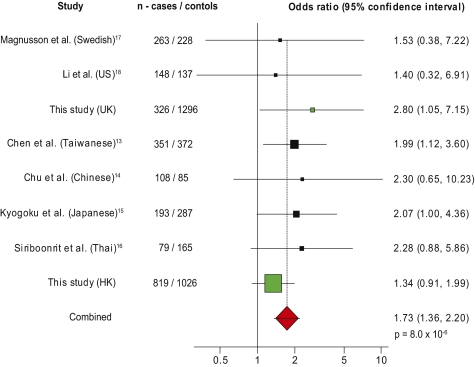
We compared the genotypes of 819 patients with SLE from Hong Kong with 1,026 ethnically matched controls, the largest study of this FCGR2B SNP in SLE performed so far. When we combined the results with other studies of Southeast Asians in a meta-analysis, this enhanced the significance of the association of FcγRIIbT232 homozygosity with SLE with an OR of 1.7 (P = 8.0 × 10−5), despite not reaching significance alone (Table S1 and Fig. S1). We also compared the genotypes of 326 Caucasian SLE patients with 1,296 controls. Homozygosity for FcγRIIbT232 was significantly associated with SLE (P = 0.014) (Table S2 and Fig. S2). Meta-analysis of our study with the published Caucasian SLE case-control studies gave an OR for FcγRIIbT232 homozygotes of 2.06 (Fig. S2) compared with 1.70 in Asians, showing that FcγRIIbT232 homozygosity is a strong susceptibility factor for SLE in both ethnic groups. When studies in both ethnic groups were combined, FcγRIIbT232 homozygosity was associated with SLE with an OR of 1.73 (P = 8.0 × 10−6) (Fig. 1), making it one of the strongest genetic associations with SLE yet described (3).
Fig. 1.
Homozygosity for FcγRIIbT232 is associated with SLE. Meta-analysis of studies shows the ORs and 95% confidence intervals for FcγRIIbT232 homozygosity in SLE cases and ethnically matched controls of Asian and Caucasian ethnicity. The studies that we have performed are shown in green. The size of the box in the forest plot reflects the weighting of the study in the combined analysis (studies in Caucasians contribute less owing to the lower minor allele frequency). Analysis performed using StatsDirect software (random effects model by DerSimonian and Laird shown in ref. 37).
Malaria and FcγRIIbT232.
We first genotyped FcγRIIbT232 in 473 Kenyan children from a malaria-endemic area who had repeated episodes of uncomplicated mild malaria associated with frequent parasite exposure. We did not see an association between FcγRIIbT232 and frequency of malarial infection before or after adjustment for age, season, ethnicity, HbS phenotype, and thalassemia genotype (Tables S3, S4, S5, and S6), suggesting that this disease phenotype is not modified by FcγRIIbT232.
In this region of Kenya, most children suffer recurrent episodes of mild clinical malaria, but only some go on to develop severe malaria, the greatest cause of infant mortality across most of subSaharan Africa (21). To investigate if FcγRIIbT232 was protective against severe malaria, we genotyped 998 control children and 684 children admitted to the high-dependency unit of Kilifi District Hospital with P. falciparum parasitemia complicated by clinical features of severe malaria. When cases and controls were compared, individuals homozygous for FcγRIIbT232 were protected against severe malaria (OR = 0.50; P = 1.0 × 10−3) (Table 1). These findings were replicated in an independent, temporally distinct case-control analysis of samples collected from the same region (OR = 0.62; P = 0.019) (Table 1), giving a combined OR of 0.56 (P = 7.1 × 10−5). The genotypes for both control groups were in Hardy–Weinberg equilibrium, but those of both case groups were not (P = 0.001 and P = 0.027 for case groups 1 and 2, respectively) consistent with FcγRIIbT232 being subject to selection pressure from severe malaria.
Table 1.
Distribution of genotype frequencies for FcγRIIbT232 in controls and severe malarial cohorts of Kenyan children
| Malarial control group 1 (n = 998) |
Severe malaria case group 1 (n = 684) |
Control group 2 (n = 1,706) |
Severe malaria case group 2 (n = 731) |
Combined |
|||||||
| n | Frequency | n | Frequency | P value | n | Frequency | n | Frequency | P value | P value | |
| II | 475 | 0.476 | 345 | 0.504 | 875 | 0.513 | 391 | 0.534 | |||
| TT | 87 | 0.087 | 31 | 0.045 | 4.1 × 10−3*† | 125 | 0.073 | 34 | 0.047 | 0.048* | |
| IT | 436 | 0.436 | 308 | 0.450 | 706 | 0.414 | 306 | 0.419 | |||
| TT | 87 | 0.087 | 31 | 0.0453 | 1.0 × 10−3‡ | 125 | 0.073 | 34 | 0.047 | 0.019‡ | 7.1 × 10−5§ |
| IT and II | 911 | 0.913 | 653 | 0.955 | OR = 0.50 | 1,581 | 0.927 | 696 | 0.953 | OR = 0.62 | OR = 0.56 |
| (0.33–0.76) | (0.42–0.91) | (0.42–0.74) | |||||||||
II denotes individuals homozygous for FcγRIIbI232, TT denotes individuals homozygous for FcγRIIbT232, and IT denotes heterozygotes.
*P value was calculated by χ2 test using 2 × 3 contingency table (df = 2).
†Genotype was not associated with specific features of severe malaria, including hematocrit, parasitemia, conscious level, or respiratory symptoms (multiple logistic regression) (Table S8).
‡P value and OR calculated by χ2 test using 2 × 2 contingency table (df = 1). Numbers in parentheses refer to 95% confidence intervals.
§Combined P value from meta-analysis of the two cohorts using StatsDirect software.
A polymorphism in the promoter region of FCGR2B, rs3219018, has been associated with changes in FcγRIIb expression and SLE in Caucasians (22), and it might have an additional effect on susceptibility to infection. We genotyped 96 Kenyan controls and found no minor alleles, despite finding a MAF of 0.16 in 90 U.K. Caucasians. With a minor allele frequency of less than 0.005, this SNP cannot be responsible for the effects seen in our malarial case-control cohorts.
Bacteremia and FcγRIIbT232.
FcγRIIb may influence susceptibility to bacterial infection, which is also a major contributor to the high infant mortality rate in subSaharan Africa (21). FcγRIIb-deficient mice show increased phagocytosis of opsonized bacteria and are protected from pneumococcal infection (23), whereas mice overexpressing FcγRIIb on macrophages are more susceptible to it (8). Moreover, humans homozygous for FcγRIIbT232 show increased phagocytosis of Streptococcus pneumoniae in vitro (19). We, therefore, genotyped two groups of children admitted to Kilifi District Hospital with blood culture-proven bacterial infection as well as controls recruited from the same study area. No significant association was found between FcγRIIb homozygosity and protection against bacterial infection (Table S7).
Discussion
We have shown that FcγRIIbT232 is associated with susceptibility to SLE with an OR of 1.7. This effect size, when reviewed in the context of recent genome-wide association studies as well as candidate and linkage studies, is the fifth strongest described for a common polymorphism. Thus, the OR reported for MHC class II (DR2 and DR3) is 2.0, and the OR for a polymorphism in IRF5 (encoding IFN regulatory factor 5) is 1.8; however, a recently reported SNP in ITGAM (alpha M integrin) has an OR of 1.6 (3). The increased MAF of rs1050501 in individuals of Southeast Asian and African descent may, therefore, contribute to the increased prevalence and severity of SLE, which has long been noted in these ethnic groups (1).
We have also shown that FcγRIIbT232 is associated with protection against severe malarial infection. The proportion of children with severe malaria who are homozygous for TT is one-half of that in controls, indicating that possession of the TT genotype reduces the chances of acquiring severe malaria by ∼50%. A number of mechanisms may contribute to this protective effect. Macrophages derived from both FcγRIIbT232 individuals and FcγRIIb-deficient mice phagocytose malarial parasites more avidly (19). Such uptake may be increased by antimalarial antibodies—in mice, FcγRIIb deficiency enhances antibody responses (5), including those to malaria (19), and thus, children with FcγRIIbT232 may develop better humoral immunity against malaria.
TNFα suppresses P. chabaudi chabaudi in mice (24), and TNF-R1 knockout mice are more susceptible to this infection (25). TNFα also increases clearance of plasmodium infection by macrophages (26) and enhances killing of P. falciparum and P. vivax through the actions of intermediates such as nitric oxide (27)—nitric oxide, in turn, correlates with resistance in children infected with P. falciparum (28). FcγRIIb-deficient mice produce higher levels of TNFα both at baseline and after infection with P. chabaudi chabaudi, as do FcγRIIbT232 human macrophages (19), and thus, TNFα might help drive the protection associated with FcγRIIbT232; however, after severe malaria is established, its role in human disease is likely to be complex (29). Indeed, it has been suggested that the delicate balance between proinflammatory and antiinflammatory mediators required to survive repeated malarial infections modulates the immune system and protects against autoimmune disease, explaining why SLE is less common in malaria-endemic Africa than in people of African descent living in Europe and the United States (30).
We did not see an association of FcγRIIbT232 with bacteremia in Kenyan children. This observation, together with the fact that bacterial infection has been a major selection pressure in Caucasian as well as African populations, suggests that it is not responsible for ethnic differences in FCGR2B genotypes. It does not, however, exclude genetic variation in FcγRIIb from playing a role in bacterial infection. It is quite possible that different bacterial infections are influenced in different ways by FcγRIIb, depending on the specific mechanism used by the immune system for their clearance; this includes the predominant IgG isotypes that they induce, which bind with different affinities to Fc receptors (5). Even in a single infection, FcγRIIb can have different effects on pathogenesis; mice deficient in the receptor are protected from primary streptococcal peritonitis but susceptible to septic shock induced by the same organism (23). In addition, epistatic interactions with polymorphisms in other FCGR genes, some of which also differ in MAF between racial groups, may influence the effect of FcγRIIbT232 genotype on infections. Thus, although alteration of susceptibility to bacterial mortality is unlikely to drive the ethnic differences in the FcγRIIb genotype that we observe, we have not excluded a more subtle role for it in bacterial infection; definition of this role would require the analysis of larger and more narrowly defined cohorts.
The high mortality from malaria has resulted in the strongest known force for evolutionary selection in the recent history of the human genome (20). The most widely known examples of this are the retention of the sickle cell and thalassemia traits: in Kenyan children from Kilifi, sickle-cell trait protects against severe malaria with an odds ratio of 0.17 (31), whereas heterozygous and homozygous alpha thalassemia confer a protective effect with odds ratios of 0.57 and 0.73, respectively (32). With an odds ratio of 0.56, homozygous FcγRIIbT232 has a similar protective effect against malaria to heterozygous thalassaemia, and thus, it could explain the higher MAF of FcγRIIbT232 seen in Africans and Southeast Asians. Malaria seems to have driven retention of a polymorphism predisposing to a polygenic autoimmune disease, and this may begin to explain the ethnic differences seen in frequency of that disease.
Materials and Methods
Subjects.
Southeast SLE cases and controls.
Nine hundred and twenty-two Hong Kong SLE patients were recruited from three Hong Kong hospitals. Medical records were reviewed to confirm that subjects met the criteria of the American College of Rheumatology (ACR) for SLE diagnosis (33). Control samples (1,116) were obtained from the Hong Kong Red Cross. Patients and controls were all self-reported Chinese ethnicity living in Hong Kong. The study was approved by the Institutional Review Board of the University of Hong Kong and Hospital Authority, Hong Kong West Cluster, New Territory West Cluster, and Hong Kong East Cluster, and all patients gave informed consent.
Caucasian SLE cases and controls.
Caucasian control individuals (1,296) were collected in Cambridge, United Kingdom, by the National Blood Service, and all were self-reported as Caucasian (described previously in ref. 11); 381 Caucasian SLE cases were collected in London, United Kingdom, and these patients were diagnosed with SLE according to clinical and serological features defined by the ACR. They were self-classified as Caucasian (34).
Mild malaria subjects.
Five hundred and eighteen children were recruited from the Ngerenya area of Kilifi and monitored from October 1998 to September 2003 by weekly surveillance in the community (35). Malaria was defined as fever (an axillary temperature of >37.4 °C) or a clinical history of fever in association with a slide positive for P. falciparum parasites at any density. During the 62,427 weeks of patient follow-up, there were 2,270 episodes of mild malaria. Details of age, season, ethnicity, hemoglobin S, and α-thalassemia genotype were available for this cohort, allowing for adjustment for potential confounding factors.
Severe malaria cases and controls.
The first group of severe malaria cases (severe malaria case group 1) was comprised of children admitted to the high-dependency unit at Kilifi District Hospital with P. falciparum malaria, and these cases were complicated by one or more clinical features of severity (coma, prostration, multiple seizures, severe malarial anemia, and/or hyperparasitemia) between 1992 and 1997 (32). The first group of Kenyan controls (severe malaria control group 1) was derived from cord-blood samples that were collected at Kilifi District Hospital during the period of 1992–2002. The second group of severe malaria cases (severe malaria case group 2) was comprised of a nonoverlapping cohort of children meeting the same criteria as children in case group 1 who were admitted to the same hospital during the period of 2000–2008. The second group of controls (control group 2) consisted of infants who were residents of the same study area as cases and were recruited between 2006 and 2007. Both control groups were selected to reflect the population served by Kilifi District Hospital and were confirmed to be well-matched to cases with regard to location of residence and ethnic group, the two most significant confounding factors of which we are aware. Our results may underestimate the protective effect of FcγRIIbT232 if a significant proportion of controls subsequently develop severe malaria, but it was confirmed that none of the controls from either group were admitted to Kilifi District Hospital with severe malaria, making this unlikely.
Bacteremia cases and controls.
The first group of bacteremia cases (bacterial case group 1) were children (<13 years) admitted to Kilifi District Hospital with blood culture positive for bacterial infection (Gram positive and negative) between 1998 and 2002 (36). The most frequent organisms were S. pneumoniae (22%), Salmonella typhi (14%), Haemophilus influenza (12%), and Escherichia coli (9%). The controls for these cases (bacterial control group 1) were frequency matched to a subset of the cases (two per case) on the basis of time (recruited within 14 days), location of home, age, and sex. Not all cases were matched for logistic reasons; the subset of the controls used was randomly selected. The second group of bacteremia cases (bacterial case group 2) was comprised of children meeting the same criteria admitted between 2003 and 2008. The controls used for these cases were the same as those used for the second group of malarial cases (control group 2) and therefore, reflect the geography and ethnicity of the population served by Kilifi District Hospital. It is likely that some children in the control groups could have developed bacteremia warranting hospitalization before or after recruitment. The risk of a child being hospitalized with bacteraemia between 0 and 5 years was ∼25 in 1,000 in Kilifi at this time (36), suggesting that around 2.5% of the controls may have been affected. As described for malaria above, such misclassification of a small proportion of controls would lead to a conservative bias, underestimating the significance of our finding.
Ethical approval for the collection of the Kenyan samples was given by the Kenya Medical Research Institute (KEMRI) National Scientific Steering and Research Committees.
Genotyping Methods.
rs 1050501.
Genomic DNA from the mild and first cohort of severe malaria cases and the Caucasian controls was genotyped by sequencing as previously described (11). The sequencing reactions were performed using Applied Biosystems BigDye chemistry, and the sequences were analyzed using an ABI 3700 capillary sequencer. Analysis of the sequence traces was performed using Sequencher software and was double-scored by a second operator. Genotypes were scored for 473 of 518 mild malaria cases and 684 of 734 severe malaria cases.
For the remaining cohorts, PCR was performed using the following primers: sense 5′-CTA-AGA-GGA-GCC-CTT-CCC-TAT-GT-3′ and antisense 5′-AAT-ACG-GGC-CTA-GAT-CTG-AAT-GTG-3′ (18). The PCR products were purified using exonuclease I and shrimp alkaline phosphatase (Exosap; GE Healthcare) and then, were genotyped using an Applied Biosystems Custom TaqMan Human SNP Genotyping Assay in accordance with the manufacturer's protocol. All genotyping data were double-scored by two independent scorers to minimize error. In addition, 96 samples were genotyped by both methods with 100% concordance between assays. Genotypes were scored for 326 of 381 Caucasian SLE cases, 819 of 876 Hong Kong SLE cases, 1,026 of 1,116 Hong Kong controls, 998 of 1,038 malaria controls in the first cohort (malaria control group 1), 809 of the first cohort of 857 bacterial cases (bacterial case group 1), 1,706 of 1,810 cord-blood control samples (control group 2), 730 of 741 cases in the second severe malaria cohort (severe malarial case group 2), and 948 of 964 cases in the second bacterial cohort (bacterial case group 2).
rs 3219018.
A nested PCR amplified a 15-kb region specific for FCGR2B, which was followed by amplification of the promoter region, with primers as previously described (22). The PCR products were purified using Exosap and sequenced using an ABI 3730 sequencer. Sequences were analyzed using Sequencher software and called by two independent researchers.
Statistics.
Genotypes were compared by two-sided χ2 tests, as indicated in the table legends, using Graphpad Prism. A recessive model was used for two reasons. First, previous genetic studies had suggested that homozygosity for FcγRIIbT232 was associated with SLE (13, 15). Second, the polymorphism disables the receptor (11, 12), and thus, functional deficiency of the receptor in minor allele homozygotes would be consistent with a recessive effect.
Previous studies in Southeast Asians suggested an association of FcγRIIbT232 homozygosity with SLE with an OR of 2–2.3. Our Hong Kong SLE study had 95% power to detect an OR of 2.0, and our Caucasian study was powered to 44% for this OR. Our studies were considerably better powered than previous studies (13–18), and the findings were strengthened by meta-analysis with published data. Meta-analyses were performed using StatsDirect software.
Power calculations for the malaria-association studies were based on an assumption that the effect size of FcγRIIbT232 on susceptibility to severe malaria might be similar to that seen in thalassaemia. Thus, with an OR of 0.57, malaria case group 1 and control group 1 had a power of 77%, whereas malaria case group 2 and control group 2 had a power of 82%. The first bacteraemia study was powered to 70% for an OR of 0.57, whereas the second group of cases and controls had a power of 89%.
Supplementary Material
Acknowledgments
We thank all patients and controls involved in the study, including the staff and patients at the KEMRI–Wellcome Trust Programme in Kilifi (this paper is published with the permission of the Director of KEMRI). We would also like to thank Kirk A. Rockett, MalariaGEN Research Manager at the Wellcome Trust Centre for Human Genetics, for collation of DNA samples. Financial support was provided by the Wellcome Trust (Programme Grants 083650/Z/07/Z and 077092) and the National Institute for Health Research Cambridge Biomedical Research Centre. The Cambridge Institute for Medical Research is in receipt of Wellcome Trust Strategic Award 079895. This project was also funded by Wellcome Trust Grants 076934 (to T.N.W.), 081835 (to J.A.G.S.), and 079082 (to B.C.U.). T.N.W. and B.C.U. also receive funds from BioMalpar Network 6 Network of Excellence. L.C.W. is a Medical Research Council (MRC) Clinical Training Fellow, K.G.C.S. is a Lister Prize Fellow, and E.J.C. is an MRC Doctoral Training Account student.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915133107/DCSupplemental.
References
- 1.Lau CS, Yin G, Mok MY. Ethnic and geographical differences in systemic lupus erythematosus: An overview. Lupus. 2006;15:715–719. doi: 10.1177/0961203306072311. [DOI] [PubMed] [Google Scholar]
- 2.Alarcón-Segovia D, et al. Familial aggregation of systemic lupus erythematosus, rheumatoid arthritis, and other autoimmune diseases in 1,177 lupus patients from the GLADEL cohort. Arthritis Rheum. 2005;52:1138–1147. doi: 10.1002/art.20999. [DOI] [PubMed] [Google Scholar]
- 3.Harley IT, Kaufman KM, Langefeld CD, Harley JB, Kelly JA. Genetic susceptibility to SLE: New insights from fine mapping and genome-wide association studies. Nat Rev Genet. 2009;10:285–290. doi: 10.1038/nrg2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 5.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 6.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 7.McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 2005;307:590–593. doi: 10.1126/science.1105160. [DOI] [PubMed] [Google Scholar]
- 8.Brownlie RJ, et al. Distinct cell-specific control of autoimmunity and infection by FcgammaRIIb. J Exp Med. 2008;205:883–895. doi: 10.1084/jem.20072565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pritchard NR, et al. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcgammaRII. Curr Biol. 2000;10:227–230. doi: 10.1016/s0960-9822(00)00344-4. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, et al. Polymorphisms in IgG Fc receptor IIB regulatory regions associated with autoimmune susceptibility. Immunogenetics. 2000;51:429–435. doi: 10.1007/s002510050641. [DOI] [PubMed] [Google Scholar]
- 11.Floto RA, et al. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat Med. 2005;11:1056–1058. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 12.Kono H, et al. FcgammaRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum Mol Genet. 2005;14:2881–2892. doi: 10.1093/hmg/ddi320. [DOI] [PubMed] [Google Scholar]
- 13.Chen JY, et al. Association of a transmembrane polymorphism of Fcgamma receptor IIb (FCGR2B) with systemic lupus erythematosus in Taiwanese patients. Arthritis Rheum. 2006;54:3908–3917. doi: 10.1002/art.22220. [DOI] [PubMed] [Google Scholar]
- 14.Chu ZT, et al. Association of Fcgamma receptor IIb polymorphism with susceptibility to systemic lupus erythematosus in Chinese: A common susceptibility gene in the Asian populations. Tissue Antigens. 2004;63:21–27. doi: 10.1111/j.1399-0039.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 15.Kyogoku C, et al. Fcgamma receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: Contribution of FCGR2B to genetic susceptibility. Arthritis Rheum. 2002;46:1242–1254. doi: 10.1002/art.10257. [DOI] [PubMed] [Google Scholar]
- 16.Siriboonrit U, et al. Association of Fcgamma receptor IIb and IIIb polymorphisms with susceptibility to systemic lupus erythematosus in Thais. Tissue Antigens. 2003;61:374–383. doi: 10.1034/j.1399-0039.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 17.Magnusson V, et al. Polymorphisms of the Fc gamma receptor type IIB gene are not associated with systemic lupus erythematosus in the Swedish population. Arthritis Rheum. 2004;50:1348–1350. doi: 10.1002/art.20151. [DOI] [PubMed] [Google Scholar]
- 18.Li X, et al. A novel polymorphism in the Fcgamma receptor IIB (CD32B) transmembrane region alters receptor signaling. Arthritis Rheum. 2003;48:3242–3252. doi: 10.1002/art.11313. [DOI] [PubMed] [Google Scholar]
- 19.Clatworthy MR, et al. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc Natl Acad Sci USA. 2007;104:7169–7174. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adjuik M, et al. Cause-specific mortality rates in sub-Saharan Africa and Bangladesh. Bull World Health Organ. 2006;84:181–188. doi: 10.2471/blt.05.026492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blank MC, et al. Decreased transcription of the human FCGR2B gene mediated by the -343 G/C promoter polymorphism and association with systemic lupus erythematosus. Hum Genet. 2005;117:220–227. doi: 10.1007/s00439-005-1302-3. [DOI] [PubMed] [Google Scholar]
- 23.Clatworthy MR, Smith KGC. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J Exp Med. 2004;199:717–723. doi: 10.1084/jem.20032197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumaratilake LM, et al. A synthetic tumor necrosis factor-alpha agonist peptide enhances human polymorphonuclear leukocyte-mediated killing of Plasmodium falciparum in vitro and suppresses Plasmodium chabaudi infection in mice. J Clin Invest. 1995;95:2315–2323. doi: 10.1172/JCI117923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Langhorne J. Tumor necrosis factor alpha p55 receptor is important for development of memory responses to blood-stage malaria infection. Infect Immun. 2000;68:5724–5730. doi: 10.1128/iai.68.10.5724-5730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muniz-Junqueira MI, dos Santos-Neto LL, Tosta CE. Influence of tumor necrosis factor-alpha on the ability of monocytes and lymphocytes to destroy intraerythrocytic Plasmodium falciparum in vitro. Cell Immunol. 2001;208:73–79. doi: 10.1006/cimm.2001.1770. [DOI] [PubMed] [Google Scholar]
- 27.Rockett KA, Awburn MM, Cowden WB, Clark IA. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991;59:3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anstey NM, et al. Nitric oxide in Tanzanian children with malaria: Inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–567. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grau GE, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 30.Butcher G. Autoimmunity and malaria. Trends Parasitol. 2008;24:291–292. doi: 10.1016/j.pt.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Williams TN, et al. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192:178–186. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams TN, et al. Both heterozygous and homozygous alpha+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005;106:368–371. doi: 10.1182/blood-2005-01-0313. [DOI] [PubMed] [Google Scholar]
- 33.Tan EM, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 34.Cunninghame Graham DS, et al. Polymorphism at the TNF superfamily gene TNFSF4 confers susceptibility to systemic lupus erythematosus. Nat Genet. 2008;40:83–89. doi: 10.1038/ng.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyakeriga AM, et al. Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis. 2004;190:439–447. doi: 10.1086/422331. [DOI] [PubMed] [Google Scholar]
- 36.Berkley JA, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.