Abstract
MicroRNAs (miRNAs) are noncoding RNAs that regulate global gene expression. miRNAs often act synergistically to repress target genes, and their dysregulation can contribute to the initiation and progression of a variety of cancers. The clinical relationship between global expression of miRNA and mRNA in cancer has not been studied in detail. We used whole-genome microarray analyses of CD138-enriched plasma cells from 52 newly diagnosed cases of multiple myeloma to correlate miRNA expression profiles with a validated mRNA-based risk stratification score, proliferation index, and predefined gene sets. In stark contrast to mRNAs, we discovered that all tested miRNAs were significantly up-regulated in high-risk disease as defined by a validated 70-gene risk score (P < 0.01) and proliferation index (P < 0.05). Increased expression of EIF2C2/AGO2, a master regulator of the maturation and function of miRNAs and a component of the 70-gene mRNA risk model, is driven by DNA copy number gains in MM. Silencing of AGO2 dramatically decreased viability in MM cell lines. Genome-wide elevated expression of miRNAs in high-risk MM may be secondary to deregulation of AGO2 and the enzyme complexes that regulate miRNA maturation and function.
Keywords: DICER1, expression profile, multiple myeloma, risk stratification, system biology
MicroRNAs (miRNAs) belong to a class of noncoding small RNAs with mature sequences that contain ≈22 nucleotides (1). As repressors of gene expression, miRNAs can bind to the 3′ untranslated region (3′UTR) of an mRNA and inhibit its translation or induce its degradation (1).
Dysregulation of miRNA is involved in cancer initiation and progression (2, 3), and miRNA expression profiles have prognostic implications (4–6). Inhibiting miRNA has proved effective in vivo (7) and therefore could be a novel therapeutic strategy for cancer (8, 9). To date, few studies have investigated the roles of miRNA in multiple myeloma (MM), a plasma cell dyscrasia that homes to and expands in the bone marrow and produces disease manifestations that include osteolytic bone destruction with hypercalcemia, anemia, immunosuppression, and end-organ damage (10). Several miRNAs have been implicated in survival and growth of myeloma cells and myeloma tumor growth. For instance, miR-21 is a target of Stat3 and thus a critical component in IL-6/Stat3-dependent survival and growth pathways of myeloma cells (11). In addition, IL-6 inhibitor SOCS1 and p53 pathway component p300-CBP-associated factor are targets of multiple miRNAs, including miR-106b-25 cluster, miR-32, miR-181a/b, and miR-19a/b (12); suppression of these miRNAs inhibited myeloma tumor growth in nude mice (12).
Applying miRNA expression profiles, Pichiorri et al. (12) identified miRNAs that were differentially expressed in plasma cells of healthy donors, subjects with a benign precursor to MM (monoclonal gammopathy of undetermined significance), and patients with MM. In other miRNA expression profiling studies, Roccaro et al. (13) determined that miR-15/16 were down-regulated in relapsed/refractory MM and regulated tumor proliferation in MM cell lines, and Lionetti et al. (14) identified 16 miRNAs sensitive to DNA copy number.
To further investigate the potential involvement of miRNA in MM, we performed integrative analyses of both miRNA expression profiles and protein-coding gene expression profiles (GEPs) of myeloma cells from newly diagnosed patients. Global increases in miRNA expression were seen in cases with high-risk MM, which sharply contrasts with discriminate expression changes of only selected miRNAs that are seen in other cancers (4, 5, 15). The association between poor prognosis and elevated global expression of miRNAs was reinforced by our observation that viability of MM cell lines was dramatically reduced upon silencing of EIF2C2/AGO2, a master regulator of miRNA maturation and function (16–18). Our findings suggest that all expressed miRNAs, instead of selected miRNAs, synergistically function together to regulate MM disease progression.
Results
Overview of miRNA Expression Profiles.
The miRNA expression profiles were normalized based on spiked-in controls spotted in the Agilent platform. Briefly, the intensities of positive controls on each array were averaged, and then miRNA signals were normalized by equalizing these averages among all arrays (details in SI Text and Fig. S1). Six miRNAs presented in the Agilent miRNA microarray—hsa-miR-560, hsa-miR-565, hsa-miR-768–3p, hsa-miR-768–5p, hsa-miR-801, and hsa-miR-128b—were not in miRBase (19) release 12.0 and were discarded; 464 human miRNAs remained. A total of 95 human miRNAs were identified as expressed, which we defined as an intensity >log2100 in at least one sample (from patients with MM or healthy donors) (Dataset S1). Most human miRNAs were absent or expressed at very low levels in our samples.
Total miRNA Expression Levels Were Higher in Myeloma Cells than in Normal Plasma Cells.
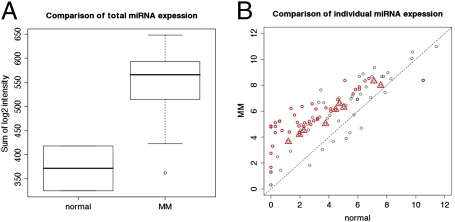
The total expression levels of miRNAs in CD138+ plasma cell samples of 52 patients newly diagnosed with MM (Table 1) were compared to those in samples from two healthy donors. The total miRNA expression level, which was determined by the mean expression levels of 95 expressed miRNAs, was higher in samples from patients with MM than in samples from healthy donors (P = 0.01, one-sided Wilcoxon test; Fig. 1A). Among 40 miRNAs with expression levels that were significantly different [statistical analysis of microarray (SAM) (20); false discovery rate (FDR) < 0.1) in myeloma cells from levels in healthy cells, 39 were consistently expressed at higher levels in samples from patients newly diagnosed with MM than in samples from healthy donors; only one of the 40 miRNAs was expressed at lower levels in samples from patients than in samples from healthy donors (Fig. 1B and Table S1). Furthermore, with quantitative PCR (qPCR), 10 randomly selected miRNAs (miR-15b, miR-16, miR-17–5p, miR-19b, miR-21, miR-22, miR-29c, let-7a, let-7d, and let-7f) were measured in purified plasma cells from three healthy donors and 10 patients with MM (miR-19b and let-7f were analyzed in cells from two healthy donors because of limited cells for all 10 analyses). All miRNAs except one were expressed at higher levels in plasma cells from patients than from healthy donors (Fig. S2). These data consistently suggest that higher total expression levels of miRNAs might be associated with MM disease initiation.
Table 1.
Clinical features of 52 patients with MM
| Clinical features | n/N (%) |
| Age ≥ 65 years | 17/52 (32.7) |
| Albumin ≥ 3.5 g/dL | 38/52 (73.1) |
| B2M ≥ 3.5 mg/dL | 29/51 (56.9) |
| B2M ≥ 5.5 mg/dL | 17/51 (33.3) |
| CRP ≥ 8 mg/L | 1/52 (1.9) |
| Hemoglobin ≥ 10 g/dL | 36/51 (70.6) |
| Creatinine ≥ 2 mg/dL | 5/51 (9.8) |
| LDH ≥ 190 U/L | 8/51 (15.7) |
B2M, β2 microglobulin; CRP, C-reactive protein; LDH, lactate dehydrogenase.
Fig. 1.
Expression levels of 95 expressed miRNAs in plasma cells from patients with MM compared with those from healthy donors. (A) Global expression of miRNAs in normal samples (n = 2) was significantly lower (P = 0.01) than in MM samples (n = 52). (B) Scatter plot of mean expression levels of miRNAs in MM samples compared with normal samples. Red triangles mark nine miRNAs on chromosome 13. Red circles mark 39 miRNAs statistically significantly up-regulated in MM. Blue circle marks one miRNA statistically significantly down-regulated in MM. Dashed line represents y = x.
Surprisingly, although chromosome 13 is deleted in ≈50% of patients with MM (21, 22), two of the nine expressed miRNAs mapping to chromosome 13 were expressed at significantly higher levels in myeloma samples than in normal samples (FDR < 0.1), and the other seven were expressed at marginally higher levels in myeloma samples than in normal samples (Fig. 1B and Table S1). This observation was consistent with a recent study reporting that four miRNAs from chromosome 13 (miR-15a, miR-19b, miR-20a, and miR-92a) were expressed at higher levels in samples from MM patients than in those from healthy donors; none were expressed at lower levels (12). Of note, our list of differentially expressed miRNAs did not completely overlap with that of Pichiorri et al. (12) (Table S1). This discrepancy may be due to use of different statistical methods (SAM in the current study and t test in Pichiorri et al. study), different sample sizes, and different experimental platforms.
Total miRNA Expression Level Was Associated with GEP-Defined Risk Score and Proliferation Index.
We previously used GEPs to define risk scores and proliferation indexes for MM disease prognosis according to expression levels of 70 and 11 genes, respectively (23) (SI Text). Higher risk scores and higher proliferation indexes were associated with shorter survival of MM patients. Taking advantage of paired miRNA and GEPs for each of 52 patients, we investigated the potential association between global miRNA expression levels and prognosis by linking total miRNA expression in an individual patient's sample with the risk score and proliferation index defined by the GEP of the same sample. Total miRNA expression level was significantly associated with risk score (P = 0.003) and proliferation index (P = 0.03). A high risk score and a high proliferation index were both significantly associated with an unfavorable clinical outcome. This observation suggests that high expression levels of total miRNA potentially confers an inferior clinical outcome.
Total miRNA Expression Level Was Associated with High-Risk Cancer Gene Sets.
Gene set enrichment analysis (GSEA) (24) was used to identify the gene sets that were significantly associated with total miRNA expression level. We calculated the correlations between total miRNA expression level and expression levels of individual protein-coding genes and then used GSEA to associate the correlations with gene sets. GSEA used the Molecular Signatures Database (MSigDB; http://www.broad.mit.edu/gsea/msigdb) containing five categories: positional gene sets, curated gene sets, motif gene sets, computational gene sets, and gene ontology gene. Table 2 lists some of the most significant and interesting gene sets in each category (full list in Dataset S2).
Table 2.
Enriched gene sets by GSEA*
| P value | ES | FDR | Rank | |
| c2: Curated gene sets | ||||
| CANCER_UNDIFFERENTIATED_META_UP | <1E-16 | 0.57 | <1E-14 | 1 |
| HUMAN_MITODB_6_2002 | <1E-16 | 0.25 | <1E-14 | 3 |
| MITOCHONDRIA | <1E-16 | 0.24 | <1E-14 | 4 |
| STEMCELL_EMBRYONIC_UP | <1E-16 | 0.20 | <1E-14 | 7 |
| STEMCELL_NEURAL_UP | <1E-16 | 0.20 | <1E-14 | 8 |
| TARTE_PLASMA_BLASTIC | <1E-16 | 0.41 | <1E-14 | 9 |
| ZHAN_MM_CD138_PR_VS_REST | 1.45E-14 | 0.66 | 1.62E-12 | 14 |
| STEMCELL_HEMATOPOIETIC_UP | 1.13E-07 | 0.09 | 3.27E-06 | 54 |
| c4: Computational gene sets | ||||
| GNF2_CCNA2 | <1E-16 | 0.60 | <1E-15 | 1 |
| GNF2_CCNB2 | <1E-16 | 0.62 | <1E-15 | 2 |
| GNF2_CDC2 | <1E-16 | 0.59 | <1E-15 | 3 |
| GNF2_CDC20 | <1E-16 | 0.65 | <1E-15 | 4 |
| GNF2_CENPF | <1E-16 | 0.59 | <1E-15 | 5 |
| GNF2_PCNA | <1E-16 | 0.62 | <1E-15 | 6 |
| GNF2_RRM1 | <1E-16 | 0.56 | <1E-15 | 7 |
| GNF2_SMC4L1 | <1E-16 | 0.52 | <1E-15 | 8 |
| MORF_AATF | <1E-16 | 0.34 | <1E-15 | 9 |
| MORF_AP2M1 | <1E-16 | 0.32 | <1E-15 | 10 |
*Selected; see Dataset S2 for a full list. ES, enrichment score (statistic generated by GSEA to measure an overlap between two gene sets); FDR, false discovery rate.
Remarkably, the gene set “CANCER_UNDIFFERENTIATED_META_UP” was the most highly enriched in the category of curated gene sets. This gene set contained genes up-regulated in multiple types of undifferentiated cancers (Table 2) (25). Consistent with this gene set, the ninth most enriched gene set was TARTE_PLASMA_BLASTIC, which is overexpressed in plasmablasts (a type of undifferentiated plasma cell) (Table 2) (26). Further supporting the association of up-regulated total miRNA level and undifferentiated cells, a few gene sets related to stem cells (the most undifferentiated cells) were significantly enriched. Specifically, STEMCELL_EMBRYONIC_UP, STEMCELL_NEURAL_UP, STEMCELL_HEMATOPOIETIC_UP, and STEMCELL_COMMON_UP, which contained genes up-regulated in at least one of three distinct types of stem cells (27), were ranked as the seventh, eighth, 54th, and 208th most enriched gene sets, respectively. Of note, the other stem cell signature, STEMCELL_COMMON_DN, which contained genes down-regulated in all of three types of stem cells, was not significantly enriched. Taken together, these observations strongly indicate that high total miRNA expression level may be associated with the high-grade undifferentiated stage of cancer, which tends to behave more aggressively than the low-grade counterparts (25).
Supporting the observed association of high expression of total miRNA with proliferation index, the 14th most enriched gene set was ZHAN_MM_CD138_PR_VS_REST (Table 2), which is up-regulated in the proliferation molecular subgroup of MM (28). Furthermore, in the category of computational gene sets, the majority of the 10 most enriched sets (Table 2) were composed of cell-proliferation genes. Of note, hundreds of cancer-related gene sets in the category of computational gene sets were significantly associated with high miRNA expression (Dataset S2).
Our analysis revealed that high expression of total miRNAs was associated with a variety of high-risk gene sets, where were observed signatures of undifferentiated cancer cells and cell cycle.
Expression of Individual miRNAs Was Associated with GEP-Defined Risk Score and Proliferation Index.
We investigated associations of each of the 95 expressed human miRNAs with risk score and proliferation index. Consistent with the observations above, no individual miRNA was significantly negatively associated with either GEP-defined risk score or proliferation index; however, 28 miRNAs significantly (FDR < 0.1) were positively associated with risk score and two miRNAs with proliferation index (Table 3). The two miRNAs associated with proliferation index were also associated with risk score. Remarkably, among the 10 expressed miRNAs that mapped to chromosome 13, eight miRNAs were significantly positively associated with risk score and one with proliferation index.
Table 3.
Association of expression level of individual miRNAs, risk score (RS), and proliferation index (PI)
| Pearson's correlation coefficient | P value | FDR | |
| Association with RS | |||
| hsa-miR-106a | 0.54 | 3.13E-05 | 7.42E-04 |
| hsa-miR-17–5p | 0.55 | 2.68E-05 | 7.42E-04 |
| hsa-miR-18a | 0.57 | 8.68E-06 | 7.42E-04 |
| hsa-miR-20a | 0.55 | 2.83E-05 | 7.42E-04 |
| hsa-miR-142–3p | 0.54 | 4.31E-05 | 8.19E-04 |
| hsa-miR-20b | 0.53 | 5.20E-05 | 8.24E-04 |
| hsa-miR-25 | 0.52 | 8.12E-05 | 1.10E-03 |
| hsa-miR-106b | 0.51 | 1.30E-04 | 1.55E-03 |
| hsa-miR-142–5p | 0.49 | 2.37E-04 | 2.50E-03 |
| hsa-miR-103 | 0.48 | 3.53E-04 | 3.36E-03 |
| hsa-miR-19a | 0.46 | 6.24E-04 | 5.39E-03 |
| hsa-miR-107 | 0.44 | 1.07E-03 | 7.83E-03 |
| hsa-miR-125a | 0.44 | 1.02E-03 | 7.83E-03 |
| hsa-miR-92 | 0.42 | 1.70E-03 | 1.15E-02 |
| hsa-miR-15a | 0.40 | 3.14E-03 | 1.99E-02 |
| hsa-miR-181b | 0.40 | 3.43E-03 | 2.04E-02 |
| hsa-miR-19b | 0.39 | 4.25E-03 | 2.37E-02 |
| hsa-miR-16 | 0.38 | 5.10E-03 | 2.68E-02 |
| hsa-miR-29b | 0.38 | 5.37E-03 | 2.68E-02 |
| hsa-let-7i | 0.38 | 5.84E-03 | 2.77E-02 |
| hsa-miR-148a | 0.35 | 1.11E-02 | 4.84E-02 |
| hsa-miR-331 | 0.35 | 1.12E-02 | 4.84E-02 |
| hsa-let-7c | 0.33 | 1.77E-02 | 7.00E-02 |
| hsa-miR-193b | 0.33 | 1.77E-02 | 7.00E-02 |
| hsa-miR-21 | 0.31 | 2.49E-02 | 9.11E-02 |
| hsa-miR-99a | 0.31 | 2.45E-02 | 9.11E-02 |
| hsa-miR-26b | 0.31 | 2.71E-02 | 9.55E-02 |
| hsa-miR-125b | 0.30 | 2.86E-02 | 9.71E-02 |
| Association with PI | |||
| hsa-miR-107 | 0.45 | 7.81E-04 | 5.36E-02 |
| hsa-miR-18a | 0.44 | 1.13E-03 | 5.36E-02 |
FDR, false discovery rate.
In addition to the statistically significant positive associations with risk score and proliferation index, many more positive associations (risk score, n = 77; proliferation index, n = 77) were identified than negative associations (risk score, n = 18; proliferation index, n = 18). Not all associations were statistically significant; the difference in positive and negative associations (77 vs. 18) was significant (P = 1.3E-9; one-sided proportional test). Furthermore, the means of Pearson's correlation coefficients were 0.19 for risk score and 0.13 for proliferation index, both of which were significantly greater than expected by permutation test (P < 1E-10; one-sided t test). These observations suggested that although some individual miRNAs alone could not significantly contribute to disease progression, their collective synergy might significantly contribute to MM disease progression.
Unsupervised Clustering Stratified Patients According to High Risk and Low Risk.
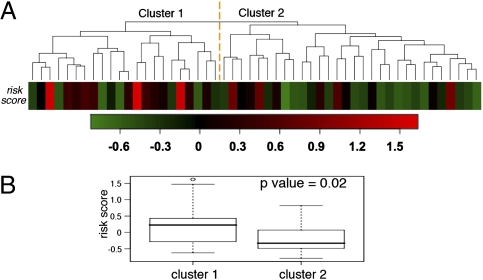
We applied unsupervised hierarchical clustering analysis to miRNA expression profiles. The 52 patients clearly separated into two clusters on the basis of expression levels of 95 expressed miRNAs (Fig. 2 and Table S2). The patients in cluster 1 had significantly higher GEP-defined risk scores than those in cluster 2 (P = 0.02; one-sided t test), suggesting that overall miRNA expression profiles were associated with risk scores. Of note, proliferation indexes were not significantly different between the clusters (P = 0.4; two-sided t test), indicating that the unsupervised clustering could not stratify the patient samples according to high or low proliferation index.
Fig. 2.
Unsupervised hierarchical clustering of samples from 52 MM patients based on miRNA expression profiles. (A) Patients’ samples were clearly grouped into two clusters. Colored bars indicate the patients’ GEP-defined risk scores (Table S2 lists miRNAs up- or down-regulated in the two clusters). (B) GEP-defined risk scores were significantly different between the two clusters.
CDKN1A/p21Waf1/Cip1 Was a Target of Multiple miRNAs.
We investigated the targets of the expressed miRNAs in Tarbase, a comprehensive database for experimentally supported animal miRNA targets (29). We found that CDKN1A/p21Waf1/Cip1 was an experimentally supported target of four distinct miRNAs (miR-106a, miR-106b, miR-17–5p, and miR-20b) among the 95 miRNAs expressed in myeloma cells; interestingly, all four miRNAs were associated with risk score (Table 3). No other single gene was targeted by more than three of the expressed miRNAs, according to Tarbase. Western blotting and luciferase reporter assays confirmed that p21Waf1/Cip1 was repressed in the JJN3 myeloma cell line after transfection of mimics of miR-106a, miR-106b, miR-17–5p, and miR-20b (Fig. S3 A and B). P21Waf1/Cip1 is a cyclin-dependent kinase inhibitor and functions as a regulator of cell cycle progression at G1, and numerous studies have reported it as a tumor suppressor gene in MM (30–32). In 272 patients newly diagnosed with MM, p21Waf1/Cip1 expression level was highly significantly associated with overall survival (P = 1E-11; hazard ratio = 0.12) (Fig. S3C).
Silencing of EIF2C2/AGO2 and DICER1 Arrested Growth and Promoted Apoptosis in Myeloma Cell Lines.
Consistent with the multiple lines of evidence that global increased expression of miRNAs in MM is associated with disease outcome, we previously reported that AGO2, a master regulator of miRNA genesis and functionality (16–18) and of B-cell differentiation (18), was an important marker for MM disease prognosis in the model for GEP-defined risk score (23). Furthermore, AGO2 is sensitive to DNA copy number, and the copy number of its locus is significantly associated with disease outcome (33) (Fig. S4).
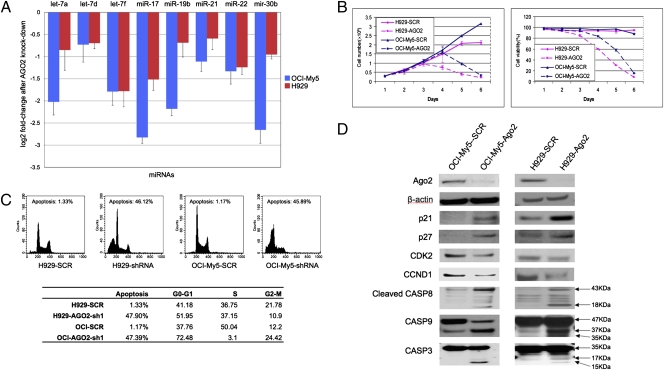
To validate the functional roles of AGO2 in myeloma cells, we used two AGO2 shRNAs to knock down AGO2 in two myeloma cell lines (H929 and OCI-My5); Western blots confirmed AGO2 knockdown in both cell lines (Fig. 3D). We used qPCR to measure changes in expression of eight randomly selected miRNAs on chromosomes 8, 9, 11, 13, 17, 22, and X before and after AGO2 silencing. All of the miRNAs were down-regulated after AGO2 silencing in both cell lines (Fig. 3A). Expression of AGO2 did not, however, significantly correlate with total expression of miRNAs in the primary tumor samples (P > 0.05). This suggested that AGO2 might not be the only factor regulating global expression of miRNAs in myeloma cells.
Fig. 3.
Effects of silencing of AGO2 on myeloma cells. (A) All eight randomly selected miRNAs were down-regulated after AGO2 knockdown. Error bars indicate standard error. (B) Cell proliferation and viability in OCI-My5 and H929 cells. Error bars indicate standard error. (C) Silencing of AGO2 induced cell cycle arrest and apoptosis. (D) Western blot analysis of proliferation- and apoptosis-associated proteins.
Cell survival experiments showed that AGO2 silencing did not inhibit cell proliferation until day 3 in H929 cells and day 4 in OCI-My5 cells; nevertheless, a dramatic decrease in cell viability was observed in cells expressing AGO2 shRNA (on day 4 in H929 cells and day 5 in OCI-My5 cells), as compared with control cells (58–60% vs. 93–96%; P < 0.05). On day 6, most cells expressing AGO2 shRNA had died, but control cells continued to proliferate (Fig. 3B).
We further investigated the effects of silencing AGO2 on cell-cycle progression by using flow cytometry analysis on day 4 in H929 cells and on day 5 in OCI-My5 cells after doxycycline induction. Silencing of AGO2 significantly enhanced G0 to G1 phase accumulation and led to cell-cycle arrest in both myeloma cell lines (Fig. 3C). We also used Western blotting to examine effects of AGO2 silencing on cell-cycle proteins p21Waf1/Cip1, p27Kip1, CDK2, and CCND1 (Fig. 3D). Silencing of AGO2 enhanced protein expression of cyclin-dependent kinase inhibitors p21Waf1/Cip1 and p27Kip1. These proteins might then inhibit CDK2 and CCND1 expression, which are important for the G1- to S-phase transition. Our results suggested that AGO2 silencing resulted in enhanced cell-cycle arrest that was mediated by dysregulated p21Waf1/Cip1, p27 Kip1, CDK2, and CCND1. Induction of p21Waf1/Cip1 expression might result from decreased activity of miR-106a, miR-106b, miR-17–5p, and miR-20b following AGO2 silencing, as discussed above.
Flow-cytometric cell-cycle analysis showed that silencing of AGO2 increased sub-G0 DNA content and induced strong apoptosis compared with that in controls (47.9% vs. 1.33% in H929; 47.39% vs. 1.17% in OCI-My5; Fig. 3C). Western blot analyses of apoptotic mechanisms showed strong activation of caspase-3, -8, and -9 in cells expressing AGO2 shRNA (Fig. 3D), which indicated that AGO2 knockdown induced apoptosis via activation of caspase signaling. The results suggested that silencing of AGO2 induced apoptosis mediated by activation of caspase-3, -8, and -9.
To further validate that total miRNA expression elevation drives MM disease progression, we knocked down DICER1, another master regulator of miRNA genesis that cleaves double-stranded RNA precursors, generating short RNAs that are then transferred to Argonaute proteins (34). Two DICER1 shRNAs were used to knock down DICER1 in OCI-My5. Western blots confirmed DICER1 knockdown in OCI-My5 (Fig. S5A). Similar to the results of AGO2 knockdown, DICER1 knockdown decreased the viability of the cells, significantly enhanced G0- to G1-phase accumulation, led to cell-cycle arrest, and greatly increased apoptosis (Fig. S5 B and C).
Discussion
Multiple lines of evidence from integrating miRNA expression profiles and mRNA expression profiles suggest that overall expression of miRNAs contribute to the progression of MM. Furthermore, among 28 individual miRNAs significantly associated with GEP-defined risk score and two significantly associated with proliferation index, all were positively correlated with risk score and proliferation index. Surprisingly, miRNAs mapping to chromosome 13 were significantly positively associated with risk score and proliferation index, even though one allele of chromosome 13 was deleted in ≈50% of MM samples (21, 22).
Our results and reports from Pichiorri et al. (12) suggested that miR-15a was up-regulated in newly diagnosed MM. On the other hand, Roccaro et al. (13) reported miR-15a was down-regulated in relapsed/refractory MM and regulated tumor proliferation in MM cell lines. Notably, Lionetti et al. (14) observed significant correlation between expression of miR-15a and the corresponding DNA locus in MM cell lines, which are more similar to MM cells in advanced-stage disease, but no significant correlation in newly diagnosed MM. Taken together, these studies suggest that miR-15a potentially plays different roles in primary tumors and advanced tumors, and may acquire more roles as the disease develops. These hypotheses warrant further study.
We did not identify a significant association between p53 and miR-34a, miR-29a, miR-29b, miR15a, and miR-16 (P values: 0.43, 0.82, 0.57, 0.14, and 0.17, respectively). However, expression of MYC was significantly positively associated with expression levels of miR-17–5p, miR-18a, miR-19b, and miR-20a (P values: 0.015, 0.014, 0.018, and 0.007, respectively). consistent with reports that MYC can regulate the miR-17–92 cluster on chromosome 13 (35). Therefore, up-regulation of MYC might contribute to unregulated expression of the miR17-92 cluster (35), but it is not likely to contribute greatly to global up-regulation of miRNAs in high-risk MM because MYC was also reported as a repressor of global miRNA expression (36).
AGO2 was reported as a pivotal factor in miRNA maturation (16–18) and B-cell differentiation (18), and our in vitro experiment findings suggest that silencing of AGO2 might globally impair miRNA expression in MM cell lines. However, expression of AGO2 did not significantly associate with global miRNA expression. This suggested that AGO2 was not the only regulator of global up-regulation of miRNAs in high-risk MM but that multiple factors might contribute.
Kumar et al. (37) and Merritt et al. (38) suggested that impaired processing of miRNAs might be associated with high-risk lung cancer and ovarian cancer, respectively. Our finding that globally up-regulated miRNA expression was associated with high-risk MM contrasts with their findings and, therefore, strongly suggests that the global function of miRNAs in cancers could be context-dependent.
Finally, silencing of AGO2 and DICER1, two key regulators for both miRNA maturation and functionality, significantly decreased viability of myeloma cells. This confirmed that the association of total miRNA expression level and disease progression was not an artificial phenomenon resulting from coexpression of miRNAs and oncogenes. The findings shed light on a potential therapeutic strategy to block the pathways of miRNA maturation and functionality, such as AGO2 or DICER1 knockdown.
Materials and Methods
Study Subjects.
Bone marrow aspirates were obtained from 52 patients newly diagnosed with MM. All subjects provided written informed consent, acknowledging the investigational nature of the protocol and the availability of other treatment options, as required by the Institutional Review Board and the Food and Drug Administration and in line with the Helsinki Declaration. Each sample was split into two, one for mRNA expression profiling and the other for miRNA expression profiling.
mRNA Purification and Microarray Hybridization.
Myeloma plasma cells were isolated from heparinized bone marrow aspirates by CD138-based immunomagnetic bead selection (AutoMacs; Miltenyi) (39). mRNA purification, cDNA synthesis, cRNA preparation, and hybridization to the Human Genome U133Plus 2.0 GeneChip microarrays (Affymetrix) were performed as previously described (23, 28). Data are accessible through NCBI GEO database with accession number GSE17306.
miRNA Purification and Microarray Hybridization.
Total RNA was isolated with TRIzol reagent (Invitrogen) from CD138-selected plasma cells that had been snap-frozen and stored in liquid nitrogen. miRNA microarray profiling was conducted with the Human miRNA Microarray platform (Agilent) according to the Agilent version 2.0 protocol for target labeling, hybridization, washing, scanning, and image analysis (details in SI Text). Total gene signal from GeneView data files extracted with default settings in Agilent Feature Extraction was used as signal for miRNAs. (Data are accessible through NCBI GEO database with accession no. GSE17306.)
Microarray Data Analyses.
The miRNA expression data were normalized so that the average values of positive controls (n = 15) on each array were equal. Affymetrix U133Plus 2.0 gene expression data were normalized with MAS5 using default parameters in Affymetrix GeneChip operating software. All statistical analyses were performed with the statistics software R (Version 2.6.2; available from http://www.r-project.org) and R packages developed by BioConductor project (available from http://www.bioconductor.org). SAM algorithm (20) (a variant of the t test that adds a constant to stabilize variation of genes expressed at low levels) from R package siggenes was used to determine miRNAs with differential expression in MM samples and normal samples. GSEA (24) was used to identify gene sets that were significantly associated with total miRNA expression level (details in SI Text).
Analysis of miRNA by qPCR.
Total RNA was extracted by TRIzol (Invitrogen) and reverse-transcribed in 10-μL reactions in an Applied Biosystems 9700 thermocycler (Applied Biosystems). TaqMan miRNA assays were used to detect and quantify mature miRNAs by ABI PRISM 7900 analytical thermocycler (Applied Biosystems) according to the manufacturer's recommendations. Normalization was performed with mean values of RNU43, RNU44, RNU48, and Z30 (details in SI Text).
Western Blotting.
Western blotting was carried out with the Western Breeze Chemiluminescent Immunodetection protocol (Invitrogen). The following primary antibodies were used: anti-AGO2, anti-DICER1, anti-β-actin; anti-caspase-3, -8, and -9; anti-p21Waf1/Cip1; anti-p27Kip1; anti-CDK2; and anti-CCND1 (Cell Signaling Technology).
EIF2C2/AGO2 and DICER1 Knockdown.
Two synthetic double-stranded oligonucleotides specific for AGO2 (5′-GATCCCCGCAAGGATCGCATCTTCAAGGTTCAAGAGACCTTGAA GATGCGATCCTTGCTTTTTA-3′ and 5′- GATCCCCGCAGGACAAAGATGTATTAAATTCA AGAGATTTAATACATCTTTGTCCTGCTTTTTA-3′) and two synthetic double-stranded oligonucleotides specific for DICER1 (5′-GATCCCCAGAGGTACTTAGGAAATTTTTCA AGAGAAAATTTCCTAAGTACCTCTTTTTTA-3′ and 5′-GATCCCCAAGAATCAGCCT CGCAACAAATTCAAGAGATTTGTTGCGAGGCTGATTCTTTTTTTA-3′) were synthesized; a nonsense scrambled oligonucleotide (5′-GATCCCCGACACGCGACTTGTACCACTTC AAGAGAGTGGTACAAGTCGCGTGTCTTTTTA-3′) was obtained from OligoEngine. Double-stranded shRNA oligonucleotides were cloned into lentiviral pLVTH vectors (kindly provided by Didier Trono, National Center for Competence in Research, Lausanne, Switzerland) that allow doxycycline-inducible expression of the cloned fragment (SI Text).
Cell Cycle and Apoptosis Analysis.
Cell cycle and apoptosis were determined by fluorescence-activated cell sorting (FACS) (SI Text).
Cell Proliferation Assay.
H929 and OCI-My5 myeloma cell lines infected with AGO2 or DICER1 shRNAs and controls were seeded at a density of 3 × 105 cells/mL. Cell number and viability were determined by trypan blue exclusion at various time intervals.
Supplementary Material
Acknowledgments
The work was supported by Grant CA55819 from the National Cancer Institute, the Lebow Fund to Cure Myeloma, the Peninsula Community Foundation, and the Nancy and Stephen Grand Philanthropic Fund. The manuscript was edited by the Office of Grants and Scientific Publications at the University of Arkansas for Medical Sciences.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The sequences reported in this paper have been deposited in the NCBI GEO database (accession no. GSE17306).
This article contains supporting information online at www.pnas.org/cgi/content/full/0908441107/DCSupplemental.
References
- 1.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Fabbri M, Croce CM, Calin GA. MicroRNAs in the ontogeny of leukemias and lymphomas. Leuk Lymphoma. 2009;50:160–170. doi: 10.1080/10428190802535114. [DOI] [PubMed] [Google Scholar]
- 3.Visone R, Croce CM. MiRNAs and cancer. Am J Pathol. 2009;174:1131–1138. doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartels CL, Tsongalis GJ. MicroRNAs: Novel biomarkers for human cancer. Clin Chem. 2009;55:623–631. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- 5.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 6.Iorio MV, Casalini P, Tagliabue E, Ménard S, Croce CM. MicroRNA profiling as a tool to understand prognosis, therapy response and resistance in breast cancer. Eur J Cancer. 2008;44:2753–2759. doi: 10.1016/j.ejca.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 7.Krützfeldt J, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 8.Czech MP. MicroRNAs as therapeutic targets. N Engl J Med. 2006;354:1194–1195. doi: 10.1056/NEJMcibr060065. [DOI] [PubMed] [Google Scholar]
- 9.Medina PP, Slack FJ. Inhibiting microRNA function in vivo. Nat Methods. 2009;6:37–38. doi: 10.1038/nmeth0109-37. [DOI] [PubMed] [Google Scholar]
- 10.Barlogie B, Shaughnessy J, Epstein J, Sanderson R, Anaissie E, Walker R, Tricot G. In: Williams Hematology. Marshall AL, Beutler E, Kaushansky K, Kipps TJ, Seligsohn U, Prchal J, editors. New York: McGraw-Hill; 2005. pp. 1501–1533. [Google Scholar]
- 11.Löffler D, et al. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–1333. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- 12.Pichiorri F, et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci USA. 2008;105:12885–12890. doi: 10.1073/pnas.0806202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roccaro AM, et al. MicroRNAs 15a and 16 regulate tumor proliferation in multiple myeloma. Blood. 2009;113:6669–6680. doi: 10.1182/blood-2009-01-198408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lionetti M, et al. Integrative high-resolution microarray analysis of human myeloma cell lines reveals deregulated miRNA expression associated with allelic imbalances and gene expression profiles. Genes Chromosomes Cancer. 2009;48:521–531. doi: 10.1002/gcc.20660. [DOI] [PubMed] [Google Scholar]
- 15.Nicoloso MS, Kipps TJ, Croce CM, Calin GA. MicroRNAs in the pathogeny of chronic lymphocytic leukaemia. Br J Haematol. 2007;139:709–716. doi: 10.1111/j.1365-2141.2007.06868.x. [DOI] [PubMed] [Google Scholar]
- 16.Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131:1097–1108. doi: 10.1016/j.cell.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 18.O'Carroll D, et al. A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev. 2007;21:1999–2004. doi: 10.1101/gad.1565607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: Tools for microRNA genomics. Nucleic Acids Res. 2008;36(Database issue):D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaughnessy J, et al. High incidence of chromosome 13 deletion in multiple myeloma detected by multiprobe interphase FISH. Blood. 2000;96:1505–1511. [PubMed] [Google Scholar]
- 22.Tricot G, et al. Poor prognosis in multiple myeloma is associated only with partial or complete deletions of chromosome 13 or abnormalities involving 11q and not with other karyotype abnormalities. Blood. 1995;86:4250–4256. [PubMed] [Google Scholar]
- 23.Shaughnessy JD, Jr, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian A, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodes DR, et al. Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci USA. 2004;101:9309–9314. doi: 10.1073/pnas.0401994101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarte K, Zhan F, De Vos J, Klein B, Shaughnessy J., Jr Gene expression profiling of plasma cells and plasmablasts: Toward a better understanding of the late stages of B-cell differentiation. Blood. 2003;102:592–600. doi: 10.1182/blood-2002-10-3161. [DOI] [PubMed] [Google Scholar]
- 27.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: Transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 28.Zhan F, et al. The molecular classification of multiple myeloma. Blood. 2006;108:2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sethupathy P, Corda B, Hatzigeorgiou AG. TarBase: A comprehensive database of experimentally supported animal microRNA targets. RNA. 2006;12:192–197. doi: 10.1261/rna.2239606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YH, et al. Growth inhibition of a human myeloma cell line by all-trans retinoic acid is not mediated through downregulation of interleukin-6 receptors but through upregulation of p21(WAF1) Blood. 1999;94:251–259. [PubMed] [Google Scholar]
- 31.Lavelle D, Chen YH, Hankewych M, DeSimone J. Histone deacetylase inhibitors increase p21(WAF1) and induce apoptosis of human myeloma cell lines independent of decreased IL-6 receptor expression. Am J Hematol. 2001;68:170–178. doi: 10.1002/ajh.1174. [DOI] [PubMed] [Google Scholar]
- 32.Stewart JP, et al. Correlation of TACC3, FGFR3, MMSET and p21 expression with the t(4;14)(p16.3;q32) in multiple myeloma. Br J Haematol. 2004;126:72–76. doi: 10.1111/j.1365-2141.2004.04996.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, et al. Integration of DNA copy number and gene expression alteration reveal novel insights into the molecular pathogenesis and prognosis of multiple myeloma. Blood (ASH Annual Meeting Abstracts) 2008;12:250. [Google Scholar]
- 34.Jinek M, Doudna JA. A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009;457:405–412. doi: 10.1038/nature07755. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 36.Chang TC, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 38.Merritt WM, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med. 2008;359:2641–2650. doi: 10.1056/NEJMoa0803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarte K, et al. Generation of polyclonal plasmablasts from peripheral blood B cells: A normal counterpart of malignant plasmablasts. Blood. 2002;100:1113–1122. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.