Abstract
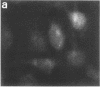
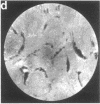
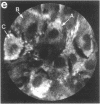
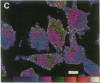
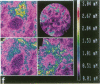
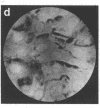
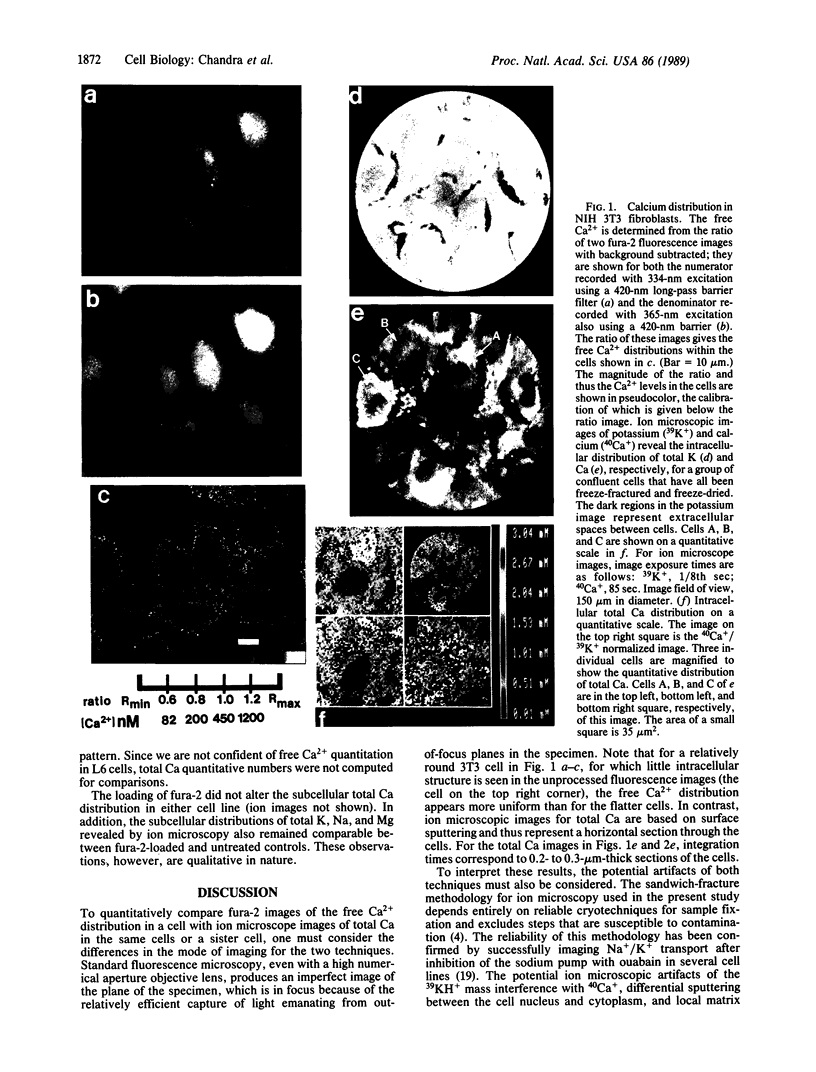
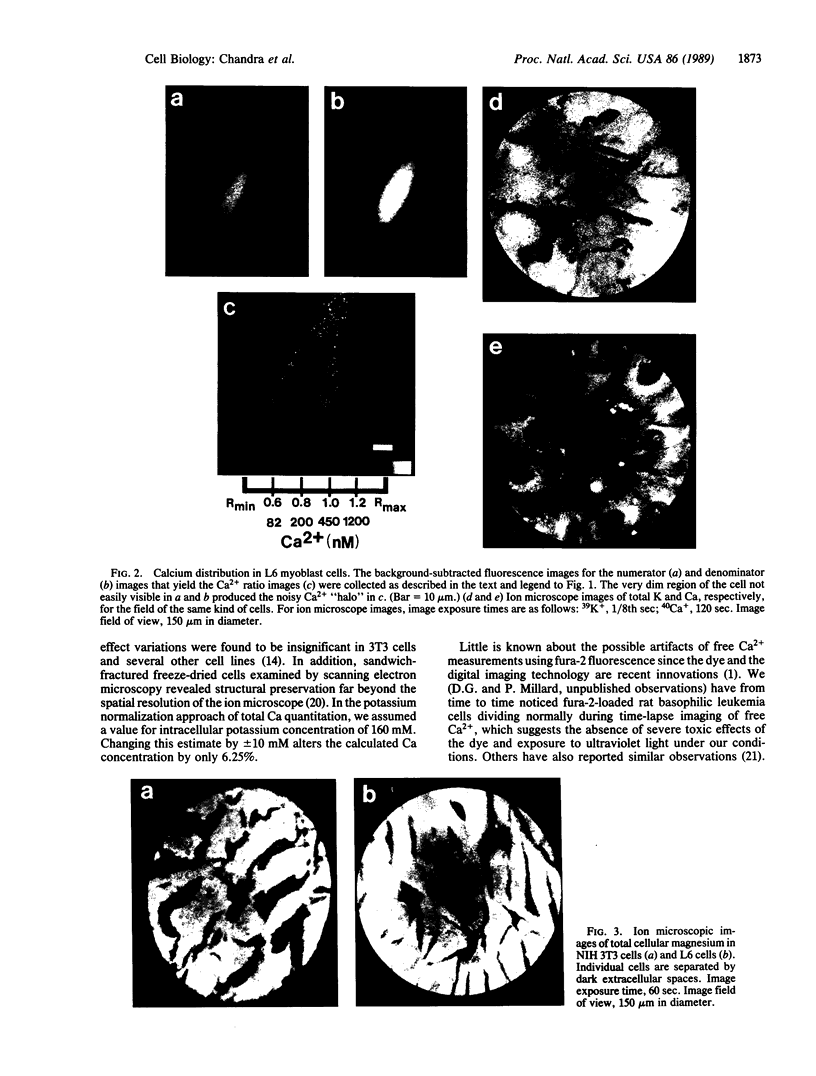
Techniques of fluorescence and ion microscopies were combined to study the free [Ca2+] and total Ca in NIH 3T3 fibroblast and L6 rat myoblast cells. Free Ca2+ measurements with the Ca2+ indicator fura-2 and digital imaging reveal an inhomogeneous distribution of free cytoplasmic Ca2+ in both cell lines. Fura-2 also reveals a difference in free Ca2+ activity between the nucleus and cytoplasm of cells. Ion microscopic observations on sister cells show that total Ca in the cytoplasm is also inhomogeneously distributed and that mean cytoplasmic levels of total Ca are higher than levels in the nuclei. In the nuclei of NIH 3T3 cells, the mean free [Ca2+] and total [Ca] were 110 +/- 30 nM and 225 +/- 43 microM, respectively, while regions in the cell cytoplasm contained up to 490 +/- 270 nM free [Ca2+] and 559 +/- 184 microM mean total [Ca]. Intracellular total Ca was greater than 3 orders of magnitude higher than intracellular free Ca2+ in either nuclear or cytoplasmic compartments. Perinuclear cytoplasmic regions in 3T3 cells contained higher free and total Ca than the cell nucleus. Loading of cells with fura-2 did not modify the subcellular distribution of total K, Na, Ca, or Mg. This combination of two powerful ion imaging techniques provides a comparison between free and total calcium in cells and introduces a different approach for examining the role of this important element in cell physiology.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Chandra S., Ausserer W. A., Morrison G. H. Evaluation of matrix effects in ion microscopic analysis of freeze-fractured, freeze-dried cultured cells. J Microsc. 1987 Dec;148(Pt 3):223–229. doi: 10.1111/j.1365-2818.1987.tb02869.x. [DOI] [PubMed] [Google Scholar]
- Chandra S., Morrison G. H. Imaging elemental distribution and ion transport in cultured cells with ion microscopy. Science. 1985 Jun 28;228(4707):1543–1544. doi: 10.1126/science.2990033. [DOI] [PubMed] [Google Scholar]
- Chandra S., Morrison G. H. Ion microscopy in biology and medicine. Methods Enzymol. 1988;158:157–179. doi: 10.1016/0076-6879(88)58054-0. [DOI] [PubMed] [Google Scholar]
- Chandra S., Morrison G. H., Wolcott C. C. Imaging intracellular elemental distribution and ion fluxes in cultured cells using ion microscopy: a freeze-fracture methodology. J Microsc. 1986 Oct;144(Pt 1):15–37. doi: 10.1111/j.1365-2818.1986.tb04670.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Gross D. J., Heppel L. A., Webb W. W. Studies on the increase in cytosolic free calcium induced by epidermal growth factor, serum, and nucleotides in individual A431 cells. J Cell Physiol. 1988 May;135(2):269–276. doi: 10.1002/jcp.1041350214. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Heppel L. A., Gross D. J., Webb W. W., Parries G. The rapid desensitization of receptors for platelet derived growth factor, bradykinin and ATP: studies on individual cells using quantitative digital video fluorescence microscopy. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1205–1212. doi: 10.1016/s0006-291x(88)80494-7. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Highsmith S., Bloebaum P., Snowdowne K. W. Sarcoplasmic reticulum interacts with the Ca(2+) indicator precursor fura-2-am. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1153–1162. doi: 10.1016/s0006-291x(86)80403-x. [DOI] [PubMed] [Google Scholar]
- Ling Y. C., Bernius M. T., Morrison G. H. SIMIPS: secondary ion mass image processing system. J Chem Inf Comput Sci. 1987 May;27(2):86–94. doi: 10.1021/ci00054a009. [DOI] [PubMed] [Google Scholar]
- Millard P. J., Gross D., Webb W. W., Fewtrell C. Imaging asynchronous changes in intracellular Ca2+ in individual stimulated tumor mast cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1854–1858. doi: 10.1073/pnas.85.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakache M., Schreiber A. B., Gaub H., McConnell H. M. Heterogeneity of membrane phospholipid mobility in endothelial cells depends on cell substrate. Nature. 1985 Sep 5;317(6032):75–77. doi: 10.1038/317075a0. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Ramseyer G. O., Morrison G. H. Relative sensitivity factors of elements in quantitative secondary ion mass spectrometric analysis of biological reference materials. Anal Chem. 1983 Oct;55(12):1963–1970. doi: 10.1021/ac00262a030. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Steinberg S. F., Bilezikian J. P., Al-Awqati Q. Fura-2 fluorescence is localized to mitochondria in endothelial cells. Am J Physiol. 1987 Nov;253(5 Pt 1):C744–C747. doi: 10.1152/ajpcell.1987.253.5.C744. [DOI] [PubMed] [Google Scholar]
- Turner L. K., Ling Y. C., Bernius M. T., Morrison G. H. Direct correlation of ion and electron microscopic images by digital image superpositioning. Anal Chem. 1987 Oct 15;59(20):2463–2468. doi: 10.1021/ac00147a005. [DOI] [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- Wroblewski J., Roomans G. M., Madsen K., Friberg U. X-ray microanalysis of cultured chondrocytes. Scan Electron Microsc. 1983;(Pt 2):777–784. [PubMed] [Google Scholar]
- Wroblewski J., Roomans G. M. X-ray microanalysis of single and cultured cells. Scan Electron Microsc. 1984;(Pt 4):1875–1882. [PubMed] [Google Scholar]