Abstract
Objective To determine the difference in outcome among elderly people with major depression who do and do not have severe white matter lesions on magnetic resonance imaging.
Design Follow up study.
Setting Two psychiatric and two general hospitals in Melbourne, Australia.
Subjects 60 depressed subjects aged over 55 referred to hospital psychiatric services with major depressive disorder meeting American Psychiatric Association (DSM-IIIR) criteria.
Main outcome measure Proportion with good outcome as determined by full recovery from initial illness and no evidence of depressive relapse or cognitive decline during follow up among those with and without lesions.
Results Mean (SD) follow up was 31.9 (9.9) months. Survival analysis showed a significant effect of severe lesions on time to poor outcome (P=0.04), with median survival 136 days in those with severe lesions compared with 315 days in those without.
Conclusion Severe white matter change on magnetic resonance imaging is associated with poor outcome in elderly depressed subjects.
Key messages
Severe deep white matter lesions on magnetic resonance imaging are common in elderly patients with depression
Patients with these lesions are at greater risk of poor long term outcome (chronicity and relapse) than those without lesions
The neuropathogical and neurochemical correlates of these white matter changes need investigation
Introduction
Major depressive disorder1 in older people often has a poor prognosis.2 Cerebral organic factors may predict poor outcome.2,3 Recent studies of the brains of elderly depressed patients have reported an excess of deep white matter lesions (but not periventricular lesions) on magnetic resonance imaging. Mild and moderate white matter lesions are common in healthy elderly people and of uncertain significance. However, severe changes are not part of normal ageing, although they are often seen in elderly depressed subjects.4 Such severe white matter lesions may predispose to the onset of first depression in some elderly people4 and have been reported to be associated with poor response to initial treatment.5,6 It is not known, however, whether white matter lesions have any influence on long term outcome in major depressive disorder. We report a follow up study of 54 elderly subjects with major depressive disorder, all of whom had magnetic resonance imaging at baseline to determine the presence and severity of deep white matter and periventricular lesions.
Subjects and methods
A more detailed account of subject selection and scanning procedure has been published.4 In brief, 60 consecutive patients aged over 55 who met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IIIR) criteria for major depressive disorder were recruited from two general and two old age psychiatric units in Melbourne over 18 months in 1992-3. All consented to magnetic resonance imaging and had a full psychiatric assessment including physical examination and screening blood tests (routine haematology and biochemical analyses including tests of thyroid function). Severity of depression was assessed with the Hamilton depression rating scale7 and cognitive function with the Cambridge cognitive examination,8 which includes the mini-mental state examination. Subjects were excluded if they had a history of stroke, transient ischaemic attacks, epilepsy, Parkinson’s disease, substance misuse, untreated hypothyroidism, cancer, insulin dependent diabetes, or other life threatening illness.
Magnetic resonance imaging
A 0.3 Tesla magnet (Fonar, Melville, New York) was used to obtain double spin echo T2 weighted (30/85/2000 TE/TE/TR) images. We took 5 mm axial slices with a 2.5 mm interslice interval and 5.1 mm coronal slices with a 0.5 mm interslice interval. Pixel size was 0.94×1.25 mm. The severity of deep white matter and periventricular lesions was rated independently by two experienced neuroradiologists (PD and BT) blind to clinical diagnosis. Both coronal and axial images were used to rate lesions on a standard 0-3 scale.9 Inter-rater reliability was assessed by using weighted κ and was 0.81 for deep white matter lesions and 0.75 for periventricular lesions.4 When the raters disagreed the rating made by PD was used for analysis.
Follow up
Subjects were followed up by experienced raters blind to the initial findings of magnetic resonance imaging. The dates of follow up varied according to clinical circumstances, but the aim was to achieve annual follow up for three years where possible. Severity of depression and cognitive function were assessed by the same methods as at baseline, and the raters also interviewed a carer and obtained history of intercurrent psychiatric illness and treatment. Initial and follow up diagnoses were made according to standard criteria for psychiatric disorder.1 Case notes were searched to determine the date of depressive relapse, return to depressive invalidism, or development of dementia where appropriate. Outcome for all subjects at their last follow up, blind to magnetic resonance imaging, was rated by DA using all available data as (a) continuously free from depression after initial recovery; (b) currently free from depression after intervening relapse of major depressive disorder; (c) “depressive invalidism”—disabling depressive symptoms not sufficient to meet criteria for major depressive disorder; (d) currently suffering from a relapse of major depressive disorder; (e) continuously ill with major depressive disorder from index episode; (f) demented; or (g) dead. For subjects who had died the date of death was entered as the date of final follow up.
Analysis of data
Subjects were divided into two groups: those with severe lesions and those with no, mild, or moderate lesions. Only severe lesions differentiate those with depression from healthy elderly people, and we hypothesised that such lesions may represent pathological brain change (as opposed to normal age related changes) and so be important in determining outcome. Data were analysed by first categorising patients in group (a) as having a good outcome and those in the other groups as having a bad outcome in accordance with established practice.2 Since some patients who have had a single relapse but made good recovery from this might also be considered as having a good outcome we reanalysed data including subjects who were well at follow up (groups a and b) as having good outcomes. We also conducted an analysis in which we included all subjects who had remained free from major depression or depressive invalidism until death as having a good outcome.
A Kaplan-Meier survival analysis was performed to take account of the variable length of follow up, as some subjects were at higher risk of relapse simply because they had been followed up for a longer time. Differences in survival rates between severe and non-severe lesion groups were determined by the log rank test. Survival time was assessed as time taken from initial recovery date until date of first relapse or death, or, for patients who remained well, until date of final follow up. Those who never recovered fully were deemed to have had a survival time of 0 days.
Results
Mean age of the 60 subjects at entry to the study was 71.2 years (SD 7.9) and 18 were men. Table 1 shows the presence and severity of lesions. Fifty four subjects were followed for between 2 and 48 months (mean 31.9 (SD 9.9) months). Six subjects refused any follow up or were lost to follow up. One subject died after two months, eight subjects were followed for 12-24 months, 20 for 24-36 months, and 25 for 36 months or more. No association was found between outcome and periventricular lesions. Table 2 shows the outcome at 32 months and the numbers with severe deep white matter lesions.
Table 1.
Presence and severity of brain lesions at start of follow up in 60 patients with major depressive disorder
| Severity of lesions | No with deep white matter lesions | No with periventricular lesions |
|---|---|---|
| None | 9 | 19 |
| Mild | 20 | 11 |
| Moderate | 15 | 13 |
| Severe | 16 | 17 |
Table 2.
Outcome after 32 months among 54 patients with major depressive disorder according to presence of severe deep white matter lesions on magnetic resonance imaging
| Outcome | Severe white matter lesions
|
Without severe white matter lesions
|
|||
|---|---|---|---|---|---|
| No (%) | 95% CI (%) | No (%) | 95% CI (%) | ||
| Continuously well* | 0 (0) | 0 to 21 | 11 (27) | 14 to 43 | |
| Recovered, one relapse and recovered | 1 (8) | 0 to 36 | 7 (17) | 7 to 32 | |
| Continuously ill or recovered, relapsed and then remained ill | 6 (46) | 19 to 75 | 14 (34) | 20 to 51 | |
| Demented/died | 6 (46) | 19 to 75 | 9 (22) | 11 to 38 | |
| Total | 13 | 41 | |||
Fisher’s exact probability test (two tailed) for comparison of good (continuously well) v bad outcomes (other categories) P = 0.048.
Subjects with severe (grade 3) deep white matter lesions had a significantly worse outcome than others and none remained continuously well (Fisher’s exact probability test P=0.048). The effect of severe lesions was significant for all three definitions of good outcome. When the eight subjects who were well at follow up after a relapse of major depressive disorder were reclassified as having a good outcome only one of the 13 subjects with severe deep white matter lesions (88%) had a good outcome compared with 18 of 41 (44%) who did not have severe lesions (P=0.02). Similarly, when the five subjects who died after remaining well from initial recovery were combined with the 11 who had been continuously well the association of severe lesions with bad outcome had a P value of 0.05.
In order to determine whether some factor related to the lesions may have affected outcome we performed analyses taking into account the potential confounding variables of age, sex, length of time with major depressive disorder before initial assessment, severity of depression, age at onset of first episode, diastolic blood pressure, and presence of cardiovascular risk factors.4 None of these variables was significantly associated with outcome. There was no association between periventricular lesions and outcome in any analysis.
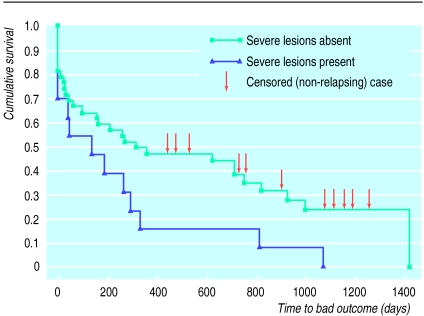
Survival analysis confirmed the effects of severe lesions on poor outcome (figure). Survival functions for the severe and non-severe lesion groups were significantly different (Log rank test statistic 3.63, df=1, P=0.04). Median survival time for the 13 subjects with lesions was 136 days (95% confidence interval 0 to 309) compared with 315 days (0 to 813) for those without lesions.
Discussion
In this two year follow up of a cohort of elderly patients with major depression and no overt cerebrovascular disease, severe deep white matter lesions predicted poor outcome. The result was not accounted for by confounding factors, although larger studies are needed to confirm a specific association between severe lesions and poor outcome.
We do not believe that the missing subjects have unduly biased the outcome as they were similar to the others in all relevant respects. Even if all six were allocated to the bad outcome group the significant association between severe lesions and bad outcome would remain. By traditional strict outcome criteria 80% of the sample did badly. However, the subjects were elderly and had required hospital treatment for depression and their outcomes are not inconsistent with earlier work on similar populations.2 The association remained when less stringent definitions of bad outcome were used. Periventricular lesions, which have been shown to be common in Alzheimer’s disease, had no effect on outcome.
Our results and previous work show that some elderly patients with major depressive disorder have evidence of damage to deep white matter structures which may have a role in the initiation, maintenance, and outcome of their condition. Limited neuropathological study of the structural basis of such white matter lesions in non-depressed subjects has shown severe lesions to be associated with demyelination, perivascular change, and arteriosclerosis.10,11 More studies are now needed on the neuropathological and neurochemical correlates of such lesions in depressed subjects. Such information may inform new treatments for this group of patients, who currently have a poor prognosis. In the interim, it seems prudent to take account of the presence of severe white matter lesions when considering the frequency of clinical monitoring and the need for prophylactic antidepressants.
Figure.
Survival analysis showing effect of white matter lesions on time to depressive relapse. Survival rates are significantly different between groups (P=0.04)
Footnotes
Funding: National Health and Medical Research Council, Australian Rotary Health Foundation, and Victorian Health Promotion Foundation.
Conflict of interest: None.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 3rd edition revised. Washington, DC: APA; 1987. [Google Scholar]
- 2.Ames D, Allen N. The prognosis of depression in old age: good, bad or indifferent? Int J Geriatr Psychiatry. 1991;6:477–481. [Google Scholar]
- 3.Jacoby R, Levy R, Bird JM. Computed tomography and the outcome of affective disorder: a follow up study of elderly patients. Br J Psychiatry. 1981;139:288–292. doi: 10.1192/bjp.139.4.288. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien J, Desmond P, Ames D, Schweitzer I, Harrigan S, Tress B. A magnetic resonance imaging study of white matter lesions in depression and Alzheimer’s disease. Br J Psychiatry. 1996;168:477–485. doi: 10.1192/bjp.168.4.477. [DOI] [PubMed] [Google Scholar]
- 5.Hickie I, Scott E, Mitchell P, Wilhelm K, Austin M-P, Bennett B. Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression. Biol Psychiatry. 1995;37:151–160. doi: 10.1016/0006-3223(94)00174-2. [DOI] [PubMed] [Google Scholar]
- 6.Simpson S, Jackson A, Baldwin RC, Burns A. Subcortical hyperintensities in late-life depression: acute response to treatment and neuropsychological impairment. International Psychogeriatrics. 1997;9:257–275. doi: 10.1017/s1041610297004432. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 8.Roth M, Tym E, Mountjoy C, Huppert F, Hendrie H, Verma S, et al. CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- 9.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5T in Alzheimer’s disease and normal ageing. Am J Roentgenology. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 10.Chimowitz MI, Estes ML, Furlan AJ, Awad IS. Further observations on the pathology of subcortical lesions identified on magnetic resonance imaging. Arch Neurology. 1992;49:747–752. doi: 10.1001/archneur.1992.00530310095018. [DOI] [PubMed] [Google Scholar]
- 11.Fazekas F, Kleimert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental MRI white matter hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]