Abstract
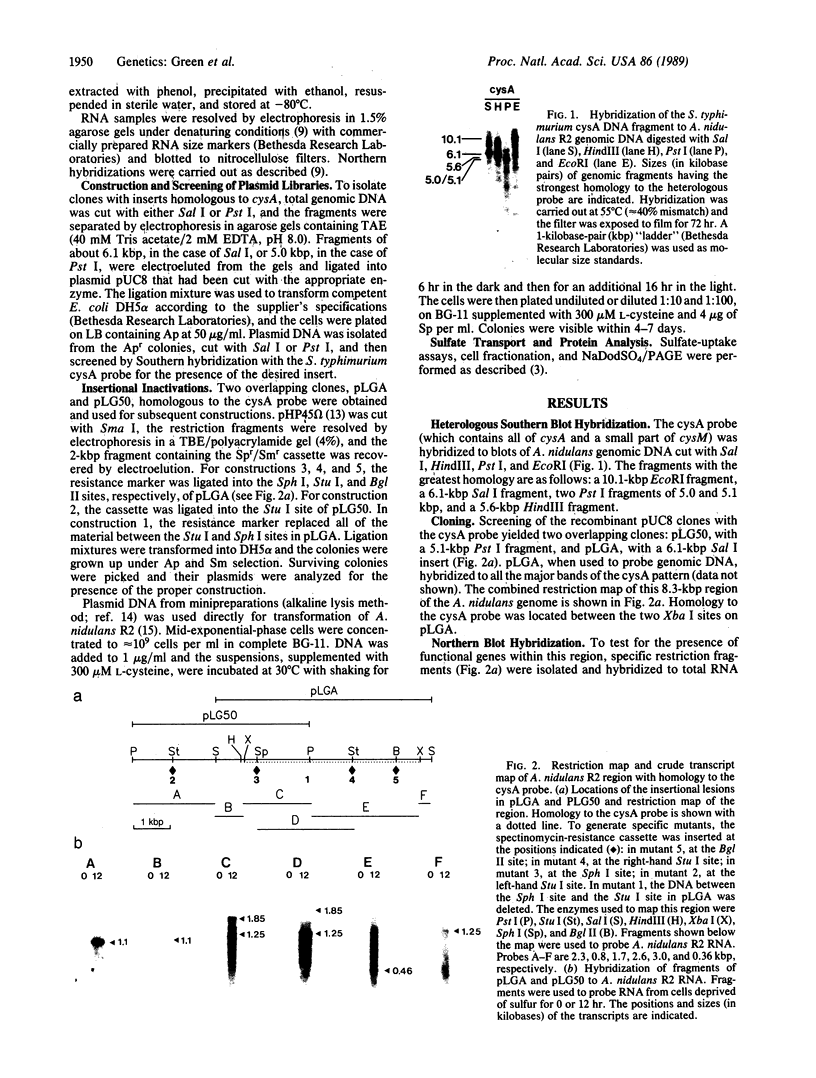
Using the cysA locus of Salmonella typhimurium as a heterologous probe, we have cloned a region of the Anacystis nidulans R2 (Synechococcus PCC 7942) genome involved in sulfate assimilation. The 8.3-kilobase-pair region encodes at least five transcripts that cannot be detected unless the cells are deprived of sulfur. One of the genes in this region has been sequenced, and the protein that it encodes is homologous to a polypeptide component of other permease systems of Escherichia coli and Salmonella. Insertional inactivation of the putative sulfate permease gene, designated cysA, as well as of other genes within this region, results in cysteine auxotrophy, reduced sulfate uptake, and altered expression of soluble and cytoplasmic-membrane polypeptides associated with sulfur starvation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. R. Cyanobacterial light-harvesting complex subunits encoded in two red light-induced transcripts. Science. 1985 Nov 1;230(4725):550–553. doi: 10.1126/science.3931221. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Johnson M. S., Husain I., Van Houten B., Thomas D. C., Sancar A. Domainal evolution of a prokaryotic DNA repair protein and its relationship to active-transport proteins. Nature. 1986 Oct 2;323(6087):451–453. doi: 10.1038/323451a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Sherman L. A. Optimal conditions for genetic transformation of the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1984 Apr;158(1):36–42. doi: 10.1128/jb.158.1.36-42.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. S., Grossman A. R. Changes in sulfate transport characteristics and protein composition of Anacystis nidulans R2 during sulfur deprivation. J Bacteriol. 1988 Feb;170(2):583–587. doi: 10.1128/jb.170.2.583-587.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Hulanicka M. D., Garrett C., Jagura-Burdzy G., Kredich N. M. Cloning and characterization of the cysAMK region of Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):322–327. doi: 10.1128/jb.168.1.322-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanjean R., Broda E. Dependence of sulphate uptake by Anacystis nidulans on energy, on osmotic shock and on sulphate stravation. Arch Microbiol. 1977 Jul 26;114(1):19–23. doi: 10.1007/BF00429625. [DOI] [PubMed] [Google Scholar]
- Kredich N. M., Hulanicka M. D., Hallquist S. G. Synthesis of L-cysteine in Salmonella typhimurium. Ciba Found Symp. 1979;(72):87–99. doi: 10.1002/9780470720554.ch6. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Szalay A. A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1983 Sep;24(1):37–51. doi: 10.1016/0378-1119(83)90129-4. [DOI] [PubMed] [Google Scholar]