Abstract
Control measures used to limit the spread of infectious disease often generate externalities. Vaccination for transmissible diseases can reduce the incidence of disease even among the unvaccinated, whereas antimicrobial chemotherapy can lead to the evolution of antimicrobial resistance and thereby limit its own effectiveness over time. We integrate the economic theory of public choice with mathematical models of infectious disease to provide a quantitative framework for making allocation decisions in the presence of these externalities. To illustrate, we present a series of examples: vaccination for tetanus, vaccination for measles, antibiotic treatment of otitis media, and antiviral treatment of pandemic influenza.
Keywords: antibiotic resistance, externalities, vaccination, emerging infectious disease, pandemic influenza
Control measures used to limit the spread of infectious disease often generate both direct effects on the targeted individuals and indirect effects on other parties. As we shall see, these side consequences, or externalities, may be either beneficial or detrimental, depending on the biology of the disease and the nature of the interventions.
An effective framework for making health policy decisions must account for both direct effects and externalities. This requirement situates the control of infectious disease as a problem in public choice economics. Several economic studies have considered the role of externalities in public health. Brito et al. (1) first posed the problem, addressing the positive externalities associated with vaccination programs. Subsequent analyses (2, 3) use this approach to illustrate the difficulty of entirely eradicating a disease by vaccination. Gersovitz and Hammer further expand the economic models and extend the analysis to public goods associated with vector control (4–6). Cook et al. (7) propose a graphical approach similar to that presented here and work out a detailed case study for cholera vaccination. However, none of these analyses address the negative externalities associated with the evolutionary process, namely the evolution and spread of antimicrobial resistance. Within the field of infectious disease epidemiology, there is a growing realization that game-theoretic problems arise around vaccine uptake (8–14) and antibiotic resistance evolution (15, 16). However, epidemiologists have yet to adopt a general framework for handling the economic externalities associated with disease control measures.
Here we fuse these two lines of research to sketch a general framework for considering the consequences of control interventions on evolving infectious diseases. To illustrate the fundamental issues that arise, we will step through a sequence of four infectious diseases. We begin by describing tetanus, for which vaccination is a pure private good and no externalities arise. Next we use measles to illustrate a case in which the externalities, namely reduced infectious pressure due to herd immunity (17), are entirely positive. We then consider otitis media, a case in which the externalities, namely selection for antibiotic resistance, are due to evolutionary processes and are purely negative. We conclude with a model of pandemic influenza, in which antiviral treatment simultaneously generates both positive and negative externalities. Each of our examples is intended to be illustrative rather than predictive, and thus we have put a high premium on simplicity; in each case, we use the most basic models possible with simple approximate parameters. Faced with an actual policy decision for a specific disease, one would do well to sacrifice the simplicity here for the realism conferred by more complex disease models. Extension is straightforward to any type of disease model, deterministic or stochastic, analytical or simulation-driven, well-mixed, age-structured, explicitly spatial, or network-based. As far as the economic modeling is concerned, the disease model is simply a “black box” that generates the marginal public benefit curves by specifying the hazard of infection and its derivatives as a function of how broadly and to whom interventions are allocated. Even when these quantities cannot be obtained analytically, numerical methods can be used for all of the calculations described herein.
Economic Method
The economic theory of public choice provides a mathematical framework for making decisions in the presence of externalities (18, 19). In this paper, we are concerned with the allocation of public health interventions: vaccinations, antibiotics, and antivirals. Due to externalities that arise with these goods, the privately obtained levels are not always socially efficient. Some sort of government intervention such as subsidies or taxes may be needed to achieve efficiency.
We begin by describing the basic economic formulation, using vaccination as an example. As we will show, this framework can also be adapted to deal with antimicrobial therapy. Consider a large population N in which a fraction q of the population is vaccinated. The annual hazard of infection is h(1, q) for those who are vaccinated and h(0, q) for those who are not. Let individual i have a utility function Ui(hi, xi), where hi is the hazard rate and xi is the amount of goods that i consumes in time period t. If i has initial wealth wi and pays p to be vaccinated, then xi = wi – p. Individual i’s private benefit from vaccination is a function vi(q), which is defined as the most money that i would be willing to pay to be vaccinated. Thus vi(q) is implicitly defined by the equation
Although the method generalizes easily to heterogeneous populations, as we will see in the otitis media example, for now consider the special case where consumers have identical utility functions of the form
When utility is of the form in Eq. 2, it follows from Eq. 1 that
If individuals must pay a price c for vaccination, they will prefer to be vaccinated if and only if the private benefits v(q) of vaccination exceed the private cost c.
Assume that the total cost of vaccinating a fraction q of the population is C(q N). In this case, the efficient level of q is that which maximizes the sum of individual utilities subject to the constraint that total consumption of goods equals the sum of individual wealths minus total resource costs of vaccinations. This sum is equal to the sum of the utilities of all of the vaccinated individuals plus the sum of the utilities of the unvaccinated, which is
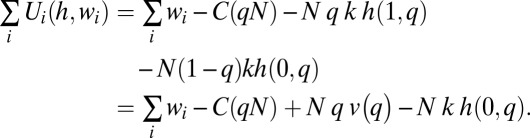 |
It follows that the efficient level of vaccination qe is that which maximizes
If we differentiate expression 4 with respect to q and divide by N, we have the following first-order necessary condition for efficiency:
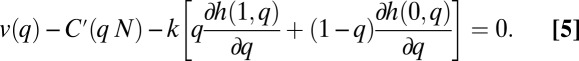 |
Let us define
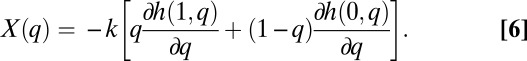 |
Thus, X(q) measures the marginal value to the population of the indirect effects of vaccination. This is the externality generated by vaccinating a single individual. Note that it is composed of two terms: the ∂h(1, q)/∂q term reflects the effect of treating one additional individual on the other treated individuals, and the ∂h(0, q)/∂q term reflects the effect of treating one additional individual on the untreated individuals. In the remainder of this paper, we look at how these terms operate for four illustrative diseases. For tetanus, both terms are zero and no externalities are generated. For measles vaccination, which generates a positive externality for the unvaccinated (but not the vaccinated), the ∂h(1, q)/∂q is zero and the ∂h(0, q)/∂q is negative. For antibiotic treatment of otitis media, which generates a negative externality for the other treated individuals (but not the untreated), the ∂h(1, q)/∂q is positive and the ∂h(0, q)/∂q is zero. For antiviral treatment of pandemic flu, which generates both positive and negative externalities, both terms are nonzero.
Suppose that individuals are required to pay the marginal cost of their vaccination. Because the private benefit of vaccination is v(q), the equilibrium level of vaccination will be qp such that v(qp) = C′(N qp). To induce an efficient outcome, the government can provide a so-called Pigouvian subsidy, subsidizing each vaccination by an amount X(qe) equal to the marginal value of the indirect effects at the efficient point.
Tetanus
With our economic framework in place, we turn to a series of examples. We begin with one of the few cases in which disease prevention is a purely private good. Tetanus is the severe prolonged contraction of skeletal muscle fibers caused by the neurotoxin tetanospasmin produced by the bacterium Clostridium tetani. Whereas tetanus kills hundreds of thousands of individuals worldwide every year, it is virtually nonexistent in industrialized nations, with a reported incidence of 50–100 cases per year in the United States over the past 30 years. This low incidence is due directly to vaccination use (20, 21).
Tetanus is not contagious, making it the only vaccine-preventable disease that is infectious but not transmissible human-to-human (20). As a result, there is no herd immunity from vaccination. In terms of our model, the hazard rate for an unvaccinated individual to become infected is simply a constant, independent of q, and thus ∂h(0, q)/∂q = 0. Furthermore, the vaccine targets the tetanospasmin toxin rather than the bacterium that produces it, thereby minimizing the selective consequences of vaccination on the bacterium itself and minimizing the risk of resistance evolution, even relative to other vaccines (22). Hence, the hazard rate for a vaccinated individual to become infected is also independent of q and so ∂h(1, q)/∂q = 0 as well. Thus, for tetanus, X(q) = 0, and there are no externalities associated with vaccination. Tetanus toxoid vaccine is a purely private good; an individual will choose to vaccinate if his or her benefit is larger than the cost, irrespective of what others are doing—and this decision has no side effects on other individuals’ risks of disease.
Measles
Next we consider a disease for which control generates positive externalities from reduced transmission. Measles is caused by a paramyxovirus and kills an estimated 242,000 people globally per year, despite an effective and readily available vaccine (23). Vaccination is the primary form of control, and generates a positive externality because high vaccination levels induce “herd immunity” (17) that reduces the risk of infection to nonvaccinated individuals.
To investigate the dependence of hazard rates on vaccination, we consider the stationary equilibrium of a basic susceptible-infectious-recovered (SIR) model of vaccination (see ref. 24). Expected lifespan is T years and population size is constant, with birth rate μ = 1/T equal to the death rate. Individuals who are vaccinated will be vaccinated at birth. We assume that the vaccine is perfectly protective, so that h(1, q) = 0 for all q. At equilibrium, those who are not vaccinated will face a constant hazard rate of contracting measles, but after recovering will not be susceptible. Let γ be the recovery rate and β the infection transmission rate. Let S(t) be the fraction of the population that is susceptible to infection, I(t) be the fraction of the population that is infectious, and R(t) be the fraction that is recovered from the disease and no longer infectious. Where 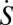 ,
, 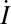 , and
, and 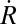 indicate derivatives with respect to time, the governing differential equations are
indicate derivatives with respect to time, the governing differential equations are
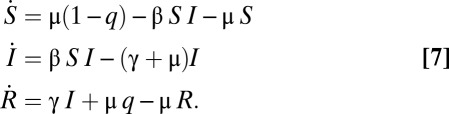 |
We define basic reproductive number R0 = β/(γ + μ). To find the equilibrium level of infection I*(q) when a fraction q is vaccinated, we take 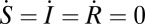 . We find that
. We find that
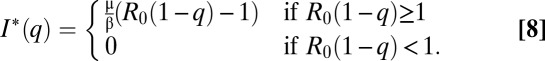 |
We see that the equilibrium fraction of infected individuals decreases linearly as the vaccinated proportion of the population increases, so long as R0(1 – q) > 1. If R0(1 – q) ≤ 1, the disease will be eradicated. The annual hazard rate for an unvaccinated, susceptible individual is given by
We assume that vaccination at the beginning of life results in lifetime immunity. Suppose that individuals who are vaccinated are asked to pay the marginal cost of their own vaccination in equal installments over their lifetimes. Let c be the marginal cost of vaccination; then the annual payment would be c/T = μ c. The annual utility gain from vaccination is v(q) = k h(0, q). At an interior equilibrium, individuals would be indifferent about being vaccinated or not. Thus the privately supported equilibrium proportion of vaccinated individuals would be qp, such that
This implies that at equilibrium the fraction of the population that is unvaccinated is
 |
The efficient level of vaccination qe is that which maximizes the sum of individual utilities subject to the constraint that total consumption of goods equals the sum of total wealths minus total resource costs of vaccination. The total annual cost of vaccinations is C(q μ N) and the total annual cost of infection is
An efficient outcome, therefore, is one that minimizes
If the marginal cost of an additional vaccination is c, the first-order condition for an efficient rate of vaccination qe is
This implies that
 |
We see from Eqs. 11 and 15 that in an efficient outcome, the fraction of the population that is left unvaccinated is just half of the equilibrium unvaccinated fraction if individuals pay the marginal cost of their vaccinations.
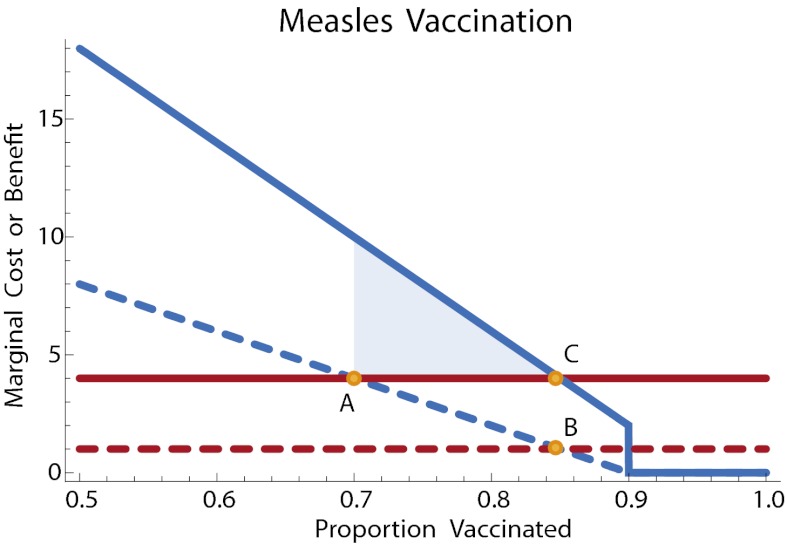
In this model, the annual externality from a vaccination is X(q) = k μ R0(1 – q). Thus, an annual subsidy of k μ R0(1 – qe) = μ(c + k)/2 for those who choose vaccination would be sufficient to induce an efficient outcome. Fig. 1 illustrates.
Fig. 1.
Measles vaccination with a cost subsidy. The annual marginal cost or benefit is plotted. Solid lines: marginal public cost (red) and marginal public benefit (blue) of vaccination. Once 90% of the population is vaccinated, the disease is eradicated and no further benefit accrues to vaccination. Dashed lines: net private cost (red) is the cost minus the subsidy; net private benefit (blue) of vaccination. Point A: private optimum without subsidy. Point B: private level of vaccination with subsidy. Point C: efficient level of vaccination. An annual subsidy brings the level of vaccination at the private optimum into line with the efficient level. The shaded region illustrates the net welfare gain due to the subsidy. The parameters in this example, with time measured in years and costs in dollars, are as follows. The recovery rate is γ = 100. So that R0 ≈ 10, the transmission parameter is γ = 1000. The annual valuation of reduced risk is k = 100; the total cost of vaccination (financial cost plus perceived risk of being vaccinated) is c = 200. Given a death rate of μ = 0.02, the annual cost is c μ = 4 and the annual subsidy is μ(c + k)/2 = 3.
As seen in previous analyses, complete eradication can be difficult even with government subsidy, due to the so-called prevalence elasticity in private demand of vaccine: Prevalence of a disease declines through increased vaccination use; the willingness to pay for vaccination decreases as well (2, 25). However, in the framework defined above, increasing vaccine prevalence through government intervention still provides a positive externality in reducing the prevalence of measles (4).
Otitis Media
Otitis media is an inflammation of the duct known as the eustachian tube, which connects the middle ear to the nasopharynx. Typically caused by ineffective clearing of Streptococcus pneumoniae or Haemophilus influenzae bacteria from the duct, otitis media is the leading cause of antibiotic prescription in children and adolescents in the United States (26). Due to the position of infection, human-to-human transmission of the infection-causing bacteria is unlikely. With asymptomatic carriage in the nasopharynx extremely common and transmission much more likely from this site than from the eustachian tube, treatment of clinical infection has little or no effect in reducing transmission.
The economics of otitis media treatment differ from measles vaccination, as there exists a negative externality of antibiotic resistance caused by overprescription of antibiotics (27–29). Although most cases of otitis media are caused by bacterial infection, some are viral in etiology and often the two are difficult to distinguish. This commonly leads to unnecessary antibiotic treatment. Moreover, most cases resolve spontaneously without treatment. The American Academy of Pediatrics now recommends that no antibiotic treatment be given for most cases in children over the age of 2 in the absence of severe illness (30).
We adjust our interpretation of the model parameters to fit the specifics of otitis media. We now interpret N as the total number of clinical cases and q as the fraction of cases that receive antibiotic therapy, and we replace the hazard rate h with a function π that gives the chance of developing complications that require further intervention beyond any initial antibiotic therapy.
There is considerable variation across individuals in their need for antibiotic treatment. To account for the individual variation in need for antibiotic treatment, we extend our economic model to consider the case in which individual utility functions differ. Suppose that there is a continuum of consumers such that the utility function of consumer t is
where t ∈ [0, 1] and k is a nondecreasing function of t.
In an efficient allocation, all of those who are treated must have at least as high a value of k as all of those who are not. If a fraction q of the cases are treated and the persons treated are chosen efficiently, those who are treated must have k(t) ≥ k(1 – q) and those who are not treated must have k(t) ≤ k(1 – q). The total cost of treatment is C(N q), and the integral of consumers’ utilities is
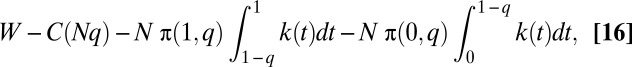 |
with  . Let us define
. Let us define 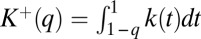 and
and 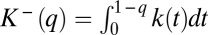 . Then expression 16 can be written as
. Then expression 16 can be written as
Taking the derivative of expression 17 with respect to q, and dividing by N, the first-order condition for efficiency is
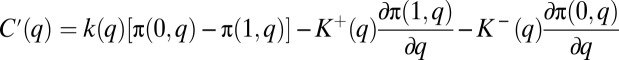 |
If the fraction q of cases are treated, the private benefits to an individual to whom the cost of infection is k(t) will be
If marginal cost is a constant c, and individuals purchase their own treatment at a price equal to the marginal cost, an equilibrium will be an outcome in which 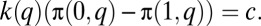 Thus, the marginal effect of a change in q on the population, X(q), is much as before but with K+(q) and K–(q) replacing the k q and k(1 – q) terms:
Thus, the marginal effect of a change in q on the population, X(q), is much as before but with K+(q) and K–(q) replacing the k q and k(1 – q) terms:
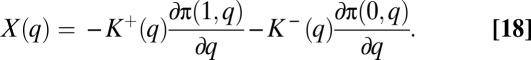 |
Antibiotic use typically selects for resistance; as usage increases, we expect the prevalence of antibiotic-resistant strains to increase and, with it, the probability of treatment failure (31). In our model, this means that the probability of complications despite treatment π(1, q) will be an increasing function of q. To determine the form of this function, we again can turn to mathematical models in disease epidemiology.
Here we use a simple susceptible-infected-susceptible (SIS) model of antibiotic use in the community to treat a human-commensal bacterium, following ref. 32. Only a small fraction α of individuals carrying the bacterium experience symptoms and are candidates for treatment; the treatment fraction q now refers to the fraction of these symptomatic cases treated. For a population of size N, we let S be the proportion of individuals not carrying the bacterial species of interest, and let Iw and Ir be the proportion of people carrying wild-type bacteria or resistant bacteria, respectively. Let γw and γr be the rates of spontaneous clearance for wild-type and resistant bacterial carriage, and let γt be the clearance rate due to treatment for wild-type carriage. Let β be the force of infection and let σ be the rate at which treated wild-type individuals develop de novo resistance. The governing differential equations are
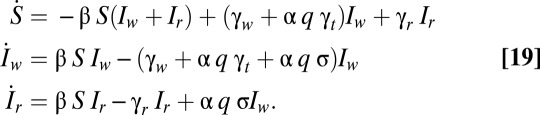 |
At steady state, sensitive and resistant strains will coexist provided that β > γw + α q γt + α q σ and κ > α q(γt + σ), where κ = γr – γw is the differential clearance rate. In this case, the equilibrium frequency of resistant bacteria [i.e., Ir/(Iw + Ir) at equilibrium] is p(q) = α q σ/(κ – α q γt) and the derivative with respect to q is p′(q) = α κ σ/(κ – α q γt)2.
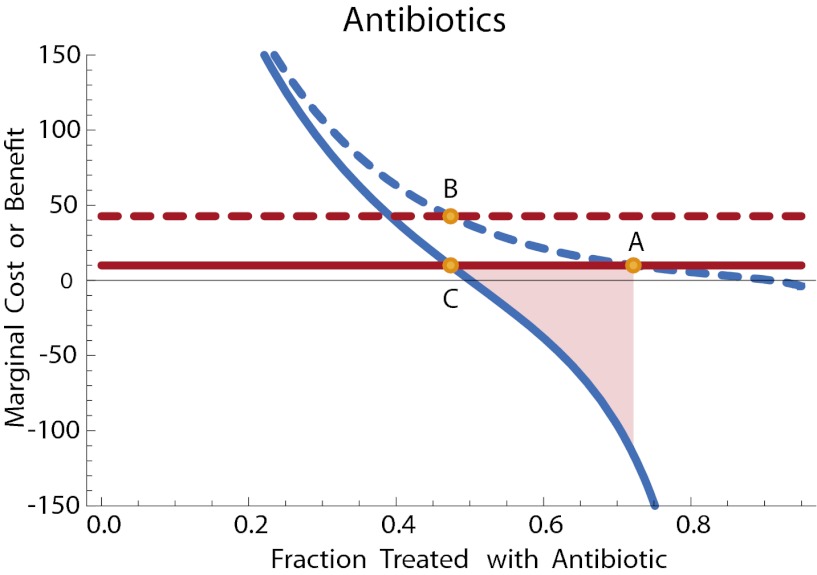
We can now compute ∂π(1, q)/∂q and ∂π(0, q)/∂q. The latter is straightforward: Assuming that there is no difference in the virulence of resistant and sensitive strains, the frequency of resistance does not directly impact untreated individuals and thus ∂π(0, q)/∂q = 0. The former term depends on the frequency of treatment failure, which in turn is proportional to the frequency of resistant bacteria. Let ρ be the chance that treatment failure leads to complications. Then ∂π(1, q)/∂q = p′(q)ρ and X(q) is negative. The efficient level of treatment qe lies below the private equilibrium, and some government intervention is required to discourage usage if we are to reach the efficient outcome. In this case, a tax of X(qe) will shift the private equilibrium to the efficient point. This optimal taxation level is illustrated in Fig. 2.
Fig. 2.
Antibiotic treatment for otitis media. Solid lines: marginal public cost (red) and marginal public benefit (blue) of antibiotic therapy. Dashed lines: net private cost with tax (red) and net private benefit (blue) of antibiotic therapy. Point A: private optimum without tax. Point B: private level of antibiotic therapy with tax. Point C: efficient level of antibiotic use. The tax brings the level of treatment at the private optimum into line with the efficient level. The shaded region illustrates the net welfare gain due to the tax. Parameters with time in days and costs in dollars are as follows: γw = 0.05 and fitness cost of resistance is a 10% increase in clearance rate so that γr = 0.055. We have γt = 0.5, σ = 0.05, α = 0.01, β = 1, ρ = 0.1. The cost of antibiotics is c = 10. The function k(t) specifying the consumer values of treatment is an exponential curve: k(t) = 5000 e–5(1–t).
Pandemic Influenza
Novel influenza A (H1N1) was first reported in Mexico City in late April 2009, and within 6 weeks (June 11) was declared a full pandemic by the World Health Organization. The most widely used antiviral agents, neuraminidase inhibitors oseltamivir and zanamivir, have demonstrated beneficial effects on H1N1 infection and are the WHO-recommended first-line and chemoprophylactic treatment for these viruses (33, 34). However, resistance to antiviral agents is a concern for public health planners (35). Resistance can evolve readily with minimal fitness costs (36), and the use of neuraminidase inhibitor antivirals has the potential to decrease the long-term effectiveness of these drugs.
In this section, we develop a simple illustrative model of pandemic influenza. Our analysis is based on ref. 37, with the simplification that we model only antiviral treatment and not antiviral prophylaxis. Our state variables are as follows: X is the fraction of susceptible individuals in the population; Ysu is the fraction of individuals infected with sensitive virus but untreated with the antiviral; Yst is the fraction of individuals infected with sensitive virus and treated with the antiviral; Yr is the fraction of individuals infected with resistant virus; and Z is the fraction of removed (recovered or dead) individuals in the population. Let γ be the recovery rate and let σ be the rate at which sensitive infections evolve de novo resistance. Let βsu, βst, and βr be the transmission parameters for untreated sensitive, treated sensitive, and resistant virus, respectively. As in the model of ref. 37, treatment reduces the infectiousness but not the duration of infection. Let q be the fraction of drug-sensitive infections that receive treatment. The governing differential equations are then
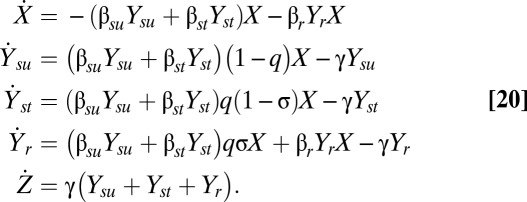 |
In the previous examples, we looked at how the treatment fraction influenced the steady-state prevalence of the disease. Such an approach is not appropriate for pandemic influenza, which sweeps through a population and then is eradicated, rather than reaching a steady endemic level. Thus, we will focus in this example on the time course of infection, tracking the number of individuals infected over time.
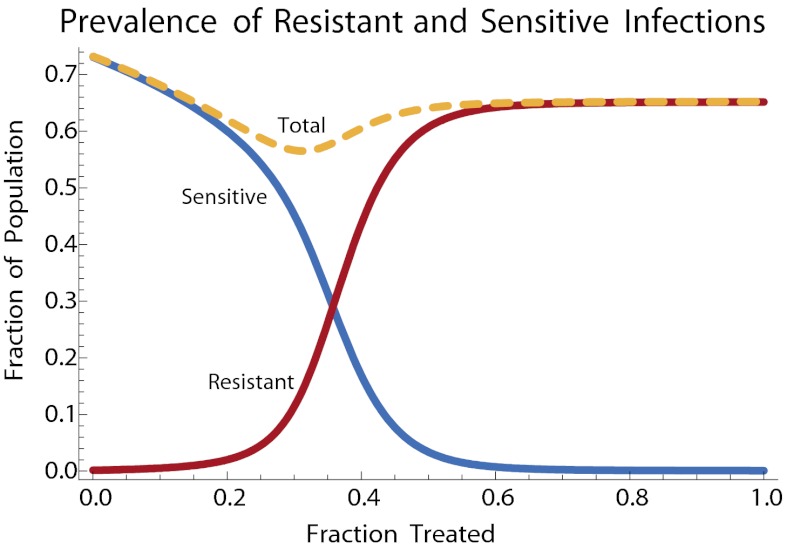
Fig. 3 shows how the fractions of individuals infected with resistant and sensitive strains, over the course of an epidemic, depend on the fraction of cases treated with antivirals. As seen in previous analyses, intermediate levels of antiviral therapy minimize the total number of cases (37) by reducing the degree to which the epidemic overshoots its eradication threshold (38).
Fig. 3.
Fraction of resistant (red) and sensitive (blue) infections for pandemic flu, as a function of the fraction of infections treated. Total fraction infected is indicated by the dashed yellow curve. Parameters follow Lipsitch et al. (37) as follows: the basic reproductive ratio R0 is 1.8 for sensitive virus. Resistant virus suffers a 10% fitness cost in the form of reduced transmission. Treatment reduces transmission of sensitive virus by 67%. The infectious period is 3.3 days, and each treated case has a 1/500 chance of developing de novo resistance. These values correspond to γ = 1/3.3, σ = 0.002, βsu = 1/1.8, βr = 0.9βsu, βst = 0.33βsu.
In our economic analysis, individuals have incentive to purchase antivirals at cost c because treatment ameliorates symptoms of influenza, such that kt and ku represent the dollar costs of a case of treated and untreated influenza, respectively. Under these assumptions, utility functions are of the form U(πt, πu, x) = x – ktπt – ku πu, where x is the amount spent on other goods, πt is the probability that the individual contracts a sensitive case and treats it, and πr is the probability that an individual contracts a case and does not treat it, either because he or she had chosen not to purchase the antiviral or because the case is resistant.
We consider two different ways in which antivirals might be dispensed. In the flu kits scenario (39), the consumer has an advance option to purchase one course of antiviral therapy to be used in the event that he or she is infected in a pandemic. For simplicity, in this scenario, we assume that no further courses of antiviral therapy will be available during the epidemic to those who did not choose to purchase them initially. In the pharmacy distribution scenario, no advance purchase is made. Instead, when an individual is infected, he or she has the option to purchase a course of antivirals for immediate use from the pharmacy, but this decision is made without knowing whether the infection is sensitive or resistant.
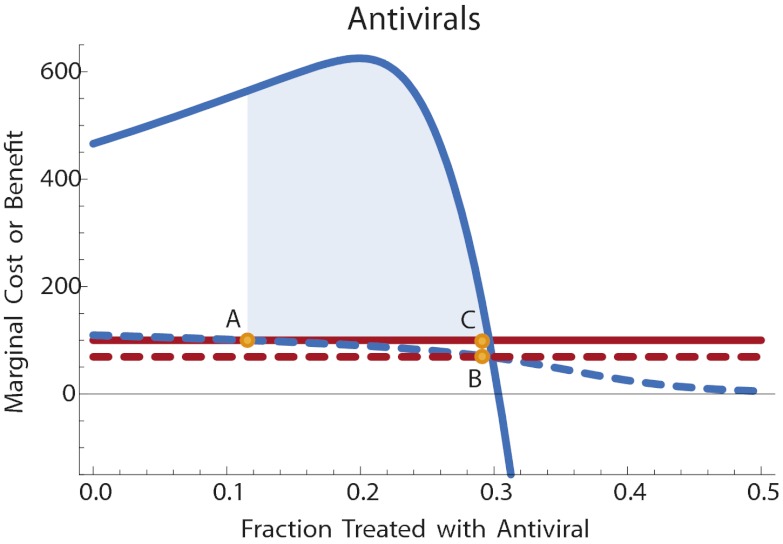
The πu and πt values can be computed directly from the fraction of the population that has been infected by each strain at the end of the epidemic, conditioned on the treatment choices of the individual. Let πr(q) be the fraction of the population that has been infected by resistant strains at the end of the epidemic and let πs(q) be the fraction of the population infected by sensitive strains. In the flu kit scenario, the purchase is made in advance of infection. Ignoring resale and exchange of antivirals, the fraction of sensitive infections treated, q, will be equal to the fraction of the population who chose to purchase the flu kit. In this case, the private benefit is v(q) = (ku – kt)πs(q). In the pharmacy distribution scenario, the purchase is deferred until the individual is known to be infected. The fraction of sensitive infections treated will be equal to the fraction of individuals who choose to purchase treatment once infected. In this case, the private benefit is conditioned on infection by some strain of flu: v(q) = (ku – kt)πs(q)/[πs(q) + πr(q)]. In each case, we can solve numerically for the private equilibrium fraction q of individuals who choose to purchase antiviral therapy. As in our previous examples, the social optimum is the point that maximizes the sum of the utilities. This can also be computed numerically. Fig. 4 and Fig. 5 illustrate.
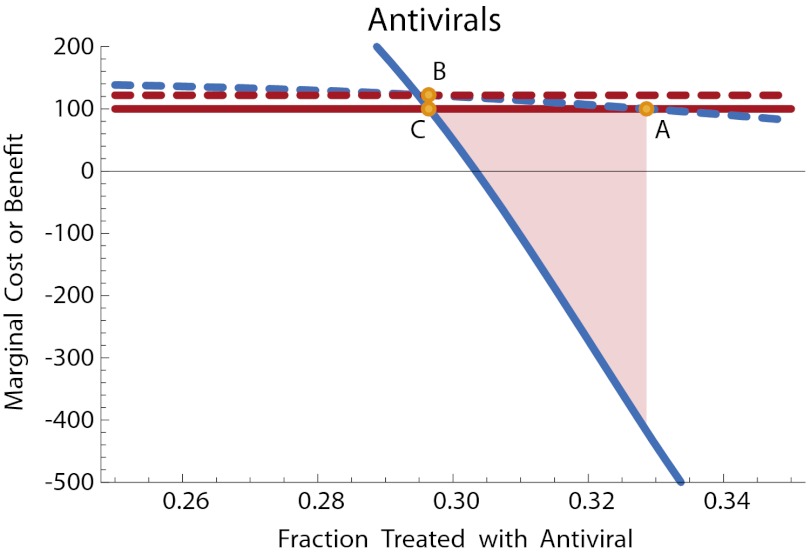
Fig. 4.
Antiviral treatment for pandemic flu, flu kit scenario. Solid lines: marginal public cost (red) and marginal public benefit (blue) of antiviral therapy. Dashed lines: net private cost with subsidy (red) and net private benefit (blue) of antiviral therapy. Point A: private optimum without subsidy. Point B: private level of antiviral therapy with subsidy. Point C: efficient level of antiviral use. The subsidy brings the level of treatment at the private optimum into line with the efficient level. The shaded region illustrates the net welfare gain due to the subsidy. Disease parameters are as in Fig. 3, with time in days and costs in dollars. Cost of antivirals is c = 100, and disease valuation parameters are kt = 850, ku = 1000.
Fig. 5.
Antiviral treatment for pandemic flu, pharmacy distribution scenario. Parameters are as in Fig. 4. Here the private market overuses antivirals and a tax is required to bring the level of treatment at the private optimum into line with the efficient level. The shaded region illustrates the net welfare gain due to the tax.
This model illustrates an interesting tradeoff. When antiviral usage is low, increasing use generates a positive externality in the form of reduced total cases. When antiviral use gets higher, this positive externality is reduced and, moreover, a negative externality arises in the form of reduced antiviral effectiveness. Depending on the distribution technology, the private market may over- or underuse antivirals relative to the efficient level. For the particular parameter values here, too few people stockpile antivirals if required to purchase them in advance of the pandemic, whereas too many use antivirals if allowed to purchase them at the time of infection. Corresponding subsidies or taxes can bring the private equilibrium into line with the efficient point, as illustrated in Fig. 4 and Fig. 5.
Summary
When coupled with mathematical models of disease, the economic theory of public choice provides a robust framework for assessing the economic impact of public health interventions. As we have illustrated, this framework allows decision makers to account for the positive and negative externalities associated with control measures such as vaccination or antimicrobial chemotherapy. Depending on the biology of the disease and the nature of the intervention, the private market may under- or overallocate. Taxes or subsidies can guide the private market to a more efficient level of intervention. Although the models presented here have been deliberately simple, the framework illustrated can be fruitfully applied to more sophisticated quantitative models of infectious disease. Doing so should help public health planners more efficiently control evolving transmissible diseases.
Acknowledgments
The authors thank Marc Lipsitch for helpful discussions. This work was supported by the National Institute of General Medical Sciences Models of Infectious Disease Agent Study Program cooperative agreement 5U01GM07649 and the MIDAS Center for Communicable Disease Dynamics 1U54GM088588 at Harvard University.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Evolution in Health and Medicine” held April 2–3, 2009, at the National Academy of Sciences in Washington, DC. The complete program and audio files of most presentations are available on the NAS web site at www.nasonline.org/Sackler_Evolution_Health_Medicine.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Brito DL, Sheshinski E, Intriligator MD. Externalities and compulsory vaccinations. J Public Econ. 1991;45:69–90. [Google Scholar]
- 2.Geoffard P-Y, Philipson T. Disease eradication: Private versus public vaccination. Am Econ Rev. 1997;87:222–230. [Google Scholar]
- 3.Francis PJ. Dynamic epidemiology and the market for vaccinations. J Public Econ. 1997;63:383–406. [Google Scholar]
- 4.Gersovitz M, Hammer JS. Infectious diseases, public policy, and the marriage of economics and epidemiology. World Bank Res Obs. 2003;18:129–157. [Google Scholar]
- 5.Gersovitz M, Hammer JS. The economic control of infectious diseases. Econ J. 2004;114:1–27. [Google Scholar]
- 6.Gersovitz M, Hammer JS. Tax/subsidy policies toward vector-borne infectious diseases. J Public Econ. 2004;89:647–674. [Google Scholar]
- 7.Cook J, et al. Using private demand studies to calculate socially optimal vaccine subsidies in developing countries. J Policy Anal Manage. 2009;28:6–28. doi: 10.1002/pam.20401. [DOI] [PubMed] [Google Scholar]
- 8.Bauch CT, Galvani AP, Earn DJD. Group interest versus self-interest in smallpox vaccination policy. Proc Natl Acad Sci USA. 2003;100:10564–10567. doi: 10.1073/pnas.1731324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauch CT, Earn DJD. Vaccination and the theory of games. Proc Natl Acad Sci USA. 2004;101:13391–13394. doi: 10.1073/pnas.0403823101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reluga TC, Bauch CT, Galvani AP. Evolving public perceptions and stability in vaccine uptake. Math Biosci. 2006;204:185–198. doi: 10.1016/j.mbs.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Galvani AP, Reluga TC, Chapman GB. Long-standing influenza vaccination policy is in accord with individual self-interest but not with the utilitarian optimum. Proc Natl Acad Sci USA. 2007;104:5692–5697. doi: 10.1073/pnas.0606774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vardavas R, Breban R, Blower S. Can influenza epidemics be prevented by voluntary vaccination? PLoS Comput Biol. 2007;3:e85. doi: 10.1371/journal.pcbi.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Boven M, Klinkenberg D, Pen I, Weissing FJ, Heesterbeek H. Self-interest versus group-interest in antiviral control. PLoS One. 2008;3:e1558. doi: 10.1371/journal.pone.0001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medlock J, Galvani AP. Optimizing influenza vaccine distribution. Science. 2009;325:1705–1708. doi: 10.1126/science.1175570. [DOI] [PubMed] [Google Scholar]
- 15.Smith DL, Levin SA, Laxminarayan R. Strategic interactions in multi-institutional epidemics of antibiotic resistance. Proc Natl Acad Sci USA. 2005;102:3153–3158. doi: 10.1073/pnas.0409523102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster KR, Grundmann H. Do we need to put society first? The potential for tragedy in antimicrobial resistance. PLoS Med. 2006;3:e29. doi: 10.1371/journal.pmed.0030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine PEM, Clarkson JA. Individual versus public priorities in the determination of optimal vaccination policies. Am J Epidemiol. 1986;124:1012–1020. doi: 10.1093/oxfordjournals.aje.a114471. [DOI] [PubMed] [Google Scholar]
- 18.Samuelson PA. The pure theory of public expenditure. Rev Econ Stat. 1954;36:387–389. [Google Scholar]
- 19.Samuelson PA. Diagrammatic exposition of a theory of public expenditure. Rev Econ Stat. 1955;37:350–356. [Google Scholar]
- 20.Centers for Disease Control. Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book) 10th Ed. Washington, DC: Public Health Foundation; 2008. [Google Scholar]
- 21.Centers for Disease Control. Diphtheria, tetanus, and pertussis: Recommendations for vaccine use and other preventive measures: Recommendations of the Immunization Practices Advisory Committee (ACIP) MMWR Morb Mortal Wkly Rep. 1991;40(RR-10):1–28. [PubMed] [Google Scholar]
- 22.Read AF, Mackinnon MJ. Pathogen evolution in a vaccinated world. In: Stearns SC, Koella JC, editors. Evolution in Health and Disease. 2nd Ed. Oxford: Oxford University Press; 2008. [Google Scholar]
- 23.World Health Organization. Measles Fact Sheet. Geneva: World Health Organization; 2007. [Google Scholar]
- 24.Keeling MJ, Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton, NJ: Princeton University Press; 2008. [Google Scholar]
- 25.Philipson T. Private vaccination and public health: An empirical examination for U.S. measles. J Hum Resour. 1996;31:611–630. [Google Scholar]
- 26.McCaig LF, Besser RE, Hughes JM. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287:3096–3102. doi: 10.1001/jama.287.23.3096. [DOI] [PubMed] [Google Scholar]
- 27.Goossens H, Ferech M, Vander Stichele R, Elseviers M ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 28.Lipsitch M. Measuring and interpreting associations between antibiotic use and penicillin resistance in Streptococcus pneumoniae. Clin Infect Dis. 2001;32:1044–1054. doi: 10.1086/319604. [DOI] [PubMed] [Google Scholar]
- 29.Lipsitch M, Samore MH. Antimicrobial use and antimicrobial resistance: A population perspective. Emerg Infect Dis. 2002;8:347–354. doi: 10.3201/eid0804.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451–1465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 31.Bergstrom CT, Feldgarden M. The ecology and evolution of antibiotic-resistant bacteria. In: Stearns SC, Koella JC, editors. Evolution in Health and Disease. 2nd Ed. Oxford: Oxford University Press; 2008. [Google Scholar]
- 32.Bonhoeffer S, Lipsitch M, Levin BR. Evaluating treatment protocols to prevent antibiotic resistance. Proc Natl Acad Sci USA. 1997;94:12106–12111. doi: 10.1073/pnas.94.22.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control. Interim Guidance on Antiviral Recommendations for Patients with Novel Influenza A (H1N1) Virus Infection and Their Close Contacts. Atlanta: Centers Dis Control; 2009. [Google Scholar]
- 34.World Health Organization. WHO Rapid Advice Guidelines on Pharmacological Management of Humans Infected with Avian Influenza A (H5N1) Virus. Geneva: World Health Organization; 2006. [Google Scholar]
- 35.Centers for Disease Control. Interim Recommendations for the Use of Influenza Antiviral Medications in the Setting of Oseltamivir Resistance Among Circulating Influenza A (H1N1) Viruses, 2008–09 Influenza Season. Atlanta: Centers Dis Control; 2009. [Google Scholar]
- 36.Weinstock DM, Zuccotti G. The evolution of influenza resistance and treatment. JAMA. 2009;301:1066–1069. doi: 10.1001/jama.2009.324. [DOI] [PubMed] [Google Scholar]
- 37.Lipsitch M, Cohen T, Murray M, Levin BR. Antiviral resistance and the control of pandemic influenza. PLoS Med. 2007;4:e15. doi: 10.1371/journal.pmed.0040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Handel A, Longini IM. Antia R., Jr. What is the best control strategy for multiple infectious disease outbreaks? Proc Biol Sci. 2007;274:833–837. doi: 10.1098/rspb.2006.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traynor K. Antiinfluenza medication kits need work, FDA advisers conclude. Am J Health Syst Pharm. 2008;65:2314–2316. doi: 10.2146/news080098. [DOI] [PubMed] [Google Scholar]