Abstract
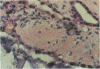
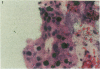
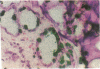
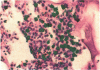
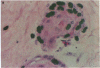
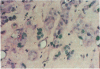
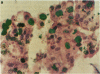
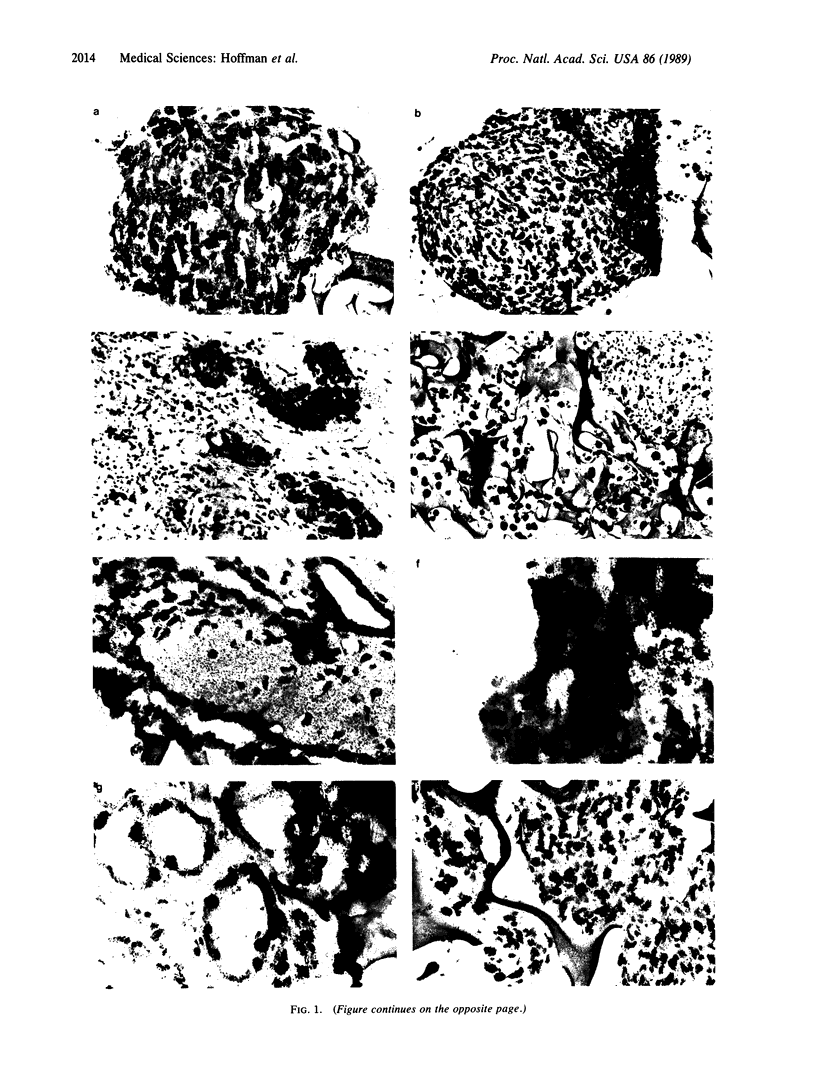
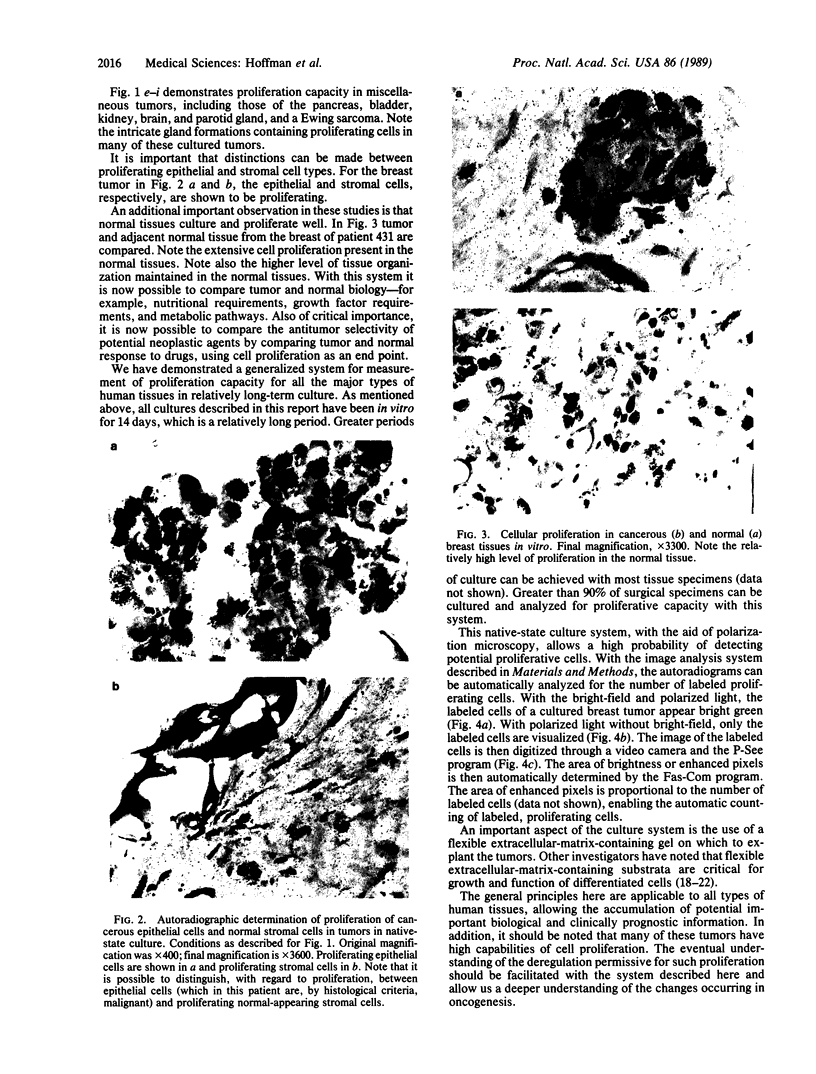
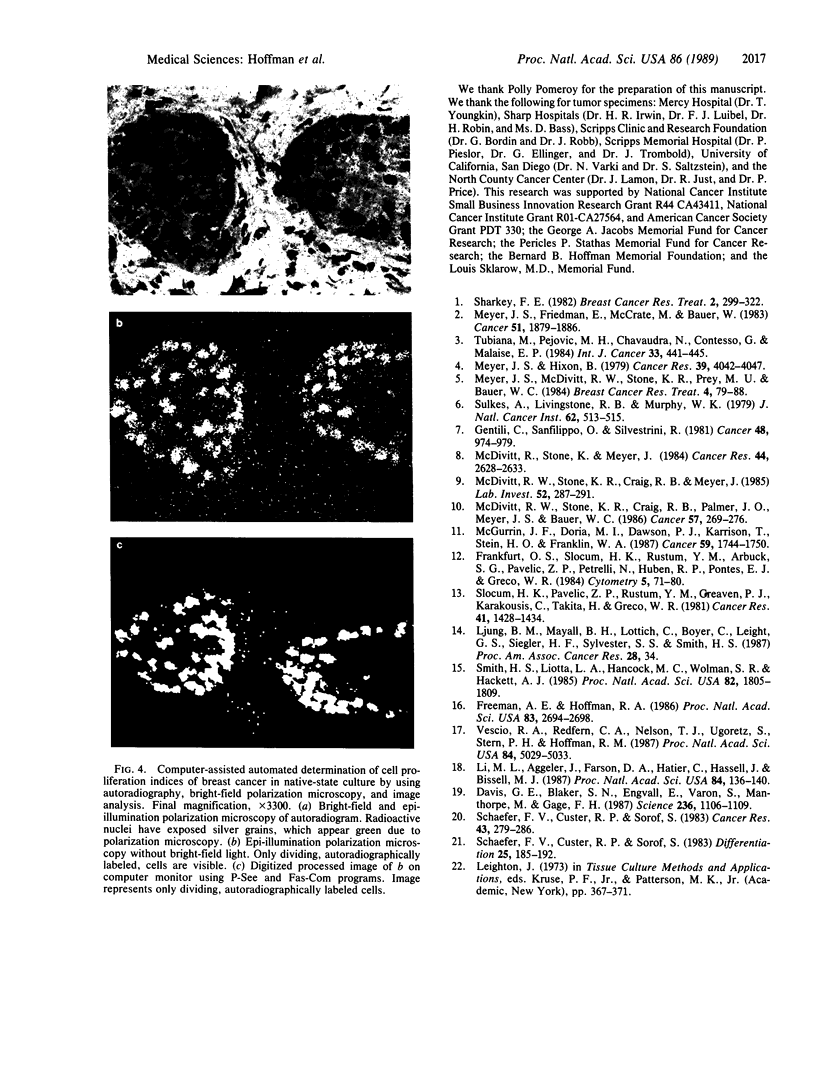
An important need in cancer research and treatment is a physiological means in vitro by which to assess the proliferation capacity of human tumors and corresponding normal tissue for comparison. We have recently developed a native-state, three-dimensional, gel-supported primary culture system that allows every type of human cancer to grow in vitro at more than 90% frequency, with maintenance of tissue architecture, tumor-stromal interaction, and differentiated functions. Here we demonstrate that the native-state culture system allows proliferation indices to be determined for all solid cancer types explanted directly from surgery into long-term culture. Normal tissues also proliferate readily in this system. The degree of resolution of measurement of cell proliferation by histological autoradiography within the cultured tissues is greatly enhanced with the use of epi-illumination polarization microscopy. The histological status of the cultured tissues can be assessed simultaneously with the proliferation status. Carcinomas generally have areas of high epithelial proliferation with quiescent stromal cells. Sarcomas have high proliferation of cells of mesenchymal organ. Normal tissues can also proliferate at high rates. An image analysis system has been developed to automate proliferation determination. The high-resolution physiological means described here to measure the proliferation capacity of tissues will be important in further understanding of the deregulation of cell proliferation in cancer as well as in cancer prognosis and treatment.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis G. E., Blaker S. N., Engvall E., Varon S., Manthorpe M., Gage F. H. Human amnion membrane serves as a substratum for growing axons in vitro and in vivo. Science. 1987 May 29;236(4805):1106–1109. doi: 10.1126/science.3576223. [DOI] [PubMed] [Google Scholar]
- Frankfurt O. S., Slocum H. K., Rustum Y. M., Arbuck S. G., Pavelic Z. P., Petrelli N., Huben R. P., Pontes E. J., Greco W. R. Flow cytometric analysis of DNA aneuploidy in primary and metastatic human solid tumors. Cytometry. 1984 Jan;5(1):71–80. doi: 10.1002/cyto.990050111. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Hoffman R. M. In vivo-like growth of human tumors in vitro. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2694–2698. doi: 10.1073/pnas.83.8.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili C., Sanfilippo O., Silvestrini R. Cell proliferation and its relationship to clinical features and relapse in breast cancers. Cancer. 1981 Aug 15;48(4):974–979. doi: 10.1002/1097-0142(19810815)48:4<974::aid-cncr2820480420>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Li M. L., Aggeler J., Farson D. A., Hatier C., Hassell J., Bissell M. J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDivitt R. W., Stone K. R., Craig R. B., Meyer J. S. A comparison of human breast cancer cell kinetics measured by flow cytometry and thymidine labeling. Lab Invest. 1985 Mar;52(3):287–291. [PubMed] [Google Scholar]
- McDivitt R. W., Stone K. R., Craig R. B., Palmer J. O., Meyer J. S., Bauer W. C. A proposed classification of breast cancer based on kinetic information: derived from a comparison of risk factors in 168 primary operable breast cancers. Cancer. 1986 Jan 15;57(2):269–276. doi: 10.1002/1097-0142(19860115)57:2<269::aid-cncr2820570214>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- McDivitt R. W., Stone K. R., Meyer J. S. A method for dissociation of viable human breast cancer cells that produces flow cytometric kinetic information similar to that obtained by thymidine labeling. Cancer Res. 1984 Jun;44(6):2628–2633. [PubMed] [Google Scholar]
- McGurrin J. F., Doria M. I., Jr, Dawson P. J., Karrison T., Stein H. O., Franklin W. A. Assessment of tumor cell kinetics by immunohistochemistry in carcinoma of breast. Cancer. 1987 May 15;59(10):1744–1750. doi: 10.1002/1097-0142(19870515)59:10<1744::aid-cncr2820591012>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Friedman E., McCrate M. M., Bauer W. C. Prediction of early course of breast carcinoma by thymidine labeling. Cancer. 1983 May 15;51(10):1879–1886. doi: 10.1002/1097-0142(19830515)51:10<1879::aid-cncr2820511021>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Hixon B. Advanced stage and early relapse of breast carcinomas associated with high thymidine labeling indices. Cancer Res. 1979 Oct;39(10):4042–4047. [PubMed] [Google Scholar]
- Meyer J. S., McDivitt R. W., Stone K. R., Prey M. U., Bauer W. C. Practical breast carcinoma cell kinetics: review and update. Breast Cancer Res Treat. 1984;4(2):79–88. doi: 10.1007/BF01806389. [DOI] [PubMed] [Google Scholar]
- Schaefer F. V., Custer R. P., Sorof S. Induction of squamous metaplasia: requirement for cell multiplication, and competition with lobuloalveolar development in cultured mammary glands. Differentiation. 1983;25(2):185–192. doi: 10.1111/j.1432-0436.1984.tb01354.x. [DOI] [PubMed] [Google Scholar]
- Schaefer F. V., Custer R. P., Sorof S. Squamous metaplasia in human breast culture: induction by cyclic adenine nucleotide and prostaglandins, and influence of menstrual cycle. Cancer Res. 1983 Jan;43(1):279–286. [PubMed] [Google Scholar]
- Slocum H. K., Pavelic Z. P., Rustum Y. M., Creaven P. J., Karakousis C., Takita H., Greco W. R. Characterization of cells obtained by mechanical and enzymatic means from human melanoma, sarcoma, and lung tumors. Cancer Res. 1981 Apr;41(4):1428–1434. [PubMed] [Google Scholar]
- Smith H. S., Liotta L. A., Hancock M. C., Wolman S. R., Hackett A. J. Invasiveness and ploidy of human mammary carcinomas in short-term culture. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1805–1809. doi: 10.1073/pnas.82.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkes A., Livingston R. B., Murphy W. K. Tritiated thymidine labeling index and response in human breast cancer. J Natl Cancer Inst. 1979 Mar;62(3):513–515. doi: 10.1093/jnci/62.3.513. [DOI] [PubMed] [Google Scholar]
- Tubiana M., Pejovic M. H., Chavaudra N., Contesso G., Malaise E. P. The long-term prognostic significance of the thymidine labelling index in breast cancer. Int J Cancer. 1984 Apr 15;33(4):441–445. doi: 10.1002/ijc.2910330404. [DOI] [PubMed] [Google Scholar]
- Vescio R. A., Redfern C. H., Nelson T. J., Ugoretz S., Stern P. H., Hoffman R. M. In vivo-like drug responses of human tumors growing in three-dimensional gel-supported primary culture. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5029–5033. doi: 10.1073/pnas.84.14.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]