Abstract
The three separate proteins that make up anthrax toxin--protective antigen (PA), edema factor (EF), and lethal factor (LF)--act in binary combinations to produce two distinct reactions in experimental animals: edema (PA + EF) and death (PA + LF). PA is believed to interact with a membrane receptor, and after proteolytic processing, to mediate endocytosis and subsequent translocation of EF or LF into the cytosol. PA can be separated, after mild trypsinolysis, into two fragments, PA65 (65 kDa) and PA20 (20 kDa). We demonstrate that trypsin-cleaved PA is capable of forming cation-selective channels in planar phospholipid bilayer membranes and that this activity is confined to the PA65 fragment; PA20, LF, and EF are devoid of channel-forming activity. These PA65 channels exhibit pH-dependent and voltage-dependent activity--a property reminiscent of the channels formed by the two-chain proteins diphtheria, tetanus, and botulinum toxins.
Full text
PDF
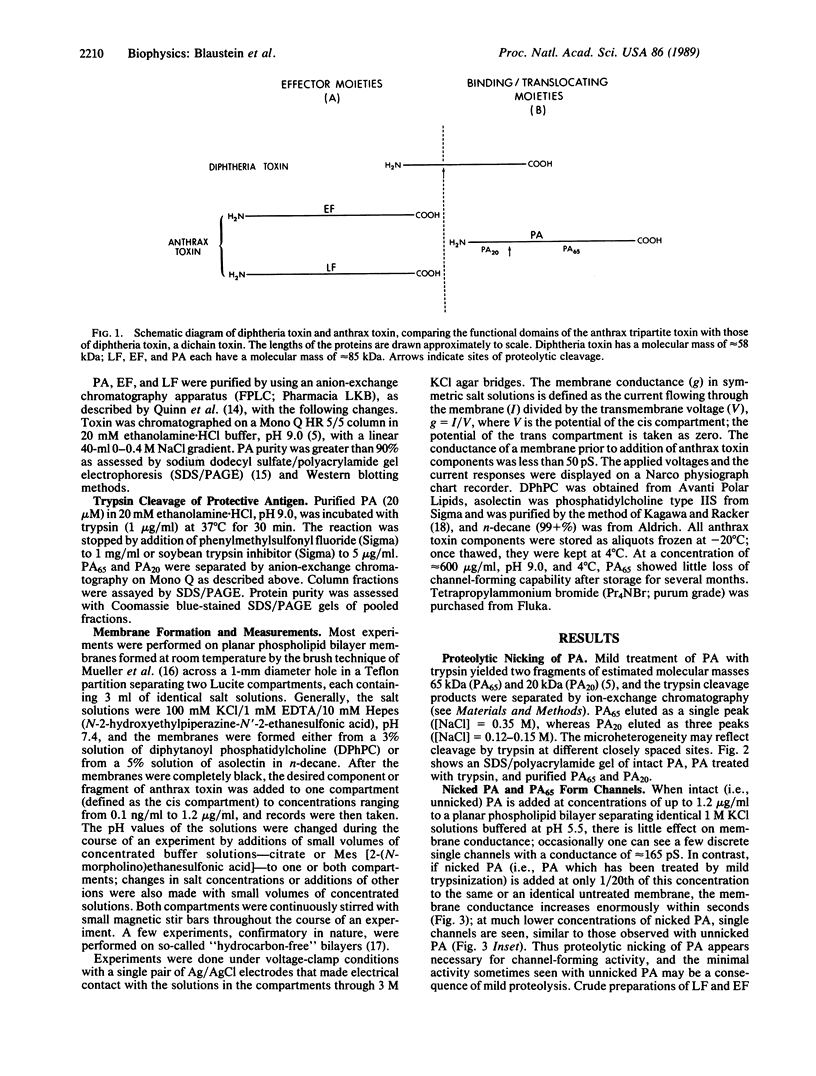
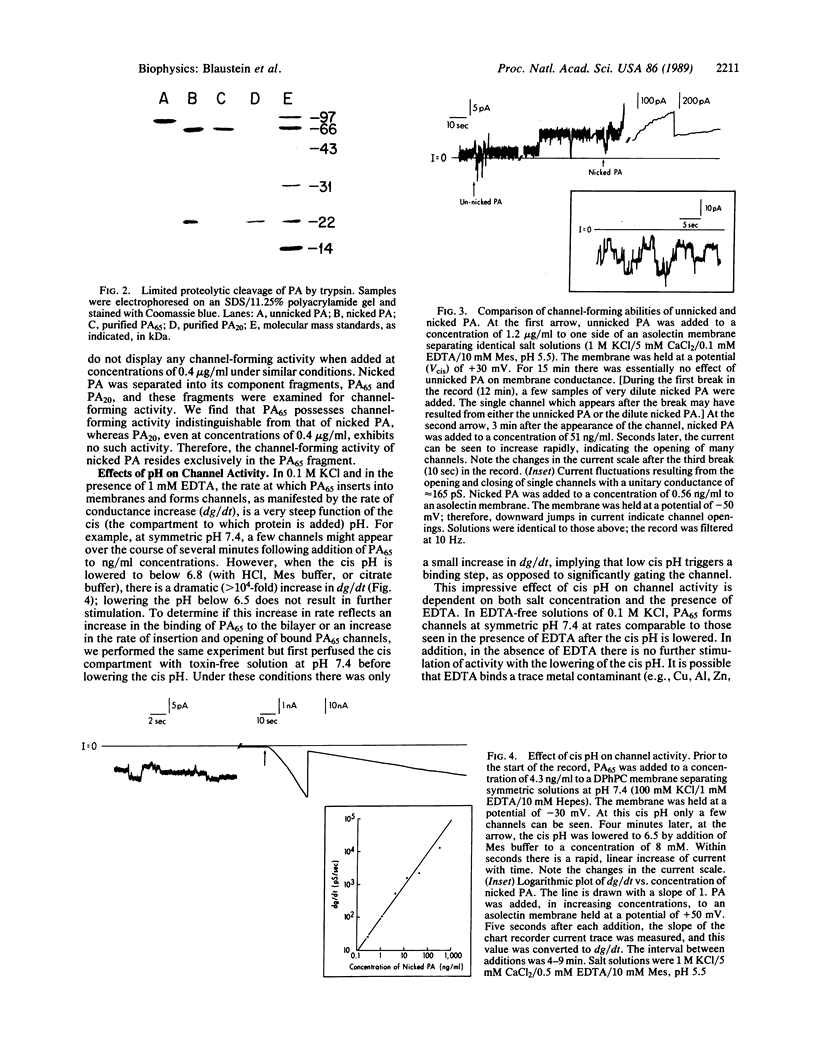
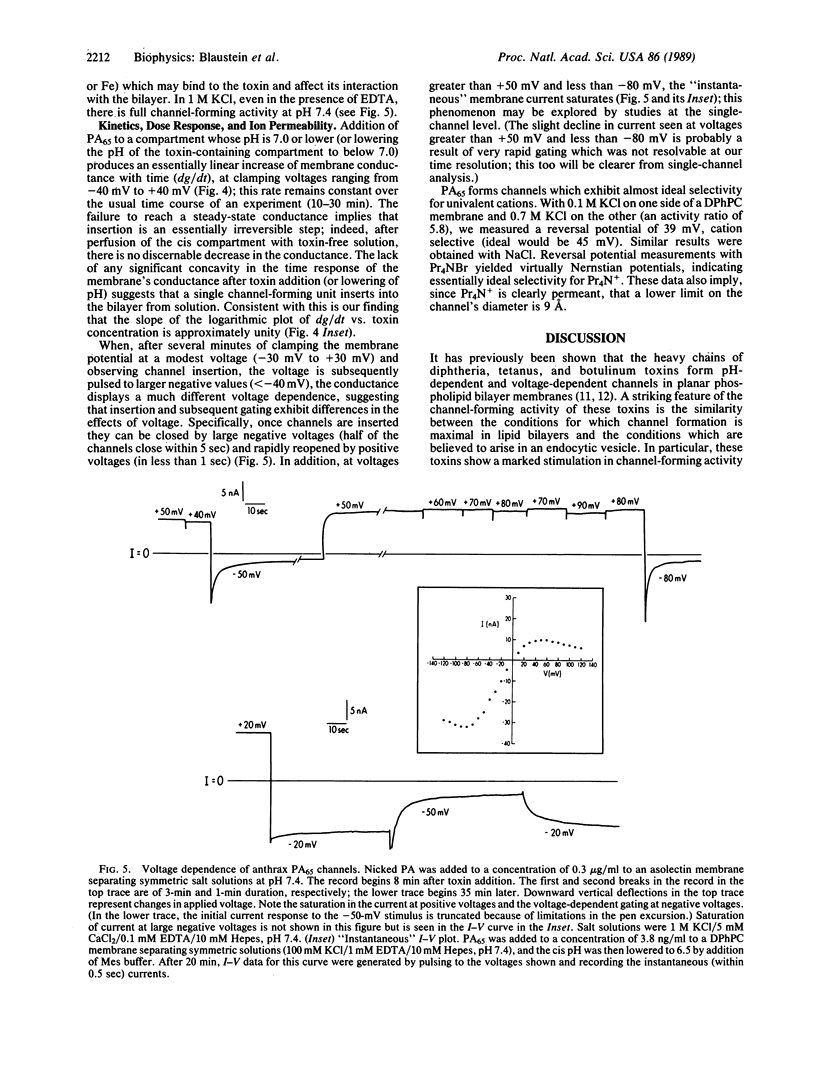

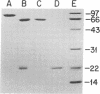
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Donovan J. J., Simon M. I., Draper R. K., Montal M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander A. M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986 Jun 5;261(16):7123–7126. [PubMed] [Google Scholar]
- Gambale F., Montal M. Characterization of the channel properties of tetanus toxin in planar lipid bilayers. Biophys J. 1988 May;53(5):771–783. doi: 10.1016/S0006-3495(88)83157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch D. H., Romero-Mira M., Ehrlich B. E., Finkelstein A., DasGupta B. R., Simpson L. L. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1692–1696. doi: 10.1073/pnas.82.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Bacillus anthracis calmodulin-dependent adenylate cyclase: chemical and enzymatic properties and interactions with eucaryotic cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:189–198. [PubMed] [Google Scholar]
- Montal M. Formation of bimolecular membranes from lipid monolayers. Methods Enzymol. 1974;32:545–554. doi: 10.1016/0076-6879(74)32053-8. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Sandvig K. How protein toxins enter and kill cells. Cancer Treat Res. 1988;37:39–73. doi: 10.1007/978-1-4613-1083-9_4. [DOI] [PubMed] [Google Scholar]
- Quinn C. P., Shone C. C., Turnbull P. C., Melling J. Purification of anthrax-toxin components by high-performance anion-exchange, gel-filtration and hydrophobic-interaction chromatography. Biochem J. 1988 Jun 15;252(3):753–758. doi: 10.1042/bj2520753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J. D., Ivins B. E. Elaboration of Bacillus anthracis antigens in a new, defined culture medium. Infect Immun. 1983 Jan;39(1):483–486. doi: 10.1128/iai.39.1.483-486.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. L. Molecular pharmacology of botulinum toxin and tetanus toxin. Annu Rev Pharmacol Toxicol. 1986;26:427–453. doi: 10.1146/annurev.pa.26.040186.002235. [DOI] [PubMed] [Google Scholar]
- Welkos S. L., Lowe J. R., Eden-McCutchan F., Vodkin M., Leppla S. H., Schmidt J. J. Sequence and analysis of the DNA encoding protective antigen of Bacillus anthracis. Gene. 1988 Sep 30;69(2):287–300. doi: 10.1016/0378-1119(88)90439-8. [DOI] [PubMed] [Google Scholar]