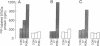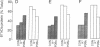Abstract
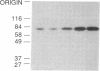
A protein of 87 kilodaltons (87 kDa) was previously identified as a major specific substrate for protein kinase C in neuronal and other tissues. We have now studied the effect of protein kinase C-catalyzed phosphorylation of this protein on its association with membranes in isolated nerve terminals (synaptosomes) from rat cerebral cortex. Incubation of synaptosomal membranes under conditions associated with activation of protein kinase C led to the release of the phosphorylated 87-kDa protein into the incubation medium. In intact synaptosomes, activation of protein kinase C by phorbol esters or by depolarization-induced Ca2+ influx caused an increased phosphorylation of the 87-kDa protein and its translocation from membrane to cytosol. This translocation showed time courses, calcium dependency, and reversibility similar to those observed for the protein kinase C-induced phosphorylation of the protein. These results suggest that protein kinase C-catalyzed phosphorylation of the 87-kDa protein is responsible for its subcellular translocation into the cytosol of nerve terminals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Albert K. A., Keum M. M., Wang J. K., Greengard P., Cohn Z. A. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature. 1988 Mar 24;332(6162):362–364. doi: 10.1038/332362a0. [DOI] [PubMed] [Google Scholar]
- Albert K. A., Nairn A. C., Greengard P. The 87-kDa protein, a major specific substrate for protein kinase C: purification from bovine brain and characterization. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7046–7050. doi: 10.1073/pnas.84.20.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K. A., Walaas S. I., Wang J. K., Greengard P. Widespread occurrence of "87 kDa," a major specific substrate for protein kinase C. Proc Natl Acad Sci U S A. 1986 May;83(9):2822–2826. doi: 10.1073/pnas.83.9.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dunkley P. R., Jarvie P. E., Heath J. W., Kidd G. J., Rostas J. A. A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res. 1986 Apr 30;372(1):115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Patel J., Kligman D. Purification and characterization of an Mr 87,000 protein kinase C substrate from rat brain. J Biol Chem. 1987 Dec 5;262(34):16686–16691. [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas S. I., Nairn A. C., Greengard P. Regional distribution of calcium- and cyclic adenosine 3':5'-monophosphate-regulated protein phosphorylation systems in mammalian brain. I. Particulate systems. J Neurosci. 1983 Feb;3(2):291–301. doi: 10.1523/JNEUROSCI.03-02-00291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas S. I., Nairn A. C., Greengard P. Regional distribution of calcium- and cyclic adenosine 3':5'-monophosphate-regulated protein phosphorylation systems in mammalian brain. II. Soluble systems. J Neurosci. 1983 Feb;3(2):302–311. doi: 10.1523/JNEUROSCI.03-02-00302.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. K., Walaas S. I., Greengard P. Protein phosphorylation in nerve terminals: comparison of calcium/calmodulin-dependent and calcium/diacylglycerol-dependent systems. J Neurosci. 1988 Jan;8(1):281–288. doi: 10.1523/JNEUROSCI.08-01-00281.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Isolation and characterization of two distinct forms of protein kinase C. J Biol Chem. 1987 Apr 5;262(10):4836–4843. [PubMed] [Google Scholar]
- Wu W. C., Walaas S. I., Nairn A. C., Greengard P. Calcium/phospholipid regulates phosphorylation of a Mr "87k" substrate protein in brain synaptosomes. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5249–5253. doi: 10.1073/pnas.79.17.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]