Abstract
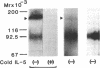
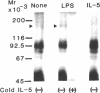
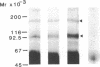
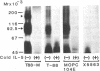
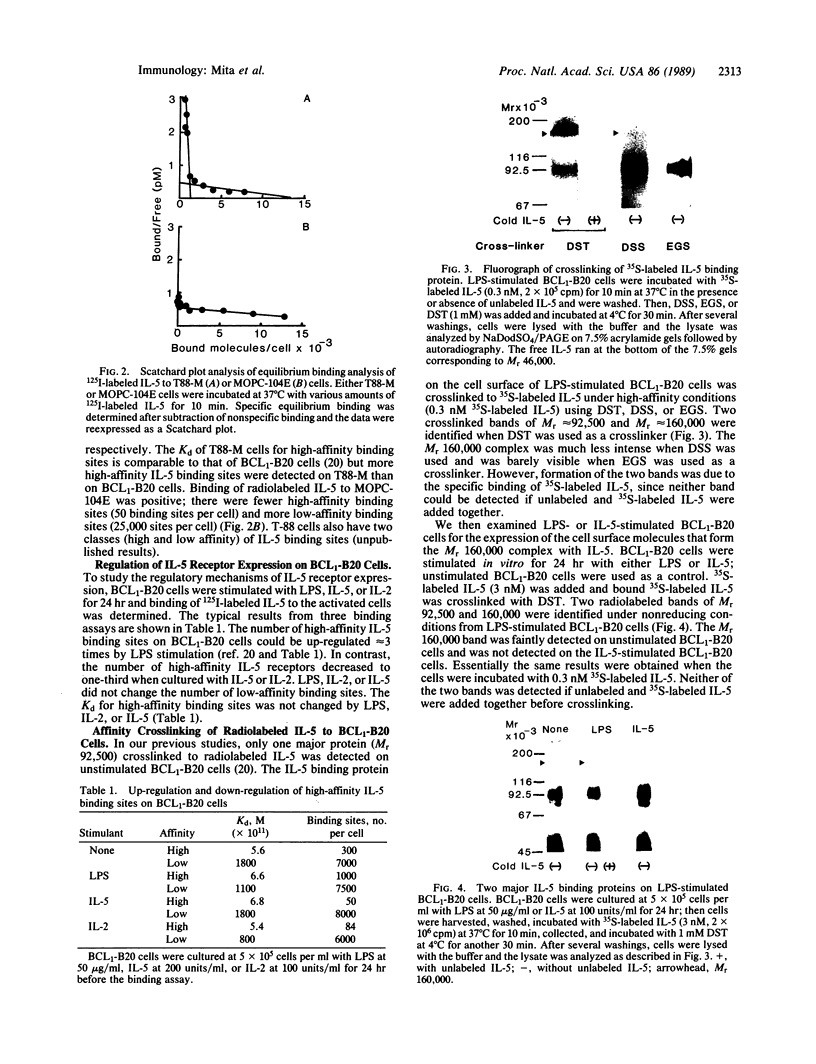
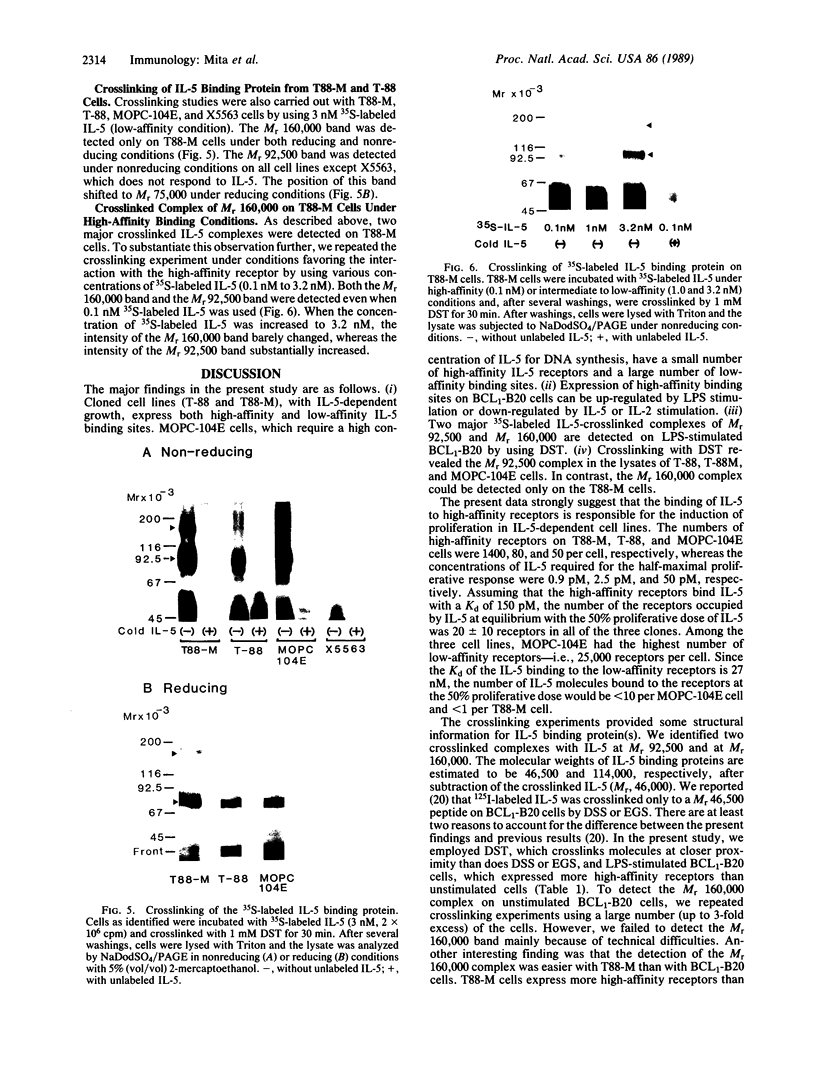
Interleukin 5 (IL-5) is a glycosylated polypeptide that acts as a key factor for B-cell growth and differentiation. We demonstrated previously that there are two classes (high and low affinity) of IL-5 receptors on murine chronic B-cell leukemic cells (BCL1-B20). Treatment of surface-bound radiolabeled IL-5 with bivalent crosslinkers identified a polypeptide of Mr 92,500. In this study, we analyzed characteristics of high-affinity IL-5 receptors on IL-5-dependent early B-cell lines (T-88 and T88-M), mouse myeloma cells (MOPC-104E), and BCL1-B20 cells. All cell lines had two classes of IL-5 binding sites, but T88-M cells bore the highest number of high-affinity receptors. The number of high-affinity IL-5 receptors on BCL1-B20 cells could be up-regulated 3-fold by lipopolysaccharide and down-regulated by IL-5. Disuccinimidyl tartarate crosslinking of 35S-labeled IL-5 to the receptors on the T88-M and lipopolysaccharide-stimulated BCL1-B20 cells revealed two major 35S-labeled components of Mr 92,500 and Mr 160,000, even when the binding of 35S-labeled IL-5 was carried out under high-affinity conditions (100 pM 35S-labeled IL-5). The Mr 92,500 component, but not the Mr 160,000 component, was detected in the lysates of MOPC-104E and T-88 cells, both of which bore a large number of low-affinity receptors and a limited number of high-affinity receptors. The results suggest that the Mr 92,500 component represents the complex of IL-5 with the low-affinity Mr 46,500 receptor, whereas the high-affinity receptor consists of the Mr 46,500 peptide and an additional peptide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma C., Tanabe T., Konishi M., Kinashi T., Noma T., Matsuda F., Yaoita Y., Takatsu K., Hammarström L., Smith C. I. Cloning of cDNA for human T-cell replacing factor (interleukin-5) and comparison with the murine homologue. Nucleic Acids Res. 1986 Nov 25;14(22):9149–9158. doi: 10.1093/nar/14.22.9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N., Kikuchi Y., Tominaga A., Takaki S., Takatsu K. BCGFII activity on activated B cells of a purified murine T cell-replacing factor (TRF) from a T cell hybridoma (B151K12). J Immunol. 1985 Jun;134(6):3944–3951. [PubMed] [Google Scholar]
- Harada N., Matsumoto M., Koyama N., Shimizu A., Honjo T., Tominaga A., Takatsu K. T cell replacing factor/interleukin 5 induces not only B-cell growth and differentiation, but also increased expression of interleukin 2 receptor on activated B-cells. Immunol Lett. 1987 Jul;15(3):205–215. doi: 10.1016/0165-2478(87)90026-5. [DOI] [PubMed] [Google Scholar]
- Harada N., Takahashi T., Matsumoto M., Kinashi T., Ohara J., Kikuchi Y., Koyama N., Severinson E., Yaoita Y., Honjo T. Production of a monoclonal antibody useful in the molecular characterization of murine T-cell-replacing factor/B-cell growth factor II. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4581–4585. doi: 10.1073/pnas.84.13.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Paul W. E. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- Kinashi T., Harada N., Severinson E., Tanabe T., Sideras P., Konishi M., Azuma C., Tominaga A., Bergstedt-Lindqvist S., Takahashi M. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature. 1986 Nov 6;324(6092):70–73. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Factors affecting B-cell growth and differentiation. Annu Rev Immunol. 1985;3:133–157. doi: 10.1146/annurev.iy.03.040185.001025. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loughnan M. S., Takatsu K., Harada N., Nossal G. J. T-cell-replacing factor (interleukin 5) induces expression of interleukin 2 receptors on murine splenic B cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5399–5403. doi: 10.1073/pnas.84.15.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita S., Harada N., Naomi S., Hitoshi Y., Sakamoto K., Akagi M., Tominaga A., Takatsu K. Receptors for T cell-replacing factor/interleukin 5. Specificity, quantitation, and its implication. J Exp Med. 1988 Sep 1;168(3):863–878. doi: 10.1084/jem.168.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K., Yoshimoto T., Katoh Y., Ono S., Matsui K., Hiroishi K., Noma T., Honjo T., Takatsu K., Higashino K. Both B151-T cell replacing factor 1 and IL-5 regulate Ig secretion and IL-2 receptor expression on a cloned B lymphoma line. J Immunol. 1988 Feb 15;140(4):1168–1174. [PubMed] [Google Scholar]
- Noma T., Mizuta T., Rosén A., Hirano T., Kishimoto T., Honjo T. Enhancement of the interleukin 2 receptor expression on T cells by multiple B-lymphotropic lymphokines. Immunol Lett. 1987 Jul;15(3):249–253. doi: 10.1016/0165-2478(87)90032-0. [DOI] [PubMed] [Google Scholar]
- Rasmussen R., Takatsu K., Harada N., Takahashi T., Bottomly K. T cell-dependent hapten-specific and polyclonal B cell responses require release of interleukin 5. J Immunol. 1988 Feb 1;140(3):705–712. [PubMed] [Google Scholar]
- Sabe H., Tanaka A., Siomi H., Koyasu S., Yonehara S., Yahara I., Tagaya Y., Sugie K., Teshigawara K., Yodoi J. Differential effects on expression of IL-2 receptors (p55 and p70) by the HTLV-I pX DNA. Int J Cancer. 1988 Jun 15;41(6):880–885. doi: 10.1002/ijc.2910410619. [DOI] [PubMed] [Google Scholar]
- Takatsu K. B-cell growth and differentiation factors. Proc Soc Exp Biol Med. 1988 Jul;188(3):243–258. doi: 10.3181/00379727-188-42732a. [DOI] [PubMed] [Google Scholar]
- Takatsu K., Harada N., Hara Y., Takahama Y., Yamada G., Dobashi K., Hamaoka T. Purification and physicochemical characterization of murine T cell replacing factor (TRF). J Immunol. 1985 Jan;134(1):382–389. [PubMed] [Google Scholar]
- Takatsu K., Tanaka K., Tominaga A., Kumahara Y., Hamaoka T. Antigen-induced T cell-replacing factor (TRF). III. Establishment of T cell hybrid clone continuously producing TRF and functional analysis of released TRF. J Immunol. 1980 Dec;125(6):2646–2653. [PubMed] [Google Scholar]
- Takatsu K., Tominaga A., Hamaoka T. Antigen-induced T cell-replacing factor (TRF). I. Functional characterization of a TRF-producing helper T cell subset and genetic studies on TRF production. J Immunol. 1980 May;124(5):2414–2422. [PubMed] [Google Scholar]
- Takatsu K., Tominaga A., Harada N., Mita S., Matsumoto M., Takahashi T., Kikuchi Y., Yamaguchi N. T cell-replacing factor (TRF)/interleukin 5 (IL-5): molecular and functional properties. Immunol Rev. 1988 Feb;102:107–135. doi: 10.1111/j.1600-065x.1988.tb00743.x. [DOI] [PubMed] [Google Scholar]
- Tominaga A., Matsumoto M., Harada N., Takahashi T., Kikuchi Y., Takatsu K. Molecular properties and regulation of mRNA expression for murine T cell-replacing factor/IL-5. J Immunol. 1988 Feb 15;140(4):1175–1181. [PubMed] [Google Scholar]
- Yamaguchi Y., Suda T., Suda J., Eguchi M., Miura Y., Harada N., Tominaga A., Takatsu K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988 Jan 1;167(1):43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Coffman R. L., Hagiwara H., Rennick D. M., Takebe Y., Yokota K., Gemmell L., Shrader B., Yang G., Meyerson P. Isolation and characterization of lymphokine cDNA clones encoding mouse and human IgA-enhancing factor and eosinophil colony-stimulating factor activities: relationship to interleukin 5. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7388–7392. doi: 10.1073/pnas.84.21.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]