Abstract
Testicular germ cell cancers in young adult men derive from a precursor lesion called carcinoma in situ (CIS) of the testis. CIS cells were suggested to arise from primordial germ cells or gonocytes. However, direct studies on purified samples of CIS cells are lacking. To overcome this problem, we performed laser microdissection of CIS cells. Highly enriched cell populations were obtained and subjected to gene expression analysis. The expression profile of CIS cells was compared to microdissected gonocytes, oogonia and cultured embryonic stem cells (ESCs) with and without genomic abberations. Three samples of each tissue type were used for the analyses. Unique expression patterns for these developmentally very related cell types revealed that CIS cells were very similar to gonocytes as only five genes distinguished these two cell types. We did not find indications that CIS was derived from a meiotic cell and the similarity to ESCs was modest compared to gonocytes. Thus we provide new evidence that the molecular phenotype of CIS cells is similar to that of gonocytes. Our data are in line with the idea that CIS cells may be gonocytes that survived in the postnatal testis. We speculate that disturbed development of somatic cells in the fetal testis may play a role in allowing undifferentiated cells to survive in the postnatal testes. The further development of CIS into invasive germ cell tumors may depend on signals from their post-pubertal niche of somatic cells, including hormones and growth factors from Leydig and Sertoli cells.
Keywords: Carcinoma in situ (CIS) testis, gonocytes, embryonic stem cells (ESC), gene expression profiling, laser microdissection
Introduction
Testicular germ cell cancer is the most common malignant disease among young adult men in Europe, affecting up to 1% of all men (1). All testicular germ cell tumors (TGCTs) of young adult men derive from carcinoma in situ (CIS). The CIS cells are believed to arise from fetal germ cells and reside dormant in the testis until they start proliferating after puberty and eventually develop into an overt tumor (2). Overt TGCTs can be divided in two major classes: the seminomas, which retain a CIS-like phenotype and germ cell features, and the more pluripotent embryonic stem cell (ESC)-like non-seminomas, which comprise tumors resembling embryonic tissues (e.g. embryonal carcinoma and teratoma) as well as extra-embryonic tissues (e.g. choriocarcinoma and yolk sac tumor).
TGCTs are part of the testicular dysgenesis syndrome (TDS) (3), a group of disorders believed to arise as a result of disturbed development of the somatic cells in the gonad, probably due to an imbalanced hormonal environment of the fetus (reviewed in (4)). The exact trigger for the neoplastic transformation is unknown, but it is probably initiated at the stage of primordial germ cells or gonocytes. This assumption is based on the morphology of CIS (5) and overlap in expression of markers in CIS, PGCs and gonocytes, but not in infantile spermatogonia and adult germ cells, including several embryonic pluripotency genes (6). In accordance, our recent study showed a striking resemblance between the gene expression profile of CIS and ESCs, as up to 34 percent of the identified CIS genes were previously reported in ESCs (7). Further, when ESCs are cultured for a prolonged time, gain of chromosome arms 17q and 12p are repeatedly observed (8). Interestingly, the same chromosomal regions are implicated in the progression of CIS to invasiveness, emphasizing the resemblance between CIS and ESCs (9;10).
When the primordial germ cells migrate through the hindgut towards the gonadal ridge, they remain sexually bipotent. After an initial proliferation in the gonadal ridge, the female germ cells, oogonia, enter meiosis while male germ cells, gonocytes, continue to proliferate until their differentiation to the quiescent pre-spermatogonia. One possible explanation for the development of CIS could be that an insufficient virilization of somatic cells surrounding the germ cells could lead to a more female-like differentiation and perhaps a premature initiation of meiosis (11).
Due to the cellularity of the testis, where CIS cells maximally constitute about 5% of the cells, it is difficult to make a satisfactory expression profile of CIS. Previous studies of global gene expression in CIS cells have analysed testis tissues containing increasing proportions of CIS cells (7), or simply compared testis tissue with CIS to normal testis tissue (12;13). While giving useful results, these approaches are limited by a considerable background noise from other cell types in the testis. We have addressed this issue by developing a fast and specific staining procedure for CIS and fetal germ cells (14), allowing laser microdissection and RNA isolation from relatively pure cell populations. This resulted in RNA of a quality sufficient to perform two rounds of amplification, producing microgram amounts of RNA, which allowed microarray analysis.
In this study, we aimed at elucidating the origin of CIS cells based on comparative gene expression profiling. For this purpose we compared gene expression profiles of microdissected CIS cells, gonocytes, and oogonia and cultured ESCs with and without genomic aberrations. To correct for contamination with RNA from Sertoli cells, in which gonocytes and CIS cells are embedded, we also microdissected Sertoli cells from tubules with CIS and included this data in the analysis.
Materials and Methods
Tissue samples and ESC lines
The Regional Committee for Medical Research Ethics in Denmark approved the use of adult testicular samples, and collection of human fetal gonads in the UK was done in agreement with the Polkinhorne guidelines, following ethical approval and informed consent of women who underwent elective abortions at 10-12 weeks of pregnancy. Adult testicular tissues containing CIS were residual tissue from orchidectomies collected at Dept. of Pathology at Rigshospitalet after diagnosis of testicular cancer. One of the normal testis RNA samples was from apparently normal tissue adjacent to a tumor, and the other two were commercial samples (Applied Biosystems/Ambion, Austin, TX, USA and Clontech, Mountain View, Ca, USA). Fetal gonads were collected in Sheffield from abortion material; the gestational age was estimated by ultrasound scanning and measurements of hand size. Cultures of the human ESC lines (H7 and Shef5) were maintained in Sheffield under direction of two of the authors (PWA and HDM). H7 cells with or without genomic aberrations were sorted using a fluorescence activated cell sorter (FACS) and only cells expressing the pluripotency marker SSEA3 (undifferentiated cells) were analyzed (see Table 1 for a more thorough description of the samples).
Table 1. Samples used for RT-PCR and microarray analysis.
Abbreviations: ESC, embryonic stem cells; Oo, microdissected oogonia; FetO, fetal ovary; Gon, microdissected gonocytes; FetT, fetal testis; CIS, microdissected carcinoma in situ; CIST, testis tissue containing CIS; -Ampli, not amplified; NT, normal testis; Sert, microdissected Sertoli cells from CIS tubules; EC, embryonal carcinoma; YST, yolk sac tumor; TER, teratoma.
| Sample ID | Age | Description | Estimated purity |
Ampli | RT-PCR | Array |
|---|---|---|---|---|---|---|
| ESC1 | - | H7 abnormal subline, SSEA3 positive |
100% | Yes | - | Yes |
| ESC2 | - | H7 normal subline, SSEA3 positive | 100% | Yes | - | Yes |
| ESC3 | - | Shef5, not FACS sorted | 100% | Yes | - | Yes |
| Oo1 | 12-13 wg | Microdissected fetal oogonia |
60% | Yes | Yes | Yes |
| Oo2 | 11-12 wg | Microdissected fetal oogonia |
60% | Yes | Yes | Yes |
| Oo3 | 10-11 wg | Microdissected fetal oogonia |
60% | Yes | Yes | Yes |
| Oo4 | 11-12 wg | Microdissected fetal oogonia |
60% | Yes | Yes | - |
| FetO4 | 11-12 wg | Total fetal ovary, same tissue as Oo4 |
- | Yes | Yes | - |
| Oo5 | 11-12 wg | Microdissected fetal oogonia |
60% | Yes | Yes | - |
| FetO5 | 11-12 wg | Total fetal ovary, same tissue as Oo5 |
- | Yes | Yes | - |
| Gon1 | 10-11 wg | Microdissected fetal gonocytes |
80% | Yes | Yes | Yes |
| Gon2 | 10-11 wg | Microdissected fetal gonocytes |
80% | Yes | Yes | Yes |
| Gon3 | 11-12 wg | Microdissected fetal gonocytes |
80% | Yes | Yes | Yes |
| Gon4 | 10-11 wg | Microdissected fetal gonocytes |
80% | Yes | Yes | - |
| FetT4 | 10-11 wg | Total fetal testis, same tissue as Gon4 |
- | Yes | Yes | - |
| Gon5 | 12-13 wg | Microdissected fetal gonocytes |
80% | Yes | Yes | - |
| FetT5 | 12-13 wg | Total fetal testis, same tissue as Gon5 |
- | Yes | Yes | - |
| CIS1 | 37 Y | Microdissected CIS from tissue with CIS + invasion (seminoma-like) |
80% | Yes | Yes | Yes |
| CIST1 | 37 Y | Same tissue as CIS1, 95% CIS tubules |
- | Yes | Yes | Yes |
| CIS2 | 30 Y | Microdissected CIS from tissue with CIS next to Classical seminoma |
80% | Yes | Yes | Yes |
| CIST2 | 30 Y | Same tissue as CIS2, 15% CIS tubules |
- | Yes | Yes | Yes |
| CIS3 | 26 Y | Microdissected CIS from tissue with CIS next to Nonseminoma, predominantly EC, immature Sertoli cells |
80% | Yes | Yes | Yes |
| CIST3 | 26 Y | Same tissue as CIS3, 60% CIS tubules |
- | Yes | Yes | Yes |
| CIS4 | 27 Y | Microdissected CIS from tissue with CIS next to Nonseminoma, predominantly EC progressing to YST, focal immature TER |
80% | Yes | Yes | - |
| CIST4 | 27 Y | Same tissue as CIS4 30% CIS tubules |
- | Yes | Yes | - |
|
CIST4 - Ampli |
27 Y | Same tissue as CIST4 not amplified | - | - | Yes | - |
| CIS5 | 55 Y | Microdissected CIS from tissue with CIS next to Nonseminoma, predominantly EC and TER |
80% | Yes | Yes | - |
| CIST5 | 55 Y | Same tissue as CIS5 95% CIS tubules |
- | Yes | Yes | - |
|
CIST5 - Ampli |
55 Y | Same tissue as CIST5 not amplified | - | - | Yes | - |
| NT1 | - | Ambion | - | Yes | - | Yes |
| NT2 | 54 Y | Areas of impaired Spermatogenesis, hyalinized tubules and lymphocyte infiltration |
- | Yes | - | Yes |
| NT3 | - | Clontech | - | Yes | - | Yes |
| Sert1 | 37 Y | From same tissue as CIS1 | 80% | Yes | - | Yes |
| Sert2 | 30 Y | From same tissue as CIS2 | 90% | Yes | - | Yes |
| Sert3 | 26 Y | From same tissue as CIS3 | 90% | Yes | - | Yes |
Preparation of cryosections for microdissection
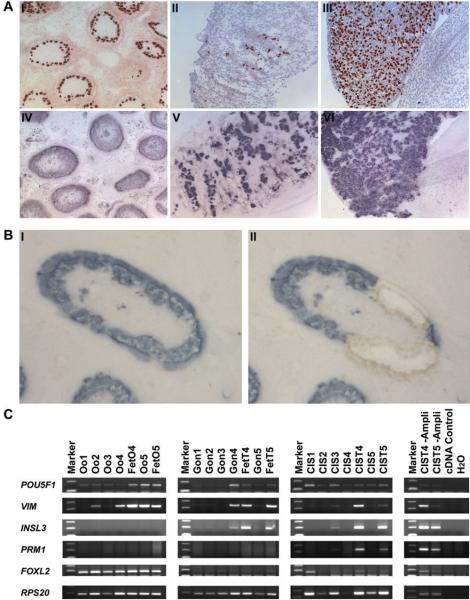
Adult testicular tissue and fetal gonads were embedded in O.C.T. compound (Sakura Fintek Europe, Zoeterwonde, NL) and snap frozen at −80°C in isopentan. Sections of 10 μm (fetal tissue) and 20 μm (adult tissue) were cut on a Shandon SME Cryotome (Life Sciences International Europe Ltd, Cheshire, UK), collected on nuclease and nucleic acid free membrane slides (Molecular Machines & Industries, Glatbrugg, CH), immediately fixed in 75% RNase free ethanol for 10 minutes and stored in absolute ethanol at −80°C. Serial sections of fetal gonads and testicular tissues containing CIS were analysed by immunohistochemistry (IHC) (Fig. 1A) for AP-2γ to identify gonocytes, oogonia and CIS (15), fetal antigen-1 (FA-1) to identify Leydig cells (16) and AMH (17) and MIC-2 (18) to identify fetal and adult Sertoli cells, respectively. An additional serial section was stained for alkaline phosphatase activity (14), which is only detectable in CIS and fetal germ cells.
Figure 1. Verification of microdissection.
A: Serial sections of a testis with CIS (I and IV), fetal testis (II and V) and fetal ovary (III and VI). Top: immunohistochemical staining for AP-2γ and bottom: alkaline phophatase expression visualized by NBT-BCIP staining. B: Frozen section with CIS stained with NBT-BCIP, before (I) and after (II) laser microdissection. C: RT-PCR on representative genes. Tissues 1-3 are the same samples used in the microarray analysis, 4 and 5 are amplified RNA from microdissected and total tissues, in the left panel, CIS 4 and 5 are unamplified whole testis samples from the same patients as in the previous panel (See Table 1 for a more thorough description). Abbreviations: Oo, microdissected oogonia; FetO, fetal ovary; Gon, microdissected gonocytes; FetT, fetal testis; CIS, microdissected carcinoma in situ; CIST, testis tissue containing CIS; -Ampli, not amplified.
Microdissection and RNA Amplification
Prior to microdissection, slides were transferred to room temperature and stained with NBT-BCIP by direct histochemistry as previously described (14). The cells were microdissected within two hours at room temperature using the MMI CellCut or SmartCut system (Olympus / Molecular Machines & Industries, Hertfordshire, UK) (Fig. 1B). Only CIS tubules with a classical appearance with CIS cells along the edge of tubules and stained areas that resembled fetal germ cells were excised in order to avoid CIS cells at a more advanced stage or unspecifically stained areas.
RNA was purified using the Ambion RNAqueous micro kit (Applied Biosystems/Ambion). The RNA quality was tested with the Bioanalyzer Picokit (Agilent Technologies, Santa Clara, CA, US), and samples were amplified in two rounds using the MessageAmp™ II aRNA Amplification Kit (Applied Biosystems/Ambion).
Microarray analysis
The following samples were analysed: three ESC samples, three microdissected oogonia samples (Oo), three microdissected gonocyte samples (Gon), three microdissected CIS samples (CIS) and three microdissected Sertoli cell samples (Sert) from tubules containing CIS. In addition, three samples of testis tissue containing CIS (CIST) from the same patients as the microdissected CIS and three normal testis samples (NT) were included (table 1). All samples underwent two rounds of amplification as described above.
For microarray analysis, we used Agilent Whole Human Genome Microarray 4×44K chips, Design Number 014850 (Agilent Technologies). Hybridisation and scanning of one-color arrays was performed as described by the manufacturer (Agilent Technologies) and analyzed using the Agilent Feature extraction software (version 9.1.3.1).
The lowess normalized, gProcessedSignal from each array was loaded into the marray and limma R/BioC package, normalized between arrays using a quantile normalization procedure and log-transformed. Normalized data was then imported into TIGRs MeV v 4.0 (19) for subsequent statistical analysis using the significance analysis of microarrays (SAM)(20) with standard settings. Partition clustering of selected gene lists was made by the Partitioning Around Medoids (PAM) clustering algorithm (R library “cluster”) and simple Correspondence Analysis (R library “MASS”) (21) was performed to elucidate the correspondence between profiles of selected genes across a set of cell types.
RT-PCR, immunohistochemistry (IHC) and in situ hybridisation (ISH)
cDNA synthesis was made with 50 ng/μL random hexamer primers. RT-PCR was performed using gene specific primers placed just upstream of the polyA site. Primer sequences, cycle numbers and annealing temperatures are summarized in Supplementary Table 1. RPS20 was used as a positive control for cDNA synthesis and PCR efficiency. Representative bands for each primerset were excised from the gels and sequenced.
Serial sections of frozen tissues (10 μm) used for microdissection were fixed in phosphate buffered formalin (4% w/v, pH 7, VWR, Bie & Berntsen, Copenhagen, DK) for 10 minutes. IHC was performed as previously described (17) with the following antibodies: AP-2γ (sc-12762, Santa Cruz Biotechnology, Santa Cruz, CA, USA), FA-1 (provided by Charlotte Harken Jensen, Odense University, Odense, DK), AMH (provided by Richard L. Cate, Biogen, Cambridge, MA, USA), MIC-2 (clone 12E7, Dako, Glostrup, DK) and MYCL1 (L-Myc (C-20) sc-790, Santa Cruz). The antibodies were diluted 1:50, 1:200, 1:150 1:50 and 1:100, respectively.
In situ hybridization was performed as previously described (22). The DNA template for the THC2340734 RNA probe was amplified using nested primers: 1st PCR: GCCAACAAGAAGGACATCATGA and GTATGGGAATGGATGGTGTGT; 2nd PCR AATTAACCCTCACTAAAGGGACATCATGACCCT and TAATACGACTCACTATAGGGTGTGTTCCCTGT (bold indicate T3 and T7 promotors).
Results
Purity of microdissected CIS and fetal germ cells
Although it was impossible to completely avoid contamination with surrounding somatic cells, we obtained enriched cell populations that, based on visual inspection, contained up to 60% oogonia, 80% gonocytes and 80% CIS cells (Fig. 1) (Table 1). Before we proceeded to microarray analysis we tested the enrichment of RNA from CIS and fetal germ cells by RT-PCR analysis of genes with cell-type-specific expression (Fig. 1C). The selected genes were: POU5F1 (OCT3/4), expressed in CIS, gonocytes and oogonia (23); VIM, expressed in Sertoli cells, endothelial cells, Leydig cells, (24) and granulosa cells (25); INSL3, specific for Leydig cells (26); FOXL2, expressed in granulosa cells and undifferentiated Sertoli cells in fetal testes and adult testes with signs of TDS (27) and PRM1 expressed in round and elongating spermatids (28). RPS20 was used as a cDNA synthesis and PCR control.
POU5F1 was, as expected, present in microdissected samples of oogonia, gonocytes and CIS and when compared to RPS20, the bands were in general stronger in the microdissected samples than in the whole tissue preparations. VIM was present in the fetal ovary, fetal testis, and whole CIS testis preparations, but weak bands were also seen in some of the microdissected oogonia, gonocyte and CIS samples, indicating contamination with neighbouring Sertoli and Granulosa cells. The Leydig cell gene, INSL3, was only detected in samples containing total testis RNA, and at a very low level in one of the CIS and a few of the gonocyte samples. FOXL2 was detected in all oogonia samples reflecting the Granulosa cell contamination, and also weakly detectable in gonocyte and CIS samples, probably due to contamination with undifferentiated and partially undervirilised Sertoli cells (27). PRM1 was as expected expressed in all CIST samples because of the presence of normal tubules with spermatogenesis, but not in any of the microdissected or fetal samples.
Based on these results, we concluded that the microdissected cell populations were adequately enriched to proceed to microarray analysis.
Evaluation of microarray gene expression profiles
Gene expression was analyzed using Agilent microarrays covering more than 41,000 unique human genes and transcripts; the analyzed samples are summarized in Table 1. The raw data has been submitted to MIAMExpress at the European Bioinformatics Institute (accession number E-TABM-488).
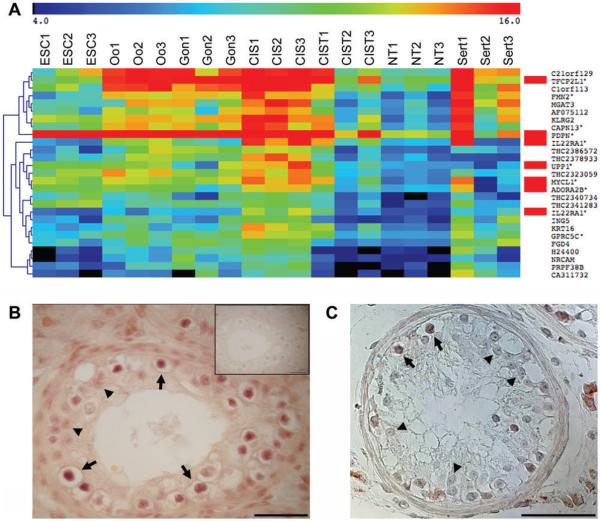
To investigate if previously described CIS markers were also identified in this dataset, we performed a 2-way SAM of microdissected CIS vs. NT (Fig. 2a). Of the 26 most significant genes (false discovery rate (FDR) = 0.1%), 5 genes had been identified as CIS markers in previous studies: PDPN (also known as M2A antigen (29)), TFCP2L1, IL22RA1, UPP1 and MYCL1 (previously MYCL) (7;13) and one (ADORA2B) had been reported in undifferentiated testicular tumors and cell lines (13). By comparison, similar 2-way SAM analyses (with the same FDR of 0.1%) on CIST vs. NT and CIST+CIS vs. NT resulted in zero significant genes specific for CIS.
Figure 2. Verification of microarray data, re-finding CIS genes.
A: 2-way SAM on CIS vs NT (delta = 0.84, FDR=0.1%) showing genes highly expressed in CIS, note that IL22RA1 is detected with 2 different oligo probes. Genes marked with red were previously described in CIS or undifferentiated testicular tumours and cell lines. The annotated genes with confirmed probe specificity are marked by asterisks. The color key at the top shows the relative expression levels from 4 to 16. A more extensive gene list (FDR<1%) can be found in Supplementary Table 2. B: Expression of MYCL1 in CIS testis. C: Expression of the THC2340734 transcript in CIS testis. Arrows indicate CIS cells and arrowheads mark Sertoli cells. Note that MYCL1 is also expressed in interstitial Leydig cells. Abbreviations: as in Fig. 1 and ESC, cultured embryonic stem cells; NT, normal testis and Sert, microdissected Sertoli cells next to CIS.
MYCL1 appeared highly expressed in CIS cells and IHC confirmed its presence in CIS cells (Fig. 2B). The heatmap of genes highly expressed in CIS included five Tentative Human Consensus (THC) sequences that appeared largely restricted to CIS cells (Fig. 2A). This was confirmed by RT-PCR for three of them, whereas one (THC2341283) did not give any bands and one (THC2378933) resulted in multiple bands (not shown). ISH with a probe for THC2340734 confirmed its high expression in CIS cells (Fig. 2C). However, all three THC transcripts only included a single exon (ensemble.org) and neither appeared to encode open reading frames.
Among the genes very high in CIS compared to NT was a Sertoli cell marker, KRT16 (IHC not shown), indicating a severe contamination of CIS samples with the neighbouring Sertoli cells. However a blast search at ENSEMBL revealed that the KRT16 oligo was only 95% identical to KRT16 (3 mismatches), but had a 100% match to two other transcripts that appeared to be non-functional KRT16 pseudogenes. To avoid similar mistakes for other genes, we checked all the oligo probes shown in the heatmaps, and found that only 77% of the oligo probes for annotated genes were unique for their intended targets (indicated by asterisks). However, since the results in this study are not based on expression of single genes but the overall profile, the lack of specificity for a subset of the oligos does not affect the results.
The CIS samples clearly included material from Sertoli cells, therefore we determined the expression profiles of microdissected Sertoli cells from CIS tubules and tested various methods for subtracting Sertoli genes from the CIS data. But irrespective of the method this also subtracted a large number of genes expressed in both Sertoli and CIS cells. Instead, we decided to include the expression profile of microdissected Sertoli cells in the heatmap to facilitate a visual comparison (Fig. 2A).
CIS cells are very similar to gonocytes
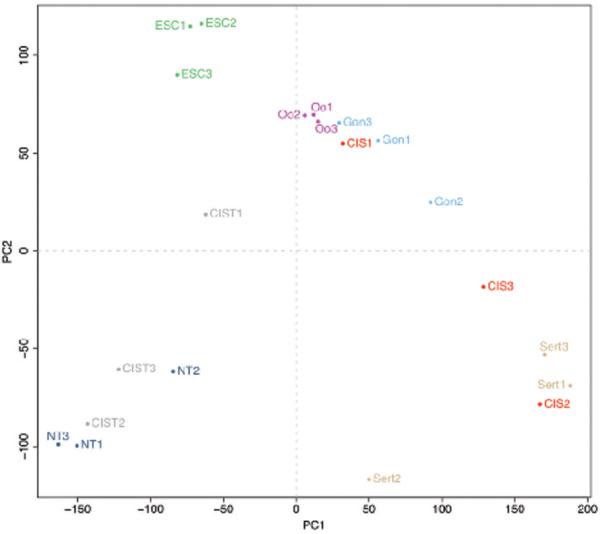
A principal component analysis of the entire data set (Fig. 3) showed that the expression profiles generally grouped by cell type: NT, ESC and Sert were the most distant groups, and Oo and Gon grouped closely together. The gene expression profiles for CIS were clearly distinct from the CIST samples, whose profiles resembled normal testis while the microdissected CIS samples grouped with fetal samples and Sertoli cells. This confirmed that the microdissection was successful in eliminating normal germ cells from the CIS gene expression profile. Interestingly, the content of CIS in CIST samples was reflected in the plot, CIST2 which contained only 15% CIS tubules were closer related to normal testis while CIST1 containing 95% CIS were much closer related to the fetal samples and microdissected CIS. The overlap between CIS and Sert was probably due to different levels of contamination with Sertoli cells. Sample clustering of the 500 most differentially expressed genes from a 6-way SAM (FDR=0%, delta=0,36) (Suppl. Fig. 1) gave similar results: the gene expression profile of CIS cells was very similar to that of gonocytes, and also related to the oogonial expression profile, whilst CIST and NT clustered together, and ESCs were more distantly related to these tissues (Suppl. Fig. 1). The similarity between CIS and gonocytes was further supported by analysis of uncorrelated shrunken centroids (USC) (30). Leaving Sertoli samples out of the USC analysis, all the CIS samples were classified as gonocytes and all CIST samples as normal testis. Thus, based on gene expression profiles, CIS cells are most similar to gonocytes.
Figure 3. Principal component analysis.
All the samples included in the microarray dataset were subjected to a principal component analysis to determine their spatial relationship to each other. Abbreviations: ESC, cultured embryonic stem cells; Oo, microdissected oogonia; Gon, microdissected gonocytes; CIS, microdissected carcinoma in situ; CIST, testis tissue containing CIS; NT, normal testis and Sert, microdissected Sertoli cells next to CIS.
We made additional analyses to characterize the dataset: Supplementary Figure 1 shows a heatmap of the 500 most differentially expressed genes from a 6-way SAM, Supplementary Figure 2 shows the most frequent profiles among these genes, and Supplementary Figure 3 shows a heatmap of genes specific for the respective cell types characterized in this study.
Identification of gene clusters possibly involved in the origin of CIS
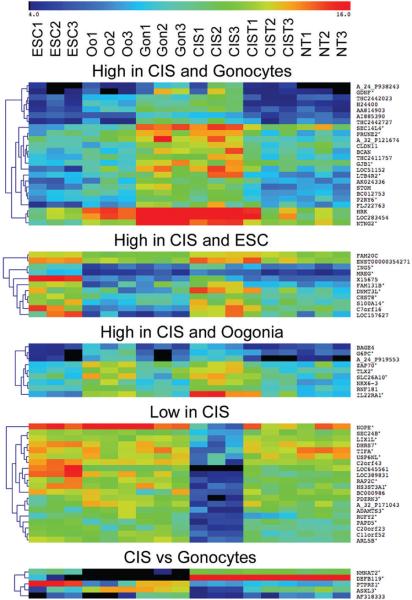
Figure 4 shows biologically interesting clusters that may provide additional knowledge on how CIS arise.
Figure 4. Biologically interesting clusters.
A 2-way SAM of interesting cell combinations. CIST was excluded in all analyses. From the top: CIS + Gonocytes vs. others (delta=1.21, FDR=0.314%), CIS + ESC vs. others (delta=1.24, FDR=0.964%), CIS + Oogonia vs. others (delta=1.33, FDR=0%), CIS vs. others (delta=3.27, FDR=0%) and CIS vs. Gonocytes (delta=3.90, FDR=0%). Annotated genes with confirmed probe specificity are marked by asterisks. The color key at the top shows the relative expression levels from 4 to 16. Abbreviations as in previous figures. Only the most differentially expressed genes are shown in Figure 4, more extensive analyses are available in supplementary Table 3.
When CIS and gonocytes were compared, only five transcripts came out as differentially expressed (FDR<1%). Two genes were upregulated in CIS: DEFB119, encoding an antimicrobial peptide, regulated by androgens and specifically expressed in the testes (31) and NMNAT2 (nicotinamide mononucleotide adenylyl-transferase-2) a central enzyme of the NAD biosynthetic pathway (32). Three genes were downregulated in CIS vs gonocytes: PTPRZ1, a protein tyrosine phosphatase receptor, which has been described in several cancer types (33); the predicted cancer associated gene ASXL3, a human homologue of the Drosophila additional sex combs (asx) gene (34), and one unannotated gene (AF318333).
An interesting finding was a large cluster of genes that were expressed at a lower level in CIS compared to the other germ cell types. This has not previously been described, as it requires pure CIS preparations. These genes may give important clues to the mechanisms of the neoplastic transformation to CIS, since their downregulation may be linked to this process.
To further study the relationships between CIS, fetal gonocytes and fetal oocytes, we performed a three-way SAM and selected the 62 most significant genes for correspondence analysis and partition clustering (Fig. 5). Comparing the three profiles of genes upregulated in CIS, gonocytes and oogonia, respectively, it was obvious that the genes representing the gonocyte and oocyte profiles were expressed at a lower level in all other cell types while the genes in the CIS profile were also highly expressed in normal adult testis and in Sertoli cells. A relatively large cluster contained genes upregulated in CIS and gonocytes, and downregulated in oogonia. Not surprisingly, among the 13 genes in this cluster, six were located on the Y-chromosome, consistent with previous observations (35) that CIS arises only in individuals with some Y chromosome material present.
Figure 5. Correspondence analysis of genes characterizing CIS, gonocytes and oogonia.
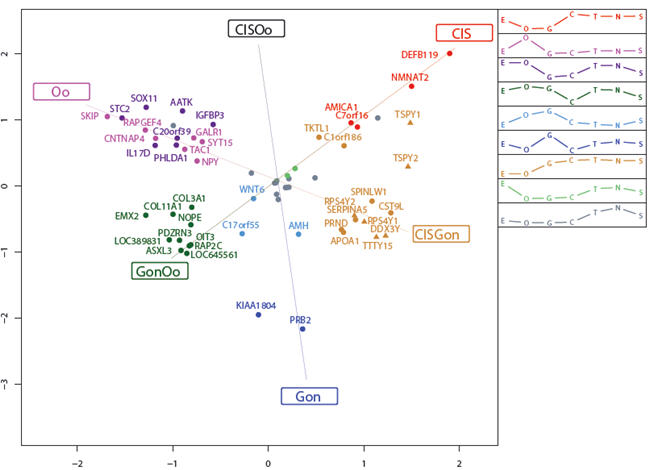
Correspondance analysis for the 62 most significant genes from a three-way significance analysis of microarrays (SAM) on the classes CIS, gonocytes and oogonia. The 62 genes were clustered into nine distinct gene groups (visualized by colouring), and the corresponding mean cluster profiles are displayed as legend. The positions of the cluster names (CIS, CISOo, etc.) represent the ideal profiles for genes expressed only in the particular cell types. Δ, Gene located on the Y chromosome. Abbreviations: E, embryonic stem cells; O, oogonia; G, gonocytes; C, carcinoma in situ; T, testis tissue containing CIS; N, normal testis; S, Sertoli cells next to CIS; CISOo, CIS and oogonia; GonOo, gonocytes and oogonia and CISGon, CIS and gonocytes.
Discussion
With this study we for the first time performed gene expression analysis on isolated CIS and fetal germ cells, allowing direct comparison of gene expression profiles of CIS cells, gonocytes and oogonia. The results were clear: no matter how the data was analyzed CIS cells always grouped with gonocytes. This study supports the proposed fetal origin of CIS, and provides the basis for a more detailed understanding of CIS.
Numerous previous studies of single genes clearly demonstrated that CIS cells in many aspects resemble gonocytes (6; 15; 29; 36-41) and that both CIS and gonocytes express a number of genes normally only seen in embryonic stem cells (23;39;40). This was confirmed in earlier microarray studies where gene expression in whole testes with CIS was compared to normal testes (7;12;13). In the study by Almstrup et al., (7) we analyzed three testis samples with increasing amounts of CIS tubules and sorted the data according to the percentage of CIS cells. Among the 100 genes most highly expressed in CIS compared to normal testis, 34 genes were also reported in ESCs. However, according to the present dataset, CIS cells are much more similar to gonocytes than to ESCs. Although we cannot exclude that the in vitro adaptation of ESCs and contamination with Sertoli cells may add to the difference between CIS and ESCs, we believe that the previously reported similarity between these cells was caused by the study design (7;12;13), which lead to a subtraction of genes expressed in both normal testis and CIS. This resulted in an overrepresentaton of pluripotency genes and thus a more pronounced similarity to ESCs.
By isolating the different cell types, we found that gonocytes and CIS cells were very similar and that both were closely related to oogonia, which have only recently diverged from the gonocytes in the sex differentiation. However, according to the correspondence analysis on genes differentially expressed between CIS, oogonia and gonocytes (Fig. 5), no genes distinguished CIS and oogonia from gonocytes, indicating that CIS do not originate from cells with oogonia characteristics, whereas several genes were highly expressed in CIS and gonocytes, but not in oogonia. Only five genes significantly distinguished CIS from gonocytes (Fig. 4) and interestingly, among these five genes, two cancer-associated genes had low expression levels in CIS. Moreover, among the genes highly expressed in CIS we found no overrepresentation of oncogenes and among the genes with low expression in CIS we found no overrepresentation of tumor suppressor genes. This indicates that CIS is not a malignant cancer cell in a classical sense but rather an arrested gonocyte.
There have been other proposals for the origin of CIS. Clark suggested that CIS may originate from a multipotent spermatogonial stem cell (42). However, human spermatogonia do not express the pluripotency genes characteristic of CIS, and although multipotent stem cells have been derived in vitro from adult human testis (43), the expression profile of these cells does not correspond to the profile of CIS cells. Most noteworthy, the CIS markers POU5F1, NANOG and CDH1 were only expressed at a low level and KLF4 and STAT3, which are expressed at a very low level in CIS according to the present dataset, were highly expressed in the spermatogonial cell population and adult germ line stem cells. Thus, this origin is rather unlikely.
Some CIS cells are hypertriploid (44;45) and this feature was suggested as an argument for their origin from spermatocytes, which duplicate their genome in preparation for meiosis (46). Alternatively, CIS cells could originate from fetal gonocytes that, in analogy to female oogonia, attempted to enter meiosis in fetal life because of insufficiently virilized microenvironment in dysgenetic testis (11). The polyploidization followed by selective gene losses and gains have been proposed by several studies as the earliest abnormality in CIS cells (10;47;48) and has been detected even in pre-CIS, an abnormal germ cell in severely dysgenetic subjects with disorders of sex differentiation (49). However, the hypertriploidy of CIS cells does not need to be related to meiosis, as most other cancers are also aneuploid. Accordingly, we found no meiosis-related genes among the genes specific for CIS. Moreover, when we selected genes highly expressed in CIS and normal adult testis, but low in gonocytes (Supplementary table 4), we did not find any genes specific for meiosis, which strongly suggest that the cell of origin is not a meiotic cell.
Pitfalls in the microdissection technology
Several challenges with the microdissection technology must be taken into consideration. Especially, it is necessary to verify the enrichment of RNA from target cells by RT-PCR before expression profiling. The preliminary verification in this study was encouraging, but also demonstrated that it is impossible to obtain completely pure cell populations by laser microdissection of tissue sections, because inclusion of material from neighbouring cells is unavoidable and cellular material from cells cut open by the microtome will inevitably leak to the surrounding tissue. Nevertheless, both RT-PCR and subsequent microarray analysis showed a substantial enrichment of RNA from target cells and expression profiling clearly showed different profiles for different cell types. We also observed specificity problems with the Agilent oligos. Many probes either matched multiple distinct transcripts or recognized opposite strands or introns in their designated targets. This underlines the importance of checking that the oligos match their targets and it further emphasizes that microarray results should always be verified. However, in this study we do not focus on expression of single genes, instead we compare global expression profiles, which leads to quite robust results that are not affected by uncertainty about the identity of a few genes.
Origin of CIS
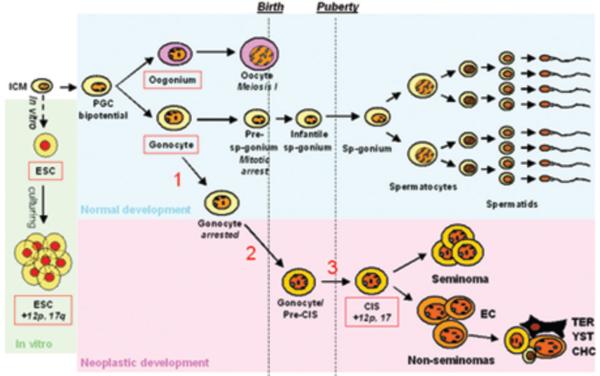
Although this study confirms previous candidate gene-based studies and clearly shows that CIS cells are very similar to gonocytes, it still remains to be determined why these gonocyte-like cells do not differentiate to spermatogonia, but persist in postnatal testes. We know many risk factors for testis cancer and virtually all are related to a poor embryonic and fetal development of the testes. Animal studies suggest that this may be related to reduced testosterone levels causing a maldevelopment of somatic cells of the testis (50). The poor function of the somatic cells affects the germ cells, which fail to differentiate properly without the appropriate paracrine signals from Sertoli and peritubular myoid cells (4). Thus, we suggest that CIS originates from gonocytes that failed to differentiate to pre-spermatogonia, due to the undermasculinized somatic cells that 1) fail to stimulate the germ cells sufficiently and 2) constitute a microenvironment that allow fetal gonocytes to survive in the postnatal testes (Fig. 6). Further investigation of the somatic compartment and its role in the progression from gonocyte to pre-spermatogonium will aid in the understanding of CIS
Figure 6. Schematic illustration of the hypothesised origin of CIS from gonocytes.
Schematic illustration of the normal male germ cell development and possible transformation to CIS. ESCs are derived from the inner cell mass of a blastocyst. Prolonged culturing often leads to an accumulation of chromosomal aberrations, especially gain of material from chromosomes 12p and 17q. During early development, primordial germ cells migrate to the gonadal ridge and develop along the female (oogonia, top) or the male (gonocytes, bottom) germ cell lineage. In the male, the gonocytes become embedded in Sertoli cells, creating testicular cords. During the period from third trimester to 3 months postnatally, the gonocytes migrate to the periphery of the tubules and differentiate to pre-spermatogonia. After puberty, the spermatogonia proliferate and start spermatogenesis. CIS cells are proposed to arise when gonocytes fail to differentiate to pre-spermatogonia (1) and fail to undergo apoptosis (2). These gonocytes or pre-CIS cells lie dormant in the testis through infancy, while genomic aberrations may occur (3), and at puberty when testosterone levels increase, they start to proliferate and genomic aberrations accumulate, especially of chromosome 12 p and 17, eventually resulting in the formation of an overt tumor. Abbreviations: ICM, inner cell mass; ESC, embryonic stem cells; PGC, primordial germ cells; Sp-gonium, spermatogonium; EC, embryonal carcinoma; TER, teratoma; YST, yolk sac tumor; CHC, chorioncarcinoma.
Supplementary Material
Aknowledgments
We would like to thank Sabina Soultanova, Ludmila Ruban, John Nielsen, Anne Jørgensen, Brian Vendelbo Hansen, Betina Nielsen, Muaber Zejnuli, Thomas Regiert, Heiko Drzonek and Ute Wirkner for skillfull technical assistance. This study was performed with financial support from the Danish Cancer Society, The Villum Kann Rasmussen Foundation, Svend Andersens Foundation and Johansens Foundation. The work by Peter Andrews and Neil J. Harrison was funded by the Medical Research Council (MRC) and Yorkshire Cancer Research (YCR).
References
- 1.Rorth M, Rajpert-De Meyts E, Andersson L, Dieckmann KP, Fossa SD, Grigor KM, Hendry WF, Herr HW, Looijenga LH, Oosterhuis JW, Skakkebaek NE. Carcinoma in situ in the testis. Scand J Urol Nephrol Suppl. 2000;(205):166–86. doi: 10.1080/00365590050509896. [DOI] [PubMed] [Google Scholar]
- 2.Skakkebaek NE, Berthelsen JG, Muller J. Carcinoma-in-situ of the undescended testis. Urol Clin North Am. 1982 Oct;9(3):377–85. [PubMed] [Google Scholar]
- 3.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001 May;16(5):972–8. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 4.Sonne SB, Kristensen DM, Novotny GW, Olesen IA, Nielsen JE, Skakkebaek NE, Rajpert-De Meyts E, Leffers H. Testicular dysgenesis syndrome and the origin of carcinoma in situ testis. Int J Androl. 2008 Apr;31(2):275–87. doi: 10.1111/j.1365-2605.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 5.Holstein AF, Korner F. Light and electron microscopical analysis of cell types in human seminoma. Virchows Arch A Pathol Anat Histol. 1974;363(2):97–112. doi: 10.1007/BF01201313. [DOI] [PubMed] [Google Scholar]
- 6.Rajpert-De Meyts E, Bartkova J, Samson M, Hoei-Hansen CE, Frydelund-Larsen L, Bartek J, Skakkebaek NE. The emerging phenotype of the testicular carcinoma in situ germ cell. APMIS. 2003 Jan;111(1):267–78. doi: 10.1034/j.1600-0463.2003.11101301.x. [DOI] [PubMed] [Google Scholar]
- 7.Almstrup K, Hoei-Hansen CE, Wirkner U, Blake J, Schwager C, Ansorge W, Nielsen JE, Skakkebaek NE, Rajpert-De Meyts E, Leffers H. Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res. 2004 Jul 15;64(14):4736–43. doi: 10.1158/0008-5472.CAN-04-0679. [DOI] [PubMed] [Google Scholar]
- 8.Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J, Meisner L, Zwaka TP, Thomson JA, Andrews PW. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004 Jan;22(1):53–4. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- 9.Kraggerud SM, Skotheim RI, Szymanska J, Eknaes M, Fossa SD, Stenwig AE, Peltomaki P, Lothe RA. Genome profiles of familial/bilateral and sporadic testicular germ cell tumors. Genes Chromosomes Cancer. 2002 Jun;34(2):168–74. doi: 10.1002/gcc.10058. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg C, van Gurp RJ, Geelen E, Oosterhuis JW, Looijenga LH. Overrepresentation of the short arm of chromosome 12 is related to invasive growth of human testicular seminomas and nonseminomas. Oncogene. 2000 Nov 30;19(51):5858–62. doi: 10.1038/sj.onc.1203950. [DOI] [PubMed] [Google Scholar]
- 11.Adamah DJ, Gokhale PJ, Eastwood DJ, Rajpert De-Meyts E, Goepel J, Walsh JR, Moore HD, Andrews PW. Dysfunction of the mitotic:meiotic switch as a potential cause of neoplastic conversion of primordial germ cells. Int J Androl. 2006 Feb;29(1):219–27. doi: 10.1111/j.1365-2605.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- 12.Biermann K, Heukamp LC, Steger K, Zhou H, Franke FE, Guetgemann I, Sonnack V, Brehm R, Berg J, Bastian PJ, Muller SC, Wang-Eckert L, et al. Gene expression profiling identifies new biological markers of neoplastic germ cells. Anticancer Res. 2007 Sep;27(5A):3091–100. [PubMed] [Google Scholar]
- 13.Skotheim RI, Lind GE, Monni O, Nesland JM, Abeler VM, Fossa SD, Duale N, Brunborg G, Kallioniemi O, Andrews PW, Lothe RA. Differentiation of human embryonal carcinomas in vitro and in vivo reveals expression profiles relevant to normal development. Cancer Res. 2005 Jul 1;65(13):5588–98. doi: 10.1158/0008-5472.CAN-05-0153. [DOI] [PubMed] [Google Scholar]
- 14.Sonne SB, Dalgaard MD, Nielsen JE, Hoei-Hansen CE, Rajpert-De Meyts E, Gjerdrum LM, Leffers H. Optimizing staining protocols for laser microdissection of specific cell types from the testis including Carcinoma in situ testis. PLoS ONE. 2009;4(5):e5536. doi: 10.1371/journal.pone.0005536. Epub 2009 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoei-Hansen CE, Nielsen JE, Almstrup K, Sonne SB, Graem N, Skakkebaek NE, Leffers H, Rajpert-De Meyts E. Transcription factor AP-2gamma is a developmentally regulated marker of testicular carcinoma in situ and germ cell tumors. Clin Cancer Res. 2004 Dec 15;10(24):8521–30. doi: 10.1158/1078-0432.CCR-04-1285. [DOI] [PubMed] [Google Scholar]
- 16.Jensen CH, Erb K, Westergaard LG, Kliem A, Teisner B. Fetal antigen 1, an EGF multidomain protein in the sex hormone-producing cells of the gonads and the microenvironment of germ cells. Mol Hum Reprod. 1999 Oct;5(10):908–13. doi: 10.1093/molehr/5.10.908. [DOI] [PubMed] [Google Scholar]
- 17.Rajpert-De Meyts E, Jorgensen N, Graem N, Muller J, Cate RL, Skakkebaek NE. Expression of anti-Mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999 Oct;84(10):3836–44. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- 18.Visfeldt J, Cortes D, Thorup JM, Byskov AG. Anti-MIC2 as a tool in examination of testicular biopsies. APMIS. 1999 Jul;107(7):631–5. doi: 10.1111/j.1699-0463.1999.tb01452.x. [DOI] [PubMed] [Google Scholar]
- 19.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003 Feb;34(2):374–8. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 20.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venables WN, Ripley BD. Modern Applied Statistics with S. Fourth ed Springer; 2002. [Google Scholar]
- 22.Nielsen JE, Hansen MA, Jorgensen M, Tanaka M, Almstrup K, Skakkebaek NE, Leffers H. Germ cell differentiation-dependent and stage-specific expression of LANCL1 in rodent testis. Eur J Histochem. 2003;47(3):215–22. doi: 10.4081/830. [DOI] [PubMed] [Google Scholar]
- 23.Rajpert-De Meyts E, Hanstein R, Jorgensen N, Graem N, Vogt PH, Skakkebaek NE. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum Reprod. 2004 Jun;19(6):1338–44. doi: 10.1093/humrep/deh265. [DOI] [PubMed] [Google Scholar]
- 24.Miettinen M, Virtanen I, Talerman A. Intermediate filament proteins in human testis and testicular germ-cell tumors. Am J Pathol. 1985 Sep;120(3):402–10. [PMC free article] [PubMed] [Google Scholar]
- 25.Czernobilsky B, Moll R, Levy R, Franke WW. Co-expression of cytokeratin and vimentin filaments in mesothelial, granulosa and rete ovarii cells of the human ovary. Eur J Cell Biol. 1985 May;37:175–90. [PubMed] [Google Scholar]
- 26.O'Shaughnessy PJ, Baker PJ, Monteiro A, Cassie S, Bhattacharya S, Fowler PA. Developmental changes in human fetal testicular cell numbers and messenger ribonucleic acid levels during the second trimester. J Clin Endocrinol Metab. 2007 Dec;92(12):4792–801. doi: 10.1210/jc.2007-1690. [DOI] [PubMed] [Google Scholar]
- 27.Hersmus R, Kalfa N, de LB, Stoop H, Oosterhuis JW, de KR, Wolffenbuttel KP, Drop SL, Veitia RA, Fellous M, Jaubert F, Looijenga LH. FOXL2 and SOX9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (DSD) J Pathol. 2008 May;215(1):31–8. doi: 10.1002/path.2335. [DOI] [PubMed] [Google Scholar]
- 28.Steger K, Pauls K, Klonisch T, Franke FE, Bergmann M. Expression of protamine-1 and -2 mRNA during human spermiogenesis. Mol Hum Reprod. 2000 Mar;6(3):219–25. doi: 10.1093/molehr/6.3.219. [DOI] [PubMed] [Google Scholar]
- 29.Sonne SB, Herlihy AS, Hoei-Hansen CE, Nielsen JE, Almstrup K, Skakkebaek NE, Marks A, Leffers H, Rajpert-De Meyts E. Identity of M2A (D2-40) antigen and gp36 (Aggrus, T1A-2, podoplanin) in human developing testis, testicular carcinoma in situ and germ-cell tumours. Virchows Arch. 2006 Aug;449(2):200–6. doi: 10.1007/s00428-006-0223-4. [DOI] [PubMed] [Google Scholar]
- 30.Yeung KY, Bumgarner RE. Multiclass classification of microarray data with repeated measurements: application to cancer. Genome Biol. 2003;4(12):R83. doi: 10.1186/gb-2003-4-12-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radhakrishnan Y, Hamil KG, Yenugu S, Young SL, French FS, Hall SH. Identification, characterization, and evolution of a primate beta-defensin gene cluster. Genes Immun. 2005 May;6(3):203–10. doi: 10.1038/sj.gene.6364184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005 Oct 28;280(43):36334–41. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- 33.Goldmann T, Otto F, Vollmer E. A receptor-type protein tyrosine phosphatase PTP zeta is expressed in human cutaneous melanomas. Folia Histochem Cytobiol. 2000;38(1):19–20. [PubMed] [Google Scholar]
- 34.Katoh M, Katoh M. Identification and characterization of ASXL3 gene in silico. Int J Oncol. 2004 Jun;24(6):1617–22. [PubMed] [Google Scholar]
- 35.Cools M, Drop SL, Wolffenbuttel KP, Oosterhuis JW, Looijenga LH. Germ cell tumors in the intersex gonad: old paths, new directions, moving frontiers. Endocr Rev. 2006 Aug;27(5):468–84. doi: 10.1210/er.2006-0005. [DOI] [PubMed] [Google Scholar]
- 36.Giwercman A, Marks A, Bailey D, Baumal R, Skakkebaek NE. A monoclonal antibody as a marker for carcinoma in situ germ cells of the human adult testis. APMIS. 1988 Aug;96(8):667–70. doi: 10.1111/j.1699-0463.1988.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 37.Jacobsen GK, Norgaard-Pedersen B. Placental alkaline phosphatase in testicular germ cell tumours and in carcinoma-in-situ of the testis. An immunohistochemical study. Acta Pathol Microbiol Immunol Scand [A] 1984 Sep;92(5):323–9. doi: 10.1111/j.1699-0463.1984.tb04411.x. [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen N, Rajpert-De Meyts E, Graem N, Muller J, Giwercman A, Skakkebaek NE. Expression of immunohistochemical markers for testicular carcinoma in situ by normal human fetal germ cells. Lab Invest. 1995 Feb;72(2):223–31. [PubMed] [Google Scholar]
- 39.Hoei-Hansen CE, Almstrup K, Nielsen JE, Sonne SB, Graem N, Skakkebaek NE, Leffers H, Rajpert-De Meyts E. Stem cell pluripotency factor NANOG is expressed in human fetal gonocytes, testicular carcinoma in situ and germ cell tumours. Histopathology. 2005 Jul;47(1):48–56. doi: 10.1111/j.1365-2559.2005.02182.x. [DOI] [PubMed] [Google Scholar]
- 40.Looijenga LH, Stoop H, de Leeuw HP, Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, Honecker F, Bokemeyer C, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003 May 1;63(9):2244–50. [PubMed] [Google Scholar]
- 41.Honecker F, Stoop H, de Krijger RR, Chris Lau YF, Bokemeyer C, Looijenga LH. Pathobiological implacations of the expression of markers ef testicular carcinoma in situ by fetal germ cells. J Pathol. 2004;203:849–857. doi: 10.1002/path.1587. [DOI] [PubMed] [Google Scholar]
- 42.Clark AT. The stem cell identity of testicular cancer. Stem Cell Rev. 2007;3(1):49–59. doi: 10.1007/s12015-007-0002-x. [DOI] [PubMed] [Google Scholar]
- 43.Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008 Oct 8; doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 44.Skakkebaek NE. Possible carcinoma-in-situ of the testis. Lancet. 1972 Sep 9;2(7776):516–7. doi: 10.1016/s0140-6736(72)91909-5. [DOI] [PubMed] [Google Scholar]
- 45.Muller J, Skakkeboek NE, Lundsteen C. Aneuploidy as a marker for carcinoma-in-situ of the testis. Acta Pathol Microbiol Scand [A] 1981 Jan;89(1):67–8. doi: 10.1111/j.1699-0463.1981.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 46.Chaganti RS, Houldsworth J. Genetics and biology of adult human male germ cell tumors. Cancer Res. 2000 Mar 15;60(6):1475–82. [PubMed] [Google Scholar]
- 47.Ottesen AM, Skakkebaek NE, Lundsteen C, Leffers H, Larsen J, Rajpert-De Meyts E. High-resolution comparative genomic hybridization detects extra chromosome arm 12p material in most cases of carcinoma in situ adjacent to overt germ cell tumors, but not before the invasive tumor development. Genes Chromosomes Cancer. 2003 Oct;38(2):117–25. doi: 10.1002/gcc.10244. [DOI] [PubMed] [Google Scholar]
- 48.Looijenga LH, Zafarana G, Grygalewicz B, Summersgill B, Debiec-Rychter M, Veltman J, Schoenmakers EF, Rodriguez S, Jafer O, Clark J, van Kessel AG, Shipley J, et al. Role of gain of 12p in germ cell tumour development. APMIS. 2003 Jan;111(1):161–71. doi: 10.1034/j.1600-0463.2003.11101201.x. [DOI] [PubMed] [Google Scholar]
- 49.Chemes H, Muzulin PM, Venara MC, Mulhmann MC, Martinez M, Gamboni M. Early manifestations of testicular dysgenesis in children: pathological phenotypes, karyotype correlations and precursor stages of tumour development. APMIS. 2003 Jan;111(1):12–23. doi: 10.1034/j.1600-0463.2003.1110104.x. [DOI] [PubMed] [Google Scholar]
- 50.Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. 2008 Feb;89(2 Suppl):e33–e38. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.