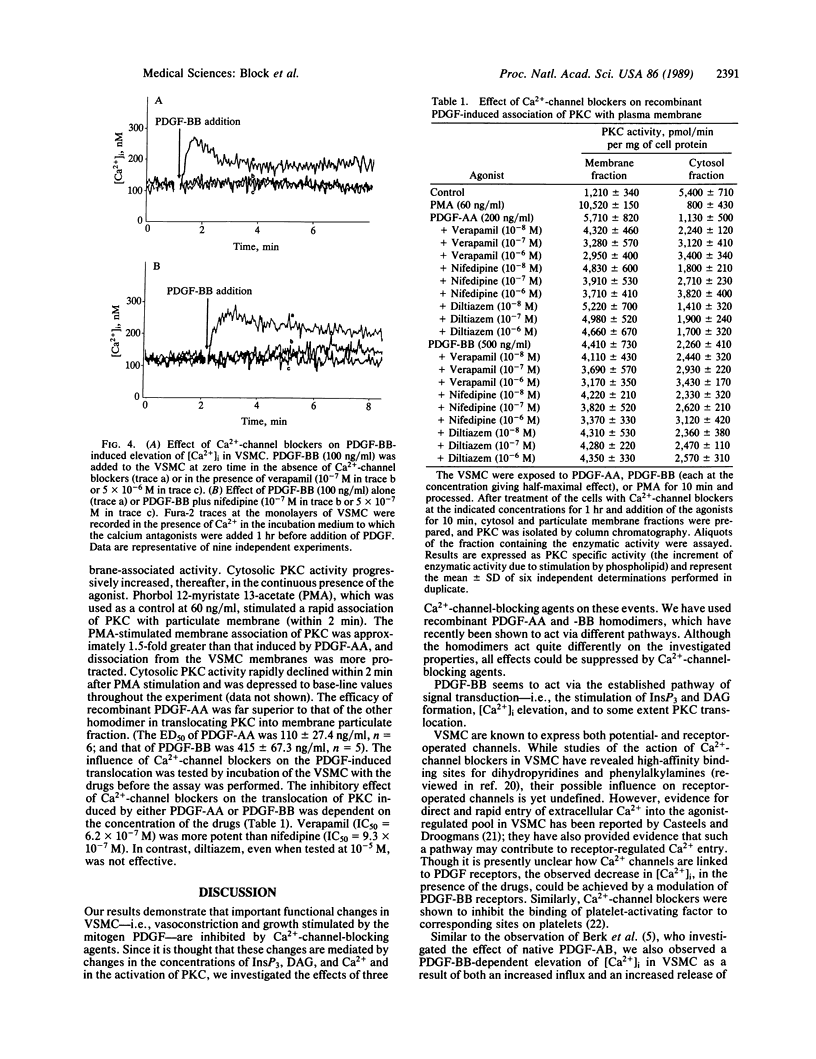
Abstract
Human platelet-derived growth factor (PDGF) is mainly composed of two polypeptide chains (PDGF-AB). All three possible dimeric forms of PDGF--i.e., PDGF-AA, PDGF-BB and PDGF-AB--exist in nature. We have used two recombinant PDGF homodimers to determine the roles of each isoform in the activation of phosphatidylinositol turnover in vascular smooth muscle cells (VSMC) isolated from rat thoracic aorta, their mitogenic effect on VSMC, and their vasoconstrictor effect on intact strips of aortic vascular tissue. Three Ca2+-channel blockers, nifedipine, verapamil, and diltiazem, were used as antagonists for investigating the PDGF-dependent changes mediated by the homodimers. PDGF-BB had a greater efficacy than PDGF-AA on inositol 1,4,5-trisphosphate release, on the formation of diacylglycerol, and on Ca2+ mobilization, which was also associated with vasoconstrictor activity and effective mitogenicity. PDGF-AA, on the other hand, was more potent than PDGF-BB in stimulating protein kinase C. In all instances, the activation of the phosphatidylinositol turnover by the two homodimers was inhibited by the Ca2+-channel blockers.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk B. C., Alexander R. W., Brock T. A., Gimbrone M. A., Jr, Webb R. C. Vasoconstriction: a new activity for platelet-derived growth factor. Science. 1986 Apr 4;232(4746):87–90. doi: 10.1126/science.3485309. [DOI] [PubMed] [Google Scholar]
- Berk B. C., Brock T. A., Webb R. C., Taubman M. B., Atkinson W. J., Gimbrone M. A., Jr, Alexander R. W. Epidermal growth factor, a vascular smooth muscle mitogen, induces rat aortic contraction. J Clin Invest. 1985 Mar;75(3):1083–1086. doi: 10.1172/JCI111772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesterman C. N., Walker T., Grego B., Chamberlain K., Hearn M. T., Morgan F. J. Comparison of platelet-derived growth factor prepared from release products of fresh platelets and from outdated platelet concentrates. Biochem Biophys Res Commun. 1983 Nov 15;116(3):809–816. doi: 10.1016/s0006-291x(83)80214-9. [DOI] [PubMed] [Google Scholar]
- Etingin O. R., Hajjar D. P. Nifedipine increases cholesteryl ester hydrolytic activity in lipid-laden rabbit arterial smooth muscle cells. A possible mechanism for its antiatherogenic effect. J Clin Invest. 1985 May;75(5):1554–1558. doi: 10.1172/JCI111860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. L., Anderson W. B. Interleukin-2 stimulates association of protein kinase C with plasma membrane. Nature. 1985 May 16;315(6016):233–235. doi: 10.1038/315233a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Platelet-derived growth factor. Mol Cell Endocrinol. 1985 Mar;39(3):169–187. doi: 10.1016/0303-7207(85)90061-9. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Benditt E. P., Schwartz S. M. Expression and developmental control of platelet-derived growth factor A-chain and B-chain/Sis genes in rat aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1524–1528. doi: 10.1073/pnas.85.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgaroli A., Milani D., Meldolesi J., Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987 Nov;105(5):2145–2155. doi: 10.1083/jcb.105.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T., Takai Y., Minakuchi R., Yu B., Nishizuka Y. Inhibitory action of chlorpromazine, dibucaine, and other phospholipid-interacting drugs on calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1980 Sep 25;255(18):8378–8380. [PubMed] [Google Scholar]
- Nilsson J., Sjölund M., Palmberg L., Von Euler A. M., Jonzon B., Thyberg J. The calcium antagonist nifedipine inhibits arterial smooth muscle cell proliferation. Atherosclerosis. 1985 Dec;58(1-3):109–122. doi: 10.1016/0021-9150(85)90059-0. [DOI] [PubMed] [Google Scholar]
- Nistér M., Hammacher A., Mellström K., Siegbahn A., Rönnstrand L., Westermark B., Heldin C. H. A glioma-derived PDGF A chain homodimer has different functional activities from a PDGF AB heterodimer purified from human platelets. Cell. 1988 Mar 25;52(6):791–799. doi: 10.1016/0092-8674(88)90421-7. [DOI] [PubMed] [Google Scholar]
- Oeken H. J., von Nettelbladt E., Zimmer M., Flockerzi V., Ruth P., Hofmann F. Cardiac sarcoplasmic reticulum contains a low-affinity site for phenylalkylamines. Eur J Biochem. 1986 May 2;156(3):661–667. doi: 10.1111/j.1432-1033.1986.tb09629.x. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Triggle D. J., Janis R. A. Calcium channel ligands. Annu Rev Pharmacol Toxicol. 1987;27:347–369. doi: 10.1146/annurev.pa.27.040187.002023. [DOI] [PubMed] [Google Scholar]
- Urthaler F. Role of calcium channel blockers in clinical medicine. Am J Med Sci. 1986 Oct;292(4):217–230. doi: 10.1097/00000441-198610000-00007. [DOI] [PubMed] [Google Scholar]
- Valone F. H. Inhibition of platelet-activating factor binding to human platelets by calcium channel blockers. Thromb Res. 1987 Mar 1;45(5):427–435. doi: 10.1016/0049-3848(87)90306-9. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Monk P. N., Consalvey S. D., Huang S. J., Dexter T. M., Downes C. P. Interleukin 3 stimulates proliferation via protein kinase C activation without increasing inositol lipid turnover. Proc Natl Acad Sci U S A. 1988 May;85(10):3284–3288. doi: 10.1073/pnas.85.10.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]