Abstract
The monosaccharide moieties found in heparin (HP) and heparan sulfate (HS), glucosamine and two kinds of uronic acids, glucuronic and iduronic acids, were efficiently synthesized by use of glucosamine hydrochloride and glucurono-6,3-lactone as starting compounds. In the synthesis of the disaccharide building block, the key issues of preparation of uronic acids (glucuronic acid and iduronic acid moieties) were achieved in 12 steps and 15 steps, respectively, without cumbersome C-6 oxidation. The resulting monosaccharide moieties were utilized to the syntheses of HP/HS disaccharide building blocks possessing glucosamine-glucuronic acid (GlcN-GlcA) or iduronic acid (GlcN-IdoA) sequences. The disaccharide building blocks were also suitable for further modification such as glycosylation, selective deprotection, and sulfation.
Keywords: synthesis, uronic acid, glucuronic acid, iduronic acid, glucosamine, heparin, heparan sulfate
1. Introduction
Heparin (HP) and Heparan sulfate (HS) are highly sulfated polysaccharides, and are the most complex carbohydrates among the glycosaminoglycan (GAG) superfamily.1 HP/HS are basically composed of a repeating α or β(1,4)-linked disaccharide unit which is derived from uronic acid, either glucuronic acid or iduronic acid, and N-acetyl-glucosamine residues. The structure is very heterogeneous and contains various substitution patterns derived from the multiple and random enzymatic modifications in their biosynthesis. The diverse micro- or domain structure, which is derived from the enzymatic modification, is considered to regulate the activity of many important biological proteins, such as growth factors, cytokines, viral proteins, and coagulation factors, through their binding interactions in many biological processes.1,2 The elucidation of the structure-function relations of HP/HS microstructures at the molecular level is, however, very difficult due to their naturally occurring structural diversity. Therefore, structurally defined HP/HS sequences (oligosaccharides) are essential for the precise understanding of the interactions of HP/HS with their target molecules. Many synthetic approaches to the synthesis of HP/HS oligosaccharides have been so far performed.3-7
Recently, a synthetic strategy for the assembly of a HP/HS oligosaccharide library has attracted much attention, because it offers a more comprehensive evaluation of HP/HS biological functions.5,6a For this accomplishment, the efficient synthesis of uronic acid moieties and disaccharide building blocks accessible to HP/HS oligosaccharides with various sugar and sulfation patterns is required. As neither idose nor iduronic acid derivatives are commercially available, their syntheses are especially important. Currently, iduronic acid moieties are synthesized from glucose or glucuronic acid as a starting material, involving C-5 epimerization and/or C-6 oxidation.7 But C-5 Epimerization sometimes needs to be carried out under harsh conditions, such as’ strong basic conditions, and the range of usable protective groups is restricted. C-6 Oxidation is also sometimes problematic in yield and repeatability. So far, many intermediates suitable for the structure of HP/HS have been developed as HP/HS building blocks.3-7
Previously, we reported the synthesis and analysis of a variety of HP/HS oligosaccharides for investigating their binding properties6, in which we clarified that an appropriate HP/HS disaccharide structure was needed for the specific binding between sugar component and protein. Although further investigation of HP/HS binding properties was needed, our previous synthetic route was not suitable for the generation of a sufficiently diverse sugar and sulfation pattern. In this study, we synthesized novel disaccharide building blocks for the systematic analysis of HP/HS binding properties.
2. Results and discussion
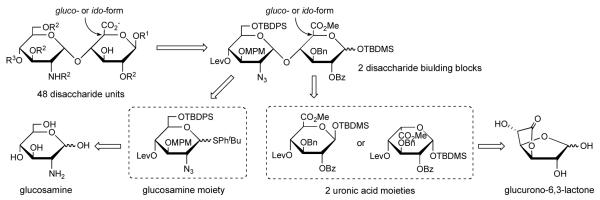
Our synthetic strategy is shown in Figure 1. The two disaccharide building blocks containing gluco- and ido-type are prepared from two common monosaccharides, d-glucosamine and d-glucurono-6,3-lactone. They contain a diverse set of protective groups for generating various sulfation patterns. O-Sulfation is achieved by selective removal of benzoyl (Bz), 4-methoxybenzyl (MPM), and/or tert-butyldiphenylsilyl (TBDPS) groups. Conversion of the azido group to amine, N-sulfate, and/or N-acetate is achieved by reduction of azido group followed by N-sulfation and/or N-acetylation. A glucosamine moiety containing different protective groups is easily prepared from glucosamine. A levulinyl (Lev) group at 4-position is introduced for the further elongation of the sugar chain. Two uronic acid moieties are prepared from glucurono-6,3-lactone, which is already oxidized at the C-6 position. The synthetic route is expected to be simple because a cumbersome oxidation step can be omitted. Conversion to ido-form is achieved by the inversion reaction at C-5 position on furanose form.
Fig. 1.
Synthetic route of heparin/heparan sulfate disaccharide structures.
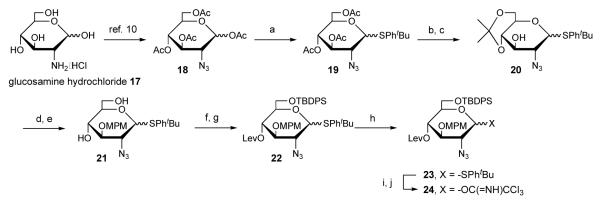
Syntheses of uronic acid moieties were carried out as shown in Scheme 1. Firstly, inexpensive glucurono-6,3-lactone 1 was transformed to 1,2-isopropylidene 2 according to the method reported previously.7m Obtained 2 was then converted to furanose 3 in 43% overall yield in 4 steps through simple protection and deprotection processes of hydroxy groups. The removal of the isopropylidene group on furanose 3 by treatment with trifluoroacetic acid (TFA) gave triol 4. Conversion to pyranose form by 1,2-selective protection was then examined. First, 1,2-cyclic acetal protection to obtain pyranose form has was used.4h,7a Unfortunately, the resulting uronic acid derivatives were a mixture of pyranose and furanose forms under the conditions of acetal formation, suggesting a lower yield of the desired pyranose form under the thermodynamic control. Thus, 1,1,3,3-tetraisopropyldisiloxanylidene (TIPDS) as a 1,2-selective protection group was chosen to yield the kinetically controlled product. 1,2-Selective cyclic reaction with 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane (TIPDSCl2) was performed under the standard conditions8 using imidazole as a base in DMF to afford pyranose form 5 in 85% yield with high pyranose-selectivity9 (α:β = 5:3). Levulinylation of pyranose 5 gave 4-O-Lev 6, which was subsequently treated with tetra-n-butylammonium fluoride (TBAF). The resultant was subjected to silylation with a TBDMS group at the 1-position, benzoylation at the 2-position, and delevulinylation to afford the glucuronic acid moiety 8 as a glycosyl acceptor. Meanwhile, ido-franose 10 was prepared by a general SN2 reaction of the triflate of furanose 3 with LevONa. After removal of the Lev group, the iduronic acid moiety 10 was treated with TFA to afford triol 11. The reaction of triol 11 with TIDPSCl2 and imidazole efficiently proceeded when CH3CN was used as a solvent at −45 °C, providing the pyranose form 12 in 78% with β-selectively. After levulinylation of 12 with LevOH, DCC, and TEA, iduronate 16 was then synthesized as a similar transformation to the sythesis of the glucuronic acid moiety 8.
Scheme 1.
Syntheses of uronic acid moieties. (a) H2SO4 in acetone, 84%; (b) TBDMSCl, imidazole in CH2Cl2; (c) 1.0 M MeONa in MeOH (d) BnOC(=NH)CCl3, TBDMSOTf, MS4Ap in CH2Cl2, 0 °C; (e) TBAF, AcOH in THF, 43% (4 steps); (f) TFA /H2O (9:1) (83%); (g) TIPDSCl2, imidazole in DMF, 85%; (h) LevOH, EDC•HCl, DMAP in CH2Cl2, 84%; (i) TBAF, AcOH in THF; (j) TBDMSCl, imidazole, MS4Ap in CH2Cl2; (k) BzCl in pyridine, 78% (3 steps); (l) H2NNH2•H2O in pyridine/AcOH (3/2), 83%; (m) Tf2O, pyridine in CH2Cl2; (n) LevONa in DMF; (o) H2NNH2•H2O in pyridine/AcOH (3/2), 56% (3 steps); (p) TFA/H2O (9:1), 95%; (q) TIPDSCl2, imidazole in CH3CN, 78%; (r) LevOH, EDC•HCl, DMAP, TEA in DMF, 68%; (s) TBAF, AcOH in THF, 90%; (t) TBDMSCl, imidazole, MS4Ap in CH2Cl2; (u) BzCl, DMAP in pyridine, 65% (2 steps); (v) H2NNH2•H2O in pyridine/AcOH (3/2), 89%.
Synthesis of the glucosamine donor 23 and 24 was carried out as shown in Scheme 2. Azido glucose 18, which was prepared according to the method reported previously10, was converted to thioglycoside 19. After removal of the acetyl group of thioglycoside 19, hydroxyl groups at 4- and 6-posotions were selectively protected with isopropylidene group. The remaining hydroxyl group was then protected with MPM group. The resultant was treated with AcOH to produce diol 21. Selectively silylation of diol 21 at 6-position with TBDPS group followed by levulinylation at 4-position gave thioglycoside donor 23. Imidate donor 24 was also prepared in the usual manner.
Scheme 2.
Synthesis of glucosamine moiety. (a) TBDMSOTf, MS AW300, HSPhtBu in CH2Cl2, 73%; (b) 1.0 M MeONa in MeOH; (c) 2-methoxypropene, CSA in acetone, 74% (2 steps); (d) MPMCl, NaH in DMF; (e) AcOH/CH2Cl2/H2O (8:1:1), 75% (2 steps); (f) TBDPSCl, imidazole in DMF; (g) LevOH, EDC•HCl, DMAP in DMF, 89% (2 steps); (h) NCS in acetone/H2O (5:2), 73%; (i) CCl3CN, Cs2CO3 in CH2Cl2.
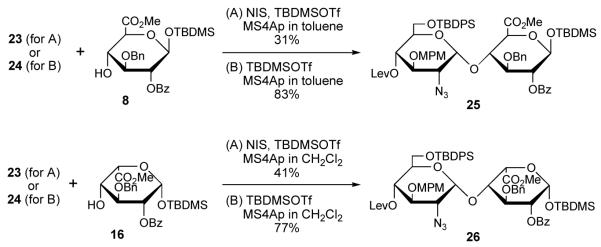
Two kinds of disaccharide building blocks were then synthesized from the prepared monosaccharide moieties (Scheme 3). Glycosylation of the thioglycoside donor 23 and glucuronate 8 was carried out by use of N-iodosuccinimide (NIS) in the presence of TBDMSOTf11 to give disaccharide 25 only in 31% yield but with high α-selectivity. Thioglycoside 23 was ineffective on the glycosylation with iduronate 16. In contrast, glycosyl imidate 24 was found to be an efficient donor in glycosylation both with glucuronate 8 and iduronate 16. These reactions proceeded smoothly to produce disaccharide 25 and 26 in 83% and 77% yields with high α-selectivity, respectively.
Scheme 3.
Synthesis of disaccharide building blocks 25 and 26. Condition (A) and (B) were used for glycosylation with thioglycoside 23 and glycosyl imidate 24, respectively.
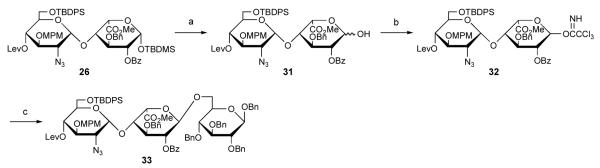
Next, we examined whether the disaccharide building blocks could apply to further modifications like sugar-chain elongation, selective deprotection, sulfation, and final deprotection, or not. One of our goals is the assembly of an HP/HS disaccharide partial structure library, in order to determine the structure-function relations of their interactions with HP/HS binding molecules using Sugar Chips. In our Sugar Chips system, a glucose moiety spacer is often used at the reducing terminal. To prepare the oligosaccharides for use in this system, we carried out syntheses of trisaccharides containing GlcN-UroA-Glc (Scheme 4) and GlcN-IdoA-Glc sequence (Scheme 5). The disaccharide building block 25 was first converted to glycosyl donor 28 via selective desilylation at 1-position followed by formation of imidate. Glycosylation of spacer 29 with obtained 28 proceeded smoothly to give trisaccharide 30 in satisfactory yield.
Scheme 4.
Synthesis of trisaccharide 30. (a) TBAF, AcOH in THF, 78%; (b) CCl3CN, Cs2CO3 in CH2Cl2; (c) TMSOTf, MS4Ap in CH2Cl2, 68% (2 steps).
Scheme 5.
Synthesis of trisaccharide 33. (a) HF•pyridine in pyridine, 73%; (b) CCl3CN, Cs2CO3 in CH2Cl2; (c) 29, TMSOTf, MS4Ap in CH2Cl2, 62% (2 steps).
Meanwhile, the disaccharide building block 26 was selectively converted to 1-OH form 31 by treatment of HF•pyridine, although there was no selectivity by treatment of TBAF. Trisaccharide 33 was prepared in a manner similar to that of trisaccharide 30.
Selective sulfation and final deprotection were examined on trisaccharide 30. The Lev group of trisaccharide 30 was selectively deprotected by hydrazine. The occurring hydroxyl group was masked with a methyl group as in our previous study6a. After removal of the TBDPS group in compound 34, the Bz group and methyl ester were hydrolyzed with NaOH. The azide group was then reduced with PMe3 and the resulting amino group was N-sulfated. Finally, all benzylic protective groups were removed by hydrogenolysis using catalytic Pd/C to give the desired trisaccharide 38.
In conclusion, we have designed novel disaccharide building blocks for HP/HS oligosaccharide precursors, which possess orthogonally cleavable protective groups and are capable of generating diverse sulfation patterns. Two disaccharide building blocks were efficiently synthesized using appropriate monosaccharide moieties. Syntheses of uronic acid moieties, which are key issues in the synthesis of HP/HS oligosaccharide, were efficiently prepared using inexpensive glucurono-6,3-lactone as a starting compound avoiding annoying oxidation processes. Selective pyaranose formation was achieved using TIDPS group as protecting group at 1,2-position. Glucuronate 8 and iduronate 16 were synthesized in 14% (12 steps) and 5% (15 steps) overall yield from glucurono-6,3-lactone, respectively. In addition, we demonstrated the synthesis of sulfated trisaccharide 38, which was achieved by selective deprotection and sulfation. Although further work will be needed to achieve such goals as sugar-chain elongation, sulfation at other positions, and deprotection, is required, various HP/HS oligosaccharides can be prepared by use of these designed disaccharide building blocks with minimal derivatization.
Experimental
General
1H- and 13C-NMR spectra were measured with a JEOL JMM-ECA600KS spectrometer. The chemical shifts in CDCl3 are given in δ values from tetramethylsilane as an internal standard. For measurement in D2O, the DHO signal (4.65 ppm) was used as a reference. Mass spectra were obtained on a micrOTOF II™ (Bruker Daltonics). Silica-gel column chromatography was carried out using silica gel 60B (Fuji Silysia, 40-63 μm) at medium pressure (2-4 kg/cm2). Precoated Kieselgel 60 F254 (Merck) was used for thin layer chromatography (TLC). Sephadex G25 fine was used for gel-filtration chromatography. All chemicals were commercial grade (Nacalai, Wako, TCI, Kanto, and Aldrich). MS4A powder was activated by heating at 350 °C in vacuo for 3 h before use.
1,2-O-isopropylidene-α-d-glucurono-6,3-lactone (2)
d-Glucurono-6,3-lactone 1 (40.0 g, 0.227 mol) was suspended in acetone (835 ml). H2SO4 (12.1 ml, 0.227 mol) was then added at room temperature. After being stirred for 38 h, the reaction mixture was neutralized with NaHCO3. After removal of insoluble materials by filtration, the filtrate was concentrated in vacuo. The residue was dissolved in AcOEt, and the resulting mixture was then washed with brine, dried over Na2SO4, filtered, and concentrated in vacuo. Crystallization from AcOEt and hexane afforded isopropylidene 2 as a white solid: Yield 41.2 g (84%). 1H-NMR (600 MHz, CDCl3) δ 6.00 (1H, d, J1,2 = 3.4 Hz, H-1), 4.96 (1H, dd, J4,3 = 2.7 Hz, J4,5 = 4.8 Hz, H-4), 4.84-4.83 (2H, m, H-2, H-3), 4.51 (1H, d, J5,4 = 4.8 Hz, H-5), 2.86 (1H, s, 5-OH), 1.53 (3H, s, CH3), 1.36 (3H, s, CH3); 13C-NMR (150 MHz, CDCl3), δ 174.7, 113.6, 106.6, 82.9, 81.2, 77.9, 70.6, 26.9, 26.5; HRMS (positive mode); Found: m/z 239.0536 [M+Na]+, Calcd. for C9H12O6Na: 239.0532.
Methyl 3-O-benzyl-1,2-O-isopropylidene-α-d-glucofuranosyluronate (3)
To a solution of compound 2 (56.9 g, 0.263 mol) in CH2Cl2 (400 ml) were added imidazole (35.8 g, 0.526 mol) and TBDMSCl (47.6 g, 0.316 mol) at room temperature. After being stirred for 7.5 h, the reaction was quenched with MeOH. The resulting mixture was washed with 1 M HCl aq., saturated NaHCO3 aq. and brine. The organic layer was dried over Na2SO4, filtered, and concentrated in vacuo. The residue was then dissolved in MeOH (500 ml) and 1 M NaOMe in MeOH (39.4 ml, 39 4 mmol) was added at 0 °C. After being stirred for 3.5 h, the reaction mixture was neutralized with 1 M HCl aq. and AcOEt was added. The organic layer was washed with 1 M HCl aq. (2 ×), water and brine, dried over Na2SO4, filtered, and concentrated in vacuo to give crude methyl 4-O-tert-butyldimethylsilyl-1,2-O-isopropylidene-α-d-glucofuranosyluronate. The crude product, benzylimidate (132 ml, 0.526 mol), and MS4A powder (50 g) was placed in 1000 mL round-bottom flask, and anhydrous CH2Cl2 (500 ml) were added at room temperature under Ar. After being stirred for 1 h, the reaction mixture was cooled to −30 °C and TBDMSOTf (7.86 ml, 34.2 mmol) was added at the same temperature. The reaction mixture was further stirred and gradually warmed to 0 °C. After 15 h, the reaction was quenched by the addition of EtOH. The resulting mixture was filtered through a celite pad and the filtrate was concentrated in vacuo. The residue was dissolved in CH2Cl2 and the mixture was washed with water and brine. The organic layer was dried over Na2SO4, filtered, and concentrated in vacuo to give crude methyl 3-O-benzyl-4-O-tert-butyldimethylsilyl-1,2-O-isopropylidene-α-d-glucofuranosylurona te. The crude product was then dissolved in THF (300 ml) and AcOH (15 ml). The mixture was cooled to −20 °C and 1 M TBAF in THF (789 ml, 0.789 mol) was added to the mixture at the same temperature. After being stirred for 3 h, the mixture was concentrated in vacuo and diluted in AcOEt. The organic layer was washed with brine (3 ×), dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 450 g, hexane:AcOEt = 6:1→2:1) afforded compound 3 as a brown oil: Yield 38.1 g (43%, 4 steps). 1H-NMR (600 MHz, CDCl3) δ 7.38-7.31 (5H, m, aromatic), 6.04 (1H, d, J1,2 = 4.1 Hz, H-1), 4.68 and 4.56 (each 1H, d, Jgem = 11.0 Hz, PhCH2), 4.64 (1H, d, J2,1 = 4.1 Hz, H-2), 4.59 (1H, dd, J5,4 = 6.1 Hz, J5,OH = 8.9 Hz, H-5), 4.42 (1H, dd, J4,3 = 3.4 Hz, J4.5 = 6.1 Hz, H-4), 4.16 (1H, d, J3.4 = 3.4 Hz, H-3), 3.76 (3H, s, COOCH3), 3.40 (1H, d, JOH,5 = 8.9 Hz, 5-OH), 1.50 (3H, s, CH3), 1.36 (3H, s, CH3); 13C-NMR (150 MHz, CDCl3), δ 173.0, 136.5, 128.5, 128.4, 128.2, 128.2, 128.0, 112.1, 105.3, 83.1, 82.1, 79.6, 72.5, 69.8, 52.6, 26.8, 26.3; HRMS (positive mode); Found: m/z 361.1268 [M+Na]+, Calcd. for C17H22O7Na: 361.1263.
Methyl 3-O-benzyl-d-glucopyranosyluronate (4)
Compound 3 (57.0 mg, 0.168 mmol) was dissolved in TFA-H2O (9:1, 1.0 ml) and stirred for 1.5 h at room temperature. After removal of volatiles, the toluene was added and azeotropically evaporated twice. Flash chromatography (silica gel: 2.5 g, toluene:acetone = 3:1) afforded compound 4 as a brown solid: Yield 41.7 mg (83%, α:β = 5:2). 1H-NMR (600 MHz, CDCl3) δ for α-anomer 7.43-7.35 (5H, m, aromatic), 5.43 (1H, s, H-1), 4.86 and 4.75 (each 1H, d, Jgem = 11.5 Hz, PhCH2), 4.49 (1H, d, J5,4 = 7.5, H-5), 4.00 (1H, dd, J4,3 = 6.8 Hz, J4,5 = 7.5 Hz, H-4), 3.89-3.73 (5H, m, H-2, H-3, COOCH3); 13C-NMR (150 MHz, CDCl3), δ for α-anomer 170.7, 137.9, 128.5, 127.9, 127.9, 91.4, 78.7, 74.2, 72.4, 70.5, 70.3, 52.5; HRMS (positive mode); Found: m/z 361.0960 [M+Na]+, Calcd. for C17H22O7Na: 321.0950.
Methyl 3-O-benzyl-1,2-O-(1,1,3,3-tetraisopropyldisiloxanylidene)-d-glucopyranosyluronate (5)
To a suspension of compound 4 (136 mg, 0.455 mmol), TIPDSCl2 (218 μl, 0.683 mmol), and MS4AP (500 mg) in anhydrous DMF (4.5 ml) was added imidazole (124 mg, 1.82 mmol) at room temperature under Ar. After being stirred for 19 h, the reaction was quenched by the addition of MeOH. The resulting mixture was diluted with AcOEt. The organic layer was washed with water, and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 450 g, hexane:EtOAc = 8:1→4:1) afforded compound 5 as a colorless oil: Yield 211 mg (85%, α:β = 5:3). 1H-NMR (600 MHz, CDCl3) δ for α-anomer 7.37-7.23 (5H, m, aromatic), 5.58 (1H, s, H-1), 4.88 and 4.72 (each 1H, d, Jgem = 11.5 Hz, PhCH2), 4.50 (1H, d, J5,4 = 8.2 Hz, H-5), 4.06 (1H, d, J2,3 = 8.2 Hz, H-2), 3.87 (1H, dd, J4,3 = 8.2 Hz, J4,5 = 8.2 Hz, H-4), 3.77 (3H, s, COOCH3), 3.76 (1H, dd, J3,2 = 8.2 Hz, J3,4 = 8.2 Hz, H-3), 2.70 (1H, s, 4-OH), 1.11-1.03 (28H, m, -Si(iPr)2- × 2); 13C-NMR (150 MHz, CDCl3), δ for α-anomer 170.4, 128.4, 128.1, 128.0, 127.8, 127.8, 127.8, 92.7, 80.3, 75.1, 74.7, 74.5, 72.3, 52.5, 17.4, 17.3, 17.2, 17.2, 17.2, 17.1, 17.0, 17.0, 13.1, 13.1, 12.8, 12.8; HRMS (positive mode); Found: m/z 563.2477 [M+Na]+, Calcd. for C26H44O8Si2Na: 563.2472.
Methyl 3-O-benzyl-4-O-levulinyl-1,2-O-(1,1,3,3-tetraisopropyldisiloxanylidene)-d-glucopyranosyluronate (6)
Compound 5 (21.4 g, 39.5 mmol) was dissolved in CH2Cl2 (200 ml) under Ar. LevOH (6.08 ml, 59.4 mmol), EDC•HCl (9.21 g, 59.4 mmol), and DMAP (7.25 g, 59.4 mmol) were then added at room temperature. After being stirred for 8.5 h, the reaction was quenched by the addition of water. The organic layer was washed with sat. NaHCO3 aq., 1 M HCl aq., and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 450 g, hexane:EtOAc = 8:1→2:1) afforded compound 6 as a colorless oil: Yield 21.1 g (84%, α:β = 5:3). 1H-NMR (600 MHz, CDCl3) δ for a-anomer) 7.35-7.27 (5H, m, aromatic), 5.58 (1H, d, J1,2 = 3.4 Hz, H-1), 5.05 (1H, dd, J4,3 = 9.5 Hz, J4,5 = 10.1 Hz, H-4), 4.89 and 4.68 (each 1H, d, Jgem = 11.5 Hz, PhCH2), 4.47 (1H, d, J5,4 = 10.1 Hz, H-5), 4.14 (1H, dd, J2,1 = 3.4 Hz, J2,3 = 8.8 Hz, H-2), 3.84 (1H, dd, J3,2 = 8.8 Hz, J3,4 = 9.5 Hz, H-3), 3.72 (3H, s, COOCH3), 2.66-2.58 (2H, m, CH3C(=O)C2H4C(=O)-), 2.49-2.31 (2H, m, CH3C(=O) C2H4C(=O)-), 2.15 (3H, s, CH3C(=O)C2H4C(=O)-), 1.17-0.91 (28H, m, -Si(iPr)2- × 2); 13C-NMR (150 MHz, CDCl3), δ for α-anomer 206.0, 171.6, 167.7, 138.4, 128.2, 128.2, 128.0, 127.7, 127.5, 92.7, 79.3, 76.1, 75.4, 74.9, 68.9, 52.8, 37.6, 29.8, 27.6, 17.4, 17.3, 17.2, 17.2, 17.0, 17.0, 17.0, 16.9, 14.3, 12.7, 12.7, 12.2; HRMS (positive mode); Found: m/z 661.2837 [M+Na]+, Calcd. for C26H44O8Si2Na: 661.2840.
Methyl (tert-butyldimethylsilyl 2-O-benzoyl-3-O-benzyl-4-O-levulinyl-β-d-glucopyranosyl)uronate (7)
Compound 6 (2.02 g, 3.16 mmol) was dissolved in THF (10 ml) and AcOH (181 μl, 3.16 mmol) was added at room temperature. A solution of TBAF in THF (6.32 ml, 1 M, 6.32 mmol) was then added. The reaction mixture was stirred for 2 h and diluted with CHCl3. The organic layer was washed with H2O, sat. NaHCO3 aq., and brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was then dissolved in CH2Cl2 (10 ml) and TBDMSCl (715 ml, 4.74 mmol) and MS4AP (1.0 g) were added at room temperature under Ar. After 1 h, imidazole (645 mg, 9.48 mmol) was then added. The reaction mixture was stirred for 19 h at −25 °C and gradually warmed to 0 °C and further stirred for 2 h. The reaction was quenched by the addition of MeOH and filtered through a celite pad. The filtrate was washed with H2O, and brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was then dissolved in pyridine (10 ml) and BzCl (550 μl, 4.74 mmol) was added at room temperature under Ar. After 13 h, the reaction was quenched by the addition of MeOH and the resulting mixture was diluted with AcOEt. The organic layer was washed with 1 M HCl aq., sat. NaHCO3 aq., and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 100 g, toluene:EtOAc = 10:1→6:1) afforded compound 7 as a white solid: Yield 1.51 g (78%, 3 steps). 1H-NMR (600 MHz, CDCl3) δ 7.99 (2H, d, J = 8.1 Hz, Bz) 7.99-7.12 (8H, m, aromatic), 5.33 (1H, dd, J4,3 = 9.5 Hz, J4,5 = 9.5 Hz, H-4) 5.31 (1H, dd, J2,1 = 7.4 Hz, J2,3 = 9.5 Hz, H-2), 4.84 (1H, d, J1,2 = 7.4 Hz, H-1), 4.65 and 4.61 (each 1H, d, Jgem = 11.5 Hz, PhCH2), 4.01 (1H, d, J5,4 = 9.5 Hz, H-5), 3.89 (1H, dd, J3,2 = 9.5 Hz, J3,4 = 9.5 Hz, H-3), 3.75 (3H, s, COOCH3), 2.71-2.66 (2H, m, CH3C(=O)C2H4C(=O)-), 2.59-2.44 (2H, m, CH3C(=O)C2H4C(=O)-), 2.17 (3H, s, CH3C(=O)C2H4C(=O)-), 0.74 (9H, s, C(CH3)3), 0.09 (3H, s, CH3), 0.00 (3H, s, CH3); 13C-NMR (150 MHz, CDCl3), δ 206.1, 171.3, 167.6, 164.7, 137.5, 133.1, 129.7, 129.6, 128.3, 128.2, 128.1, 127.9, 127.6, 96.0, 78.8, 74.5, 73.6, 72.7, 71.3, 52.8, 37.6, 29.8, 29.8, 27.6, 25.2, −4.3, −5.5; HRMS (positive mode); Found: m/z 637.2445 [M+Na]+, Calcd. for C32H42O10SiNa: 637.2445.
Methyl (tert-butyldimethylsilyl 2-O-benzoyl-3-O-benzyl-β-d-glucopyranosyl)uronate (8)
Compound 7 (2.60 g, 4.23 mmol) was dissolved in pyridine-AcOH (3:2, 10 ml) and hydrazine monohydrate (308 μl, 6.34 mmol) was added at room temperature. After 1.5 h, the reaction was quenched by the addition of acetone and the resulting mixture was diluted with AcOEt. The organic layer was washed with 1 M HCl aq., sat. NaHCO3, aq., and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 100 g, hexane:EtOAc = 3:1) afforded compound 8 as a white solid: Yield 1.80 g (83%). 1H-NMR (600 MHz, CDCl3) δ 7.99 (2H, d, J = 7.4 Hz, Bz) 7.57-7.14 (8H, m, aromatic), 5.22 (1H, dd, J2,1 = 7.4 Hz, J2,3 = 9.5 Hz, H-2), 4.81 (1H, d, J1,2 = 7.4 Hz, H-1), 4.79 and 4.75 (each 1H, d, Jgem = 11.5 Hz, PhCH2), 4.09 (1H, ddd, J4,3 = 8.8 Hz, J4,5 = 9.5 Hz, J4,OH = 2.0 Hz, H-4), 3.91 (1H, d, J5,4 = 9.5 Hz, H-5), 3.83 (3H, s, COOCH3), 3.71 (1H, dd, J3,2 = 9.5 Hz, J3,4 = 8.8 Hz, H-3), 3.18 (1H, d, JOH,4 = 2.0 Hz, 4-OH), 1.03 (9H, s, C(CH3)3), 0.08 (3H, s, CH3), 0.01 (3H, s, CH3); 13C-NMR (150 MHz, CDCl3), δ 169.9, 169.9, 133.0, 129.8, 129.6, 129.6, 128.5, 128.2, 128.2, 128.0, 128.0, 127.8, 127.6, 127.6, 96.3, 80.4, 74.5, 74.2, 74.0, 72.1, 52.7, 25.5, 25.3, 25.2, 17.3, −4.4, −5.5; HRMS (positive mode); Found: m/z 539.2078 [M+Na]+, Calcd. for C27H36O8SiNa: 539.2077.
Methyl 3-O-benzyl-1,2-O-isopropylidene-α-l-idofuranosyluronate (10)
Compound 8 (50.0 mg, 0.159 mmol) was dissolved in CH2Cl2 (3 ml) and pyridine (50.0 μl, 0.319 mmol) was added at room temperature under Ar. The mixture was cooled to −20 °C and a solution of Tf2O (50.0 μl, 0.638 mmol) in CH2Cl2 (2.0 ml) was then added dropwise over 20 min at the same temperature. After being stirred for 2 h, the reaction mixture was transferred into separating funnel. The organic layer was washed with sat. NaHCO3 aq., 3% HCl aq. and brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was then dissolved in anhydrous DMF (5 ml) and LevONa (43.0 mg, 0.319 mmol) was added at room temperature under Ar. After being stirred for 4 h, the reaction mixture was diluted with AcOEt. The organic layer was washed with water (2 ×) and brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was then dissolved in pyridine-AcOH (3:2, 5 ml) and cooled to 0°C. After addition of hydrazine monohydrate (35.0 μl, 0.738 mmol) at the same temperature, the reaction mixture was stirred for 0.5 h. The reaction was quenched by the addition of acetone and the resulting mixture was diluted with AcOEt. The organic layer was washed with sat. NaHCO3 aq. (3 ×), 10% HCl aq. (2 ×), water and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 5 g, hexane:EtOAc = 9:1→8:1) afforded compound 10 as a colorless oil: Yield 27.9 mg (56%, 3 steps). 1H-NMR (600 MHz, CDCl3) δ 7.38-7.27 (5H, m, aromatic), 6.02 (1H, d, J1,2 = 4.1 Hz, H-1), 4.74 and 4.53 (each 1H, d, Jgem = 11.6 Hz, PhCH2), 4.68 (1H, d, J2,1 = 4.1 Hz, H-2), 4.54-4.52 (3H, m, H-4, H-5), 4.20 (1H, d, J3,4 = 4.1 Hz, H-3), 3.75 (3H, s, -COOCH3), 3.35 (1H, d, JOH,5 = 2.7 Hz, 5-OH), 1.49 (3H, s, CH3), 1.35 (3H, s, CH3); 13C-NMR (150 MHz, CDCl3), δ 171.9, 136.6, 128.5, 128.5, 128.2, 127.9, 127.8, 112.5, 105.1, 82.9, 82.9, 80.1, 72.3, 69.8, 52.7, 27.0, 26.5; HRMS (positive mode); Found: m/z 361.1242 [M+Na]+, Calcd. for C17H22O7Na: 361.1263.
Methyl 3-O-benzyl-l-idopyranosyluronate (11)
Compound 10 (46.8 mg, 0.138 mmol) was dissolved in TFA/H2O (9:1, 1.0 ml) and stirred for 2 h at room temperature. After removal of volatiles, the toluene was added and azeotropically evaporated twice. Flash chromatography (silica gel: 5 g, toluene:acetone = 3:1→1:1) afforded compound 11 as a brown solid: Yield 39.1 mg (95%). 1H-NMR (600 MHz, CDCl3) δ for β-anomer of pyranose form 7.39-7.30 (5H, m, aromatic), 5.08 (1H, s, H-1), 4.64 (2H, s, PhCH2), 4.58 (1H, s, H-5), 4.05 (1H, s, H-4), 3.97 (1H, s, H-3), 3.86 (1H, s, H-2), 3.80 (3H, s, COOCH3); 13C-NMR (150 MHz, CDCl3), δ for β-anomer 170.4, 137.2, 128.5, 128.1, 127.6, 93.1, 75.3, 74.3, 72.3, 67.5, 67.2, 52.6; HRMS (positive mode); Found: m/z 321.0947 [M+Na]+, Calcd. for C14H18O7Na: 321.0950.
Methyl 3-O-benzyl-1,2-O-(1,1,3,3-tetraisopropyldisiloxanylidene)-α-l-idopyranosyluronate (12)
To a suspension of compound 11 (12.5 g, 41.9 mmol), TIPDSCl2 (20.1 ml, 62.9 mmol), and MS4Ap (20 g) in anhydrous acetonitrile (200 ml) was added imidazole (11.4 g, 0.168 mol) at −45 °C under Ar. After being stirred for 41 h, volatiles were removed in vacuo. Flash chromatography (silica gel: 450 g, toluene:EtOAc = 100:1→30:1) afforded compound 12 as a yellow oil: Yield 17.7 g (78%). 1H-NMR (600 MHz, CDCl3) δ for α-anomer 7.37-7.30 (5H, m, aromatic), 5.23 (1H, s, H-1), 4.71 and 4.58 (each 1H, d, Jgem = 12.2 Hz, PhCH2), 4.58 (1H, d, J5,4 = 1.3 Hz, H-5), 4.50 (1H, dd, J4,5 = 1.3 Hz, J4,OH = 12.2 Hz, H-4), 3.91 (1H, d, J2,3 = 3.4 Hz, H-2), 3.88 (1H, d, J3,2 = 3.4 Hz, H-3), 3.88 (1H, d, JOH,4 = 12.2 Hz, 4-OH), 3.79 (3H, s, COOCH3), 1.04-1.01 (28H, m, -Si(iPr)2- × 2); 13C-NMR (150 MHz, CDCl3), δ 169.1, 137.2, 129.7, 128.5, 128.1, 127.6, 127.6, 95.5, 76.6, 75.7, 72.3, 71.4, 67.5, 52.0, 17.3, 17.3, 17.2, 17.1, 17.1, 17.0, 17.0, 16.9, 14.5, 12.6, 12.2, 12.0; HRMS (positive mode); Found: m/z 563.2472 [M+Na]+, Calcd. for C26H44O8Na: 563.2472.
Methyl 3-O-benzyl-4-O-levulinyl-1,2-O-(1,1,3,3-tetraisopropyldisiloxanylidene)-α-l-idopyranosyluronate (13)
To a solution of compound 12 (7.31 g, 13.5 mmol) in anhydrous DMF (50 ml) were added LevOH (5.54 ml, 54.1 mmol), EDC•HCl (6.23 g, 54.1 mmol), DMAP (6.61 g, 54.1 mmol), and Et3N (7.54 ml, 54.1 mmol) at room temperature. The reaction mixture was stirred for 24 h and the reaction was quenched by the addition of MeOH. After addition of AcOEt, the organic layer was washed with water (2 ×) and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 450 g, hexane:EtOAc = 4:1→2:1) afforded compound 13 as a colorless oil: Yield 5.92 g (68%). 1H-NMR (600 MHz, CDCl3) δ 7.37-7.30 (5H, m, aromatic), 5.18 (1H, s, H-1), 5.09 (1H, dd, J4,3 = 2.7 Hz, J4,5 = 2.7 Hz, H-4), 4.80 and 4.64 (each 1H, d, Jgem = 12.2 Hz, PhCH2), 4.65 (1H, d, J5,4 = 2.7 Hz, H-5), 3.88 (1H, dd, J3,2 = 2.7 Hz, J2,3 = 2.7 Hz, H-3), 3.81 (1H, d, J2,3 = 2.7 Hz, H-2), 3.77 (3H, s, COOCH3), 2.78-2.62 (3H, m, CH3C(=O)C2H4C(=O)-), 2.53-2.50 (1H, m, CH3C(=O)C2H4C(=O)-), 2.17 (3H, s, CH3C(=O)C2H4C(=O)-), 1.04-1.01 (28H, m, -Si(iPr)2- × 2); 13C-NMR (150 MHz, CDCl3), δ 206.2, 172.0, 168.0, 137.3, 128.4, 128.4, 127.9, 127.7, 127.7, 96.0, 76.0, 73.2, 72.6, 70.3, 67.4, 52.1, 37.7, 29.6, 27.9, 17.3, 17.3, 17.3, 17.1, 17.1, 17.0, 14.4, 12.7, 12.5, 12.4; HRMS (positive mode); Found: m/z 661.2820 [M+Na]+, Calcd. for C31H50O10Si2Na: 661.2840.
Methyl 3-O-benzyl-4-O-levulinyl-l-idopyranosyluronate (14)
Compound 13 (9.43 g, 14.7 mmol) was dissolved in THF (150 ml) and AcOH (4.22 ml, 73.8 mmol) was added at room temperature. A solution of TBAF in THF (29.5 ml, 1 M, 29.5 mol) was then added. After being stirred for 2.5 h, the reaction mixture was diluted with AcOEt. The organic layer was washed with H2O, sat. NaHCO3 aq., and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 300 g, toluene:EtOAc = 10:1→1:1) afforded compound 14 as a colorless oil: Yield 5.28 g (90%, α:β = 1:2). 1H-NMR (600 MHz, CDCl3) δ for α-anomer 7.39-7.17 (5H, m, aromatic), 5.24 (1H, s, H-4), 5.04 (1H, d, J1,OH = 5.4 Hz, H-1), 4.75 and 4.65 (each 1H, d, Jgem = 11.5 Hz, PhCH2), 4.68 (1H, s, H-5), 4.12 (1H, d, JOH,1 = 5.4 Hz, OH), 3.95 (1H, s, H-3), 3.79 (3H, s, COOCH3), 3.68 (1H, s, H-2), 2.78-2.72 (2H, m, CH3C(=O)C2H4C(=O)-), 2.55-2.52 (2H, m, CH3C(=O)C2H4C(=O)-), 2.19 (3H, s, CH3C(=O)C2H4C(=O)-); 13C-NMR (150 MHz, CDCl3), δ for a-anomer 206.6, 171.1, 168.0, 136.8, 128.5, 128.2, 127.7, 93.0, 74.3, 72.7, 72.4, 68.0, 67.8, 52.6, 37.9, 29.6, 27.8; HRMS (positive mode); Found: m/z 419.1319 [M+Na]+, Calcd. for C19H24O9Na: 419.1318.
Methyl (tert-butyldimethylsilyl 2-O-benzoyl-3-O-benzyl-4-O-levulinyl-β-l-Idopyranosyl)uronate (15)
Compound 14 (5.18 g, 13.0 mmol) was dissolved in CH2Cl2 (100 ml) and TBDMSCl (3.93 g, 26.1 mmol) and MS4AP (10 g) were added at room temperature under Ar. Imidazole (2.67 g, 39.2 mmol) was then added to the mixture and the reaction mixture was stirred at −20 °C. After being stirred for 3 h, the reaction mixture was warm to room temperature and further stirred for 3.5 h. The reaction was quenched by addition of MeOH and the resulting mixture was filtered through celite pad. The filtrate was washed with H2O and brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was dissolved in pyridine (50 ml) and BzCl (3.03 ml, 26.1 mmol) and DMAP (639 mg, 5.23 mmol) were added at room temperature under Ar. After 19 h, the reaction was quenched by the addition of MeOH and the resulting mixture was diluted with AcOEt. The organic layer was washed with 1 M HCl aq. (2 ×), sat. NaHCO3 aq., and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 400 g, toluene:EtOAc = 80:1) afforded compound 15 as a colorless oil: Yield 5.25 g (65%, 2 steps). 1H-NMR (600 MHz, CDCl3) δ 8.12 (2H, d, J = 8.1 Hz, Bz) 7.57-7.16 (8H, m, aromatic), 5.23 (1H, s, H-1), 5.22 (1H, s, H-4), 5.19 (1H, s, H-2), 4.81 and 4.77 (each 1H, d, Jgem = 11.5 Hz, PhCH2), 4.68 (1H, s, H-5), 4.00 (1H, s, H-3), 3.81 (3H, s, COOCH3), 2.58-2.55 (2H, m, CH3C(=O)C2H4C(=O)-), 2.48-2.28 (2H, m, CH3C(=O)C2H4C(=O)-), 2.05 (3H, s, CH3C(=O)C2H4C(=O)-), 0.80 (9H, s, C(CH3)3), 0.14 (3H, s, CH3), 0.09 (3H, s, CH3); 13C-NMR (150 MHz, CDCl3), δ 205.8, 171.7, 167.8, 165.6, 133.1, 129.9, 129.8, 128.5, 128.2, 128.1, 127.7, 93.1, 73.9, 72.9, 72.3, 68.0, 67.0, 52.3, 37.6, 29.5, 29.5, 27.8, 25.5, −4.0, −5.2; HRMS (positive mode); Found: m/z 637.2445 [M+Na]+, Calcd. for C32H42O10SiNa: 637.2445.
Methyl (tert-butyldimethylsilyl 2-O-benzoyl-3-O-benzyl-β-l-idopyranosyl)uronate (16)
Compound 15 (64.6 mg, 0.104 mmol) was dissolved in pyridine-AcOH (3:2, 1 ml) and hydrazine monohydrate (7.60 μl, 0.157 mmol) was added at room temperature. After 5 h, the reaction was quenched by addition of acetone and the resulting mixture was diluted with AcOEt. The organic layer was washed with 1 M HCl aq., sat. NaHCO3, aq., and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 5 g, toluene:EtOAc = 50:1→30:1) afforded compound 16 as a colorless oil: Yield 48.5 mg (89%). 1H-NMR (600 MHz, CDCl3) δ 8.00 (2H, d, J = 7.4 Hz, Bz), 7.59-7.16 (8H, m, aromatic), 5.28 (1H, d, J2,3 = 2.7 Hz, H-2), 5.25 (1H, s, H-1), 4.77 and 4.73 (each 1H, d, Jgem = 11.5 Hz, PhCH2), 4.61 (1H, d, J5,4 = 2.0 Hz, H-5), 4.06 (1H, dd, J4,5 = 2.0 Hz, J4,OH = 11.5 Hz, H-4), 3.99 (1H, d, J3,2 = 2.7 Hz, H-3), 3.82 (3H, s, COOCH3), 3.08 (1H, d, JOH,4 = 11.5 Hz, OH), 0.80 (9H, s, C(CH3)3), 0.14 (3H, s, CH3), 0.08 (3H, s, CH3); 13C-NMR (150 MHz, CDCl3), δ 169.0, 165.4, 137.0, 133.3, 129.7, 129.4, 128.5, 128.4, 128.1, 127.8, 93.1, 75.9, 74.1, 72.6, 69.4, 67.8, 52.2, 25.4, −4.1, −5.3; HRMS (positive mode); Found: m/z 539.2077 [M+Na]+, Calcd. for C27H36O8SiNa: 539.2077.
4-tert-Butylphenyl 3,4,6-tri-O-acetyl-2-azido-2-deoxy-1-thio-d-glucopyranoside (19)
Compound 18(5.17 g, 13.8 mmol), 4-tert-butylthiophenol (9.56 ml, 55.4 mmol), and MSAW300 (10 g) were suspended in anhydrous CH2Cl2 (100 ml) under Ar. After being stirred for 0.5 h, the mixture was cooled to 0 °C. TBDMSOTf (5.00 ml, 27.7 mmol) was added at the same temperature. The reaction mixture was gradually warmed to room temperature and stirred for 3 days. The reaction mixture was further stirred for 1 day at 60 °C and the reaction was quenched by the addition of water. The resulting mixture was filtered through celite pad. The filtrate was washed with water, sat. NaHCO3 aq., and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 300 g, hexane:AcOEt = 8:1→2:1) afforded compound 18 as a brown oil: 4.82 g (73%, α:β = 4:1). 1H-NMR (600 MHz, CDCl3), δ for a-anomer 7.42-7.33 (4H, m, aromatic), 5.59 (1H, d, J1,2 = 5.4 Hz, H-1), 5.35 (1H, dd, J3,2 = 9.5 Hz, J3,4 = 10.2 Hz, H-3), 5.06 (1H, dd, J4,3 = 10.2 Hz, J4,5 = 8.8 Hz, H-4), 4.65-4.62 (1H, m, H-5), 4.32 (1H, dd, J6a,6b = 12.9 Hz, J6a,5 = 5.4 Hz, H-6a), 4.08-4.03 (2H, m, H-2, H-6b), 2.10 (3H, s, CH3), 2.06 (3H, s, CH3), 2.04 (3H, s, CH3), 1.30 (9H, s, C(CH3)3); 13C-NMR (150 MHz, CDCl3), δ for α-anomer 170.5, 169.8, 152.3, 134.3, 132.4, 128.6, 126.3, 86.7, 72.0, 68.6, 68.3, 61.8, 61.5, 34.5, 31.1, 20.6, 20.6, 20.6; HRMS (positive mode); Found: m/z 502.1631 [M+Na]+, Calcd. for C22H29N3O7SNa: 502.1624.
4-tert-Butylphenyl 2-azido-2-deoxy-4,6-O-isopropylidene-1-thio-d-glucopyranoside (20)
To a solution of compound 19 (49.5 g, 0.103 mol) in methanol (400 ml) was added 1 M NaOMe in MeOH (100 ml, 0.100 mol) at room temperature. After 3 h, the reaction was quenched by addition of Dowex 50W (H+). After removal of insoluble materials by filtration, the filtrate was concentrated in vacuo. The residue was then dissolved in acetone (400 ml) and 2-methoxypropene (29.6 ml, 0.309 mmol) and CSA (7.19 g, 30.9 mmol) were added at room temperature under Ar. After 12 h, AcOEt was added. The organic layer was washed with water and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 450 g, hexane:EtOAc = 10:1→3:1) afforded compound 20 as a brown oil: Yield 29.8 g (74%, 2 steps, α:β = 5:1). 1H-NMR (600 MHz, CDCl3), δ for α-anomer 7.42-7.34 (4H, m, aromatic), 5.50 (1H, d, J1,2 = 5.4 Hz, H-1), 4.25-4.21 (1H, m, H-5), 3.94 (1H, dd, J3,2 = 9.5 Hz, J3,4 = 8.8 Hz, H-3), 3.88-3.83 (2H, m, H-2, H-6a), 3.77 (1H, d, J6b,6a = 10.8 Hz, H-6b), 3.62 (1H, dd, J4,3 = 8.8 Hz, J4,5 = 9.5 Hz, H-4), 2.70 (1H, s, 3-OH), 1.52 (3H, s, CH3), 1.46 (3H, s, CH3), 1.30 (9H, s, C(CH3)3); 13C-NMR (150 MHz, CDCl3), δ for α-anomer 151.3, 132.6, 132.1, 126.2, 100.1, 88.0, 74.3, 70.9, 64.2, 64.0, 61.9, 34.5, 31.1, 28.9, 19.1; HRMS (positive mode); Found: m/z 416.1628 [M+Na]+, Calcd. for C19H27N3O4SNa: 416.1620.
4-tert-Butylphenyl 2-azido-2-deoxy-3-O-(4-methoxybenzyl)-1-thio-d-glucopyranoside (21)
To a solution of compound 20 (120 mg, 0.304 mmol) in anhydrous DMF (5 ml) were added MPMCl (82.7 μl, 0.610 mmol) and NaH (18.3 mg, 0.762 mmol) at 0 °C under Ar. The reaction mixture was stirred and gradually warmed to room temperature. After 4.5 h, the reaction was quenched by addition of methanol and the reaction mixture was diluted with AcOEt. The organic layer was washed with water and brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was then dissolved in CH2Cl2 (1 ml) and AcOH-H2O (8:1, 9 ml) was added at room temperature. After 4 h, the reaction was quenched by the addition of 1 M NaOH aq. The organic layer was washed with 1 M NaOH aq (2 ′), and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 5 g, hexane:EtOAc = 1:1) afforded compound 4-tert-butylphenyl 2-azido-2-deoxy-4,6-O-isopropylidene-3-O-(4-methoxybenzyl)-1-thio-d-glucopyranosi de as a brown oil: Yield 108 mg (75%, 2 steps, α:β = 4:1). 1H-NMR (600 MHz, CDCl3), δ for α-anomer 7.44-7.29 (6H, m, aromatic), 6.90 (2H, d, J = 8.8 Hz, MPM), 5.51 (1H, d, J1,2 = 5.4 Hz, H-1), 4.94 and 4.69 (each 1H, d, Jgem = 11.5 Hz, PhCH2), 4.24 (1H, dd, J3,2 = 8.8 Hz, J3,4 = 8.1 Hz, H-3), 3.87-3.78 (6H, m, H-2, H-6a, H-6b, OCH3), 3.64-3.61 (2H, m, H-4, H-5), 1.30 (9H, s, C(CH3)3); 13C-NMR (150 MHz, CDCl3), δ for a-anomer 151.4, 132.6, 129.8, 126.2, 114.1, 87.3, 81.1, 72.1, 70.8, 63.7, 62.0, 55.2, 34.5, 31.1; HRMS (positive mode); Found: m/z 496.1886 [M+Na]+, Calcd. for C24H31N3O5SNa: 496.1882.
4-tert-Butylphenyl 2-azido-6-O-tert-butyldiphenylsilyl-2-deoxy-4-O-levulinyl-3-O-(4-methoxybenzyl)-1-thio-d-glucopyranoside (22)
To a solution of compound 4-tert-butylphenyl 2-azido-2-deoxy-4,6-O-isopropylidene-3-O-(4-methoxybenzyl)-1-thio-d-glucopyranosi de (12.2 g, 25.7 mmol) in anhydrous DMF (100 ml) were added TBDPSCl (9.92 ml, 38.6 mmol) and imidazole (5.26 g, 7.73 mmol) at room temperature under Ar. After 5 h, the reaction was quenched by addition of methanol and the mixture was diluted with AcOEt. The organic layer was washed with water and brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was then dissolved in anhydrous DMF (120 ml) and LevOH (10.5 ml, 0.103 mol), EDC•HCl (11.9 g, 77.2 mmol), DMAP (9.44 g, 77.2 mmol) were added at room temperature under Ar. After 26 h, the reaction was quenched by the addition of MeOH and the mixture was diluted with AcOEt. The organic layer was washed with water and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 450 g, hexane:EtOAc = 8:1→3:1) afforded compound 22 as a brown oil: Yield 18.6 g (89%, 2 steps, α:β = 10:3). 1H-NMR (600 MHz, CDCl3), d for a-anomer 7.45-7.22 (16H, m, aromatic), 6.88 (2H, d, J = 8.8 Hz, MPM), 5.55 (1H, d, J1,2 = 5.4 Hz, H-1), 5.16 (1H, dd, J4,3 = 9.5 Hz, J4,5 = 9.5 Hz, H-4), 4.76 and 4.62 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.41-4.37 (1H, m, H-5), 3.94 (1H, dd, J2,1 = 5.4 Hz, J2,3 = 10.2 Hz, H-2), 3.80-3.65 (6H, m, H-3, H-6a, H-6b, OCH3), 2.61-2.58 (2H, m, CH3C(=O)C2H4C(=O)-), 2.37-2.34 (2H, m, CH3C(=O)C2H4C(=O)-), 2.15 (3H, s, CH3), 1.27 (9H, s, C(CH3)3), 1.01 (9H, s, C(CH3)3); 13C-NMR (150 MHz, CDCl3), δ for α-anomer 205.9, 171.2, 135.7, 135.5,132.0, 129.9127.6, 127.5, 126.1, 113.8, 87.2, 78.9, 74.8, 71.8, 70.5, 63.8, 62.4, 55.2, 37.7, 31.1, 29.8, 27.8, 26.6; HRMS (positive mode); Found: m/z 832.3429 [M+Na]+, Calcd. for C45H55N3O7SSiNa: 832.3428.
2-Azido-6-O-tert-butyldiphenylsilyl-2-deoxy-4-O-levulinyl-3-O-(4-methoxybenzyl)-d-glucopyranose
To a solution of compound 23 (75.5 mg, 93.2 μmol) in acetone-H2O (5:2, 1.4 ml) were added NCS (87.1 mg, 0.652 mmol) at room temperature. After 12 h, the reaction was quenched by the addition of 10% Na2S2O3 aq and the mixture was diluted with AcOEt. The organic layer was washed with 10% Na2S2O3 aq. and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 5 g, hexane:EtOAc = 6:1→4:1) afforded 2-azido-6-O-tert-butyldiphenylsilyl-2-deoxy-4-O-levulinyl-3-O-(4-methoxybenzyl)-d-glucopyranose as a colorless oil: Yield 44.8 mg (73%, α:β = 5:3). 1H-NMR (600 MHz, CDCl3), δ for α-anomer 7.69-7.23 (12H, m, aromatic), 6.87 (2H, d, J = 8.8 Hz, MPM), 5.28 (1H, dd, J1,2 = 3.4 Hz, J1,OH = 3.4 Hz, H-1), 5.14 (1H, dd, J4,3 = 9.5 Hz, J4,5 = 10.2 Hz, H-4), 4.71 and 4.60 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.40-3.97 (1H, m, H-5), 3.98 (1H, dd, J3,2 = 9.5 Hz, J3,4 = 9.5 Hz, H-3), 3.79 (3H, s, OCH3), 3.70-3.66 (2H, m, H-6a, H-6b), 3.46 (1H, dd, J2,1 = 3.4 Hz, J2,3 = 9.5 Hz, H-2), 2.84 (1H, d, OH), 2.61-2.58 (2H, m, CH3C(=O)C2H4C(=O)-), 2.37-2.32 (2H, m, CH3C(=O)C2H4C(=O)-), 2.14 (3H, s, CH3), 1.02 (9H, s, C(CH3)3); 13C-NMR (150 MHz, CDCl3), δ for α-anomer 206.1, 171.2, 135.8, 135.7, 135.6, 135.6, 129.8, 129.7, 129.6, 127.5, 127.5, 113.7, 91.8, 77.3, 74.4, 70.7, 70.5, 63.5, 62.9, 55.2, 37.7, 29.8, 27.8, 26.7; HRMS (positive mode); Found: m/z 684.2713 [M+Na]+, Calcd. for C35H43N3O8SiNa: 684.2717.
2-Azido-6-O-tert-butyldiphenylsilyl-2-deoxy-4-O-levulinyl-3-O-(4-pivaloylaminobenzyl)-d-glucopyranosyl trichloroacetimidate (24)
To a solution of 2-azido-6-O-tert-butyldiphenylsilyl-2-deoxy-4-O-levulinyl-3-O-(4-methoxybenzyl)-d-glucopyranose (5.53 g, 8.36 mmol) in CH2Cl2 (15 ml) were added trichloroacetonitrile (2.79 ml, 27.8 mmol) and Cs2CO3 (908 mg, 2.73 mmol) at 0 °C. After being stirred for 2 h, the reaction mixture was filtered throught a celite pad. The filtrate was concentrated in vacuo to afford glycosyl imidate 24, which was used to the next reaction without further purification.
Methyl [2-O-azido-6-O-tert-butyldiphenylsilyl-2-O-deoxy-4-O-levulinyl-3-O-(4-methoxyphenylmetyl)-α-d-glucopyranosyl]-(1→4)-(tert-butyldimethylsilyl 2-O-benzoyl-3-O-benzyl-β-d-glucopyranosid)uronate (25)
The glycosyl donor 24 (8.38 mmol), glycosyl accepter 8 (2.85 g, 5.51 mmol), and MS4A powder (7.0 g) were then suspended in anhydrous toluene (70 ml) under Ar. After being stirred for 2 h, the mixture was cooled to −20 °C. TBDMSOTf (506 μl, 2.20 mmol) was added at the same temperature. The reaction mixture was stirred and gradually warmed to 0 °C. After 5 h, the reaction was quenched by the addition of water. The resulting mixture was filtered through a celite pad. The filtrate was washed with water, sat. NaHCO3 aq., and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 300 g, toluene:AcOEt = 100:1→20:1) afforded compound 25 as a brown oil: Yield 5.29 g (83%). 1H-NMR (600 MHz, CDCl3), δ 8.04 (2H, d, J = 8.1 Hz, Bz), 7.65-7.19 (20H, m, aromatic), 6.88 (2H, d, J = 8.8 Hz, MPM), 5.51 (1H, d, J1′,2′ = 3.4 Hz, H-1′), 5.36-5.31 (2H, m, H-2,H-4′), 4.87 (1H, d, J1,2 = 7.4 Hz, H-1), 4.79 and 4.72 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.72 and 4.61 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.28 (1H, dd, J4,3 = 8.8 Hz, J4,5 = 9.5 Hz, H-4), 4.07 (1H, d, J5,4 = 9.5 Hz, H-5), 4.01 (1H, dd, J3,2 = 8.8 Hz, J3,4 = 8.8 Hz, H-3), 3.90 (1H, dd, J3′,2′ = 10.2 Hz, J3′,4′ = 9.5 Hz, H-3′), 3.79 (3H, s, OCH3), 3.69 (1H, dd, J6a’,5′ = 1.3 Hz, J6a’,6b’ = 11.8 Hz, H-6a’), 3.64 (1H, dd, J6b’,5′ = 1.3 Hz, J6b’,6a’ = 11.8 Hz, H-6b’), 3.56-3.53 (4H, m, OCH3, H-5′), 3.34 (1H, dd, J2′,1′ = 3.4 Hz, J2′,3′ = 10.2 Hz, H-2′), 2.66-2.63 (2H, m, CH3C(=O)C2H4C(=O)-), 2.42-2.36 (2H, m, CH3C(=O)C2H4C(=O)-), 2.17 (3H, s, CH3C(=O)C2H4C(=O)-), 1.02 (9H, s, C(CH3)3), 0.74 (9H, s, C(CH3)3), 0.06 (3H, s, CH3), 0.00 (3H, s, CH3); 13C-NMR (150 MHz, CDCl3), δ 206.3, 168.4, 163.7, 135.7, 135.6, 135.6, 129.8, 129.6, 129.6, 129.6, 129.5, 128.4, 128.3, 127.8, 127.8, 127.7, 127.6, 127.5, 113.7, 97.3, 95.9, 82.2, 77.3, 74.8, 74.7, 74.3, 74.1, 73.7, 70.8, 69.6, 62.8, 60.7, 55.2, 52.4, 37.7, 29.8, 27.8, 26.6, 25.2, −4.4, −5.4; HRMS (positive mode); Found: m/z 1182.4793 [M+Na]+, Calcd. for C62H77N3O15Si2Na: 1182.4791.
Methyl [2-O-azido-6-O-tert-butyldiphenylsilyl-2-O-deoxy-4-O-levulinyl-3-O-(4-methoxyphenylmetyl)-α-d-glucopyranosyl]-(1→4)-(tert-butyldimethylsilyl 2-O-benzoyl-3-O-benzyl-β-d-idopyranosid)uronate (26)
The glycosyl imidate 24 (0.375 mmol), glycosyl accepter 15 (97.1 mg, 0.187 mmol), and MS4A powder (800 mg) were then suspended in anhydrous CH2Cl2 (8.0 ml) under Ar. After being stirred for 3 h, the mixture was cooled to −20 °C. TBDMSOTf (21.5 μl, 93.7 mmol) was added at the same temperature. The reaction mixture was stirred and gradually warmed to room temperature. After 18 h, the reaction was quenched by the addition of water. The resulting mixture was filtered through a celite pad. The filtrate was washed with water, and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 20 g, toluene:AcOEt = 100:1→60:1) afforded compound 26 as a brown oil: Yield 169 mg (77%). 1H-NMR (600 MHz, CDCl3), δ 8.21 (2H, d, J = 7.4 Hz, Bz), 7.66 (18H, m, aromatic), 7.02 (2H, d, J = 8.8 Hz, MPM), 6.80 (2H, d, J = 8.8 Hz, MPM), 5.28 (1H, dd, J4′,3′ = 9.5 Hz, J4′,5′ = 9.5 Hz, H-4′), 5.19 (1H, s, H-1), 5.09 (1H, s, H-2), 4.84 and 4.76 (each 1H, d, Jgem = 11.5 Hz, PhCH2), 4.68 (1H, d, J1′,2′ = 3.4 Hz, H-1′), 4.51 (1H, d, J5,4 = 2.0 Hz, H-5), 4.30 (1H, d, J3,4 = 3.4 Hz, H-3), 3.92-3.90 (3H, m, H-4, PhCH2), 3.79-3.75 (4H, m, H-5′, OCH3), 3.67-3.66 (2H, m, H-6a’, H-6b’), 3.55 (1H, dd, J3′,2′ = 9.5 Hz, J3′,4′ = 9.5 Hz, H-3′), 3.45 (3H, s, OCH3), 3.31 (1H, dd, J2′,1′ = 3.4 Hz, J2′,3′ = 9.5 Hz, H-2′), 2.63-2.61 (2H, m, CH3C(=O)C2H4C(=O)-), 2.39-2.37 (2H, m, CH3C(=O)C2H4C(=O)-), 2.17 (3H, s, CH3C(=O)C2H4C(=O)-), 1.00 (9H, s, C(CH3)3), 0.80 (9H, s, C(CH3)3), 0.14 (3H, s, CH3), 0.06 (3H, s, CH3); 13C-NMR (150 MHz, CDCl3), δ 206.1, 170.7, 168.3, 166.5, 135.8, 135.7, 130.1, 129.6, 129.5, 129.5, 129.0, 128.5, 128.4, 128.2, 128.0, 127.5, 127.4, 113.6, 99.2, 93.4, 77.9, 75.1, 74.1, 73.7, 73.7, 73.0, 71.1, 69.7, 68.9, 63.4, 61.5, 55.2, 51.8, 37.7, 30.9, 29.8, 29.2, 27.8, 26.6, 25.5, −4.0, −5.3; HRMS (positive mode); Found: m/z 1182.4759 [M+Na]+, Calcd. for C62H77N3O15Si2Na: 1182.4791.
Methyl [2-O-azido-6-O-tert-butyldiphenylsilyl-2-O-deoxy-4-O-levulinyl-3-O-(4-methoxybenzyl)-α-d-glucopyranosyl]-(1→4)-(2-O-benzoyl-3-O-benzyl-d-glucopyranosyl)uronate (27)
To a solution of compound 25 (55.7 mg, 48.0 μmol) in THF (1 ml) were added AcOH (5.5 μl, 96.0 μmol) and 1 M TBAF in THF (48.0 μl, 48.0 μmol) at room temperature. After being stirred for 12 h, the reaction mixture was poured into AcOEt. The resulting mixture was washed with water and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 5 g, toluene:EtOAc = 10:1→8:1) afforded compound 27 as a colorless oil: Yield 39.5 mg (78%, α:β = 5:1). 1H-NMR (600 MHz, CDCl3), δ for α-anomer 8.11 (2H, d, J = 7.4 Hz, Bz), 7.66-7.19 (20H, m, aromatic), 6.86 (2H, d, J = 8.8 Hz, MPM), 5.65 (1H, dd, J1,2 = 3.4 Hz, J1,OH = 5.4 Hz, H-1), 5.37 (1H, d, J1′,2′ = 3.4 Hz, H-1′), 5.26 (1H, dd, J4′,5′ = 10.2 Hz, J4′,3′ = 9.5 Hz, H-4′), 5.16 (1H, dd, J2,3 = 8.1 Hz, J2,1 = 3.4 Hz, H-2), 4.85 (2H, s, PhCH2), 4.70 (1H, d, J5,4 = 7.1 Hz, H-5), 4.46 and 4.39 (each 1H, d, Jgem = 10.2 Hz, PhCH2), 4.37 (1H, dd, J3,4 = 7.4 Hz, J3,2 = 8.1 Hz, H-3), 4.19 (1H, dd, J4,5 = 7.1 Hz, J4,3 = 7.4 Hz, H-4), 3.81-3.77 (4H, m, H-3′, OCH3), 3.69-3.62 (3H, m, H-5′, H-6a’, H-6b’), 3.54 (3H, s, OCH3), 3.32 (1H, dd, J2′,3′ = 10.2 Hz, J2′,1′ = 3.4 Hz, H-2′), 3.23 (1H, d, JOH,1 = 5.4 Hz, OH), 2.65-2.62 (2H, m, CH3C(=O)C2H4C(=O)-), 2.42-2.39 (2H, m, CH3C(=O)C2H4C(=O)-), 2.16 (3H, s, CH3C(=O)C2H4C(=O)-), 1.01 (9H, s, C(CH3)3); 13C-NMR (150 MHz, CDCl3), δ 206.1, 170.9, 169.2, 159.2, 135.7, 135.6, 130.1, 129.8, 129.6, 129.6, 129.5, 129.3, 128.6, 128.4, 128.4, 127.8, 127.7, 127.5, 127.5, 113.7, 98.3, 89.9, 77.3, 75.1, 74.3, 74.1, 73.3, 72.4, 71.5, 71.0, 70.0, 62.8, 61.7, 55.2, 52.4, 37.7, 29.8, 27.8, 26.6; HRMS (positive mode); Found: m/z 1068.3932 [M+Na]+, Calcd. for C56H63N3O15SiNa: 1068.3926.
Benzyl (2-O-azido-6-O-tert-butyldiphenylsilyl-2-O-deoxy-4-O-levulinyl-3-O-(4-methoxybenzyl)-α-d-glucopyranosyl)-(1®4)-(methyl 2-O-benzoyl-3-O-benzyl-β-d-glucopyranosyluronate)-(1→6)-2,3,4-tetra-O-benzyl-β-d-glucopyranoside (30)
To a solution of 27 (54.1 mg, 51.7 μmol) in CH2Cl2 (2 ml) were added trichloroacetonitrile (51.8 μl, 517 mmol) and Cs2CO3 (16.8 mg, 51.7 μmol) at room temperature. After being stirred for 1 h, the reaction mixture was filtered through celite pad. The filtrate was concentrated in vacuo to give glycosyl imidate 28. The glycosyl imidate 28 (51.7 μmol), glucose moiety 29 (55.9 mg, 0.103 mmol), and MS4A powder (400 mg) were then suspended in anhydrous CH2Cl2 (4 ml) under Ar. After being stirred for 2.5 h, the mixture was cooled to −78 °C. TMSOTf (5.74 μl, 25.8 μmol) was then added at the same temperature. After 23 h, the reaction was quenched by the addition of water. The resulting mixture was filtered through a celite pad. The filtrate was washed with water, and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 5 g, toluene:AcOEt = 50:1→10:1) afforded compound 30 as a colorless oil: Yield 54.7 mg (68%, 2 steps). 1H-NMR (600 MHz, CDCl3), δ 7.98 (2H, d, J = 6.7 Hz, Bz), 7.65-7.13 (40H, m, aromatic), 6.88 (2H, d, J = 8.8 Hz, MPM), 5.46 (1H, d, J1′,2′ = 3.4 Hz, H-1′), 5.43 (1H, dd, J2′,3′ = 8.8 Hz, J2′,1′ = 7.4 Hz, H-2′), 5.35 (1H, dd, J4″,5″ = 9.5 Hz, J4″,3″ = 9.5 Hz, H-4″), 4.87 and 4.69 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.86 and 4.68 (each 1H, d, Jgem = 12.2 Hz, PhCH2), 4.79 and 4.70 (each 1H, d, Jgem = 10.2 Hz, PhCH2), 4.73 (1H, d, J1′,2′ = 7.4 Hz, H-1′), 4.69 and 4.64 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.65 and 4.41 (each 1H, d, Jgem = 12.2 Hz, PhCH2), 4.60 and 4.44 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.36 (1H, d, J1,2 = 7.4 Hz, H-1), 4.26 (1H, dd, J4′,5′ = 9.5 Hz, J4′,3′ = 8.8 Hz, H-4′), 4.10 (1H, dd, J6a,6b = 10.8 Hz, J6a,5 = 1.3 Hz, H-6a), 4.05 (1H, d, J5′,4′ = 9.5 Hz, H-5′), 3.97 (1H, dd, J3′,4′ = 8.8 Hz, J3′,2′ = 8.8 Hz, H-3′), 3.90 (1H, dd, J3″,4″ = 9.5 Hz, J3″,2″ = 9.5 Hz, H-3″), 3.78 (3H, s, OCH3), 3.69 (1H, dd, J6a”,6b” = 11.5 Hz, J6a”,5″ = 1.3 Hz, H-6a”), 3.64 (1H, dd, J6b”,6a” = 11.5 Hz, J6b”,5″ = 2.7 Hz, H-6b”), 3.59-3.52 (6H, m, H-3, H-6b, H-5″, OCH3), 3.44-3.41 (1H, m, H-5), 3.40 (1H, dd, J2,3 = 8.1 Hz, J2,1 = 7.4 Hz, H-2), 3.34-3.30 (2H, m, H-4, H-2″), 2.66-2.63 (2H, m, CH3C(=O)C2H4C(=O)-), 2.42-2.39 (2H, m, CH3C(=O)C2H4C(=O)-), 2.16 (3H, s, CH3C(=O)C2H4C(=O)-), 1.01 (9H, s, C(CH3)3); 13C-NMR (150 MHz, CDCl3), δ 206.1, 168.3, 164.8, 159.3, 138.4, 135.7, 135.7, 129.8, 129.6, 129.6, 129.0, 128.4, 128.3, 128.3, 128.2, 128.2, 128.1, 127.9, 127.8, 127.8, 127.7, 127.7, 127.6, 127.5, 125.2, 113.7, 102.1, 101.0, 97.4, 84.4, 82.1, 82.0, 77.7, 77.2, 75.6, 74.8, 74.7, 74.7, 74.5, 74.4, 74.1, 73.9, 72.9, 70.9, 70.7, 69.8, 68.3, 62.8, 61.4, 55.2, 52.4, 37.7, 29.8, 27.8, 26.7; HRMS (positive mode); Found: m/z 1590.6341 [M+Na]+, Calcd. for C90H97N3O20SiNa: 1590.6332.
Methyl [2-O-azido-6-O-tert-butyldiphenylsilyl-2-O-deoxy-4-O-levulinyl-3-O-(4-methoxybenzyl)-α-d-glucopyranosyl]-(1→4)-(2-O-benzoyl-3-O-benzyl-l-idopyranosyl)uronate (31)
To a solution of compound 26 (19.9 mg, 17.1 μmol) in pyridine (1 ml) was added HF•pyridine (44.4 μl, 1.71 mmol) at room temperature. After being stirred for 2 h, the reaction mixture was poured into AcOEt. The resulting mixture was washed with water and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 5 g, toluene:EtOAc = 10:1→3:1) afforded compound 31 as a colorless oil: Yield 13.1 mg (73%, α:β = 5:3). 1H-NMR (600 MHz, CDCl3), δ for α-anomer 8.18 (2H, d, J = 7.4 Hz, Bz), 7.65-7.17 (18H, m, aromatic), 7.04 (2H, d, J = 8.8 Hz, MPM), 6.82 (2H, d, J = 8.8 Hz, MPM), 5.49 (1H, d, J1.1-OH = 8.8 Hz, H-1), 5.05 (1H, d, J2,3 = 2.7 Hz, H-2), 4.89 (1H, d, J5,4 = 2.7 Hz, H-5), 4.87 (1H, d, Jgem = 11.5 Hz, PhCH2), 4.77 (1H, d, Jgem = 11.5 Hz, PhCH2), 4.68 (1H, d, J1′,2′ = 3.4 Hz, H-1′), 4.34 (1H, dd, J3,2 = 2.7 Hz, J3,4 = 3.4 Hz, H-3), 4.09 (1H, d, J1-OH,1 = 8.8 Hz, 1-OH), 4.01 (1H, dd, J4,3 = 3.4 Hz, J4,5 = 2.7 Hz, H-4), 3.96 (1H, d, Jgem = 10.8 Hz, PhCH2), 3.83 (1H, d, Jgem = 10.8 Hz, PhCH2), 3.77-3.75 (4H, m, H-6a’, OCH3), 3.72-3.68 (2H, m, H-5′, H-6b’), 3.50 (3H, s, OCH3), 3.29 (1H, dd, J2′,1′ = 3.4 Hz, J2′,3′ = 7.4 Hz, H-2′), 2.66-2.59 (2H, m, CH3C(=O)C2H4C(=O)-), 2.42-2.31 (2H, m, CH3C(=O)C2H4C(=O)-), 2.16 (3H, s, CH3C(=O)C2H4C(=O)-), 1.00 (9H, s, C(CH3)3); 13C-NMR (150 MHz, CDCl3), δ for β-anomer 206.0, 170.7, 165.8, 159.2, 135.7, 135.7, 133.2, 130.0, 129.7, 129.6, 129.5, 128.6, 128.5, 128.5, 128.3, 127.5, 127.4, 127.4, 113.6, 99.2, 93.6, 77.9, 74.7, 74.0, 73.6, 72.5, 71.2, 69.7, 68.2, 67.4, 63.2, 61.4, 55.2, 52.1, 37.7, 29.8, 27.8, 26.6; HRMS (positive mode); Found: m/z 1068.3912 [M+Na]+, Calcd. for C56H63N3O15SiNa: 1068.3926.
Benzyl (2-O-azido-6-O-tert-butyldiphenylsilyl-2-O-deoxy-4-O-levulinyl-3-O-(4-methoxybenzyl)-α-d-glucopyranosyl)-(1→4)-(methyl 2-O-benzoyl-3-O-benzyl-β-l-idopyranosyluronate)-(1→6)-2,3,4-tetra-O-benzyl-β-d-glucopyranoside (33)
To a solution of 31 (112 mg, 107 μmol) in CH2Cl2 (8 ml) were added trichloroacetonitrile (107 μl, 1.07 mmol) and Cs2CO3 (34.8 mg, 107 μmol) at 0 °C. the reaction mixture was stirred and gradually warmed to room temperature. After being stirred for 6 h, the reaction mixture was filtered through celite pad. The filtrate was concentrated in vacuo to give glycosyl imidate 32. The glycosyl imidate 32 (107 μmol), glucose moiety 29 (116 mg, 214 mmol), and MS4A powder (1.0 g) were then suspended in anhydrous CH2Cl2 (10 ml) under Ar. After being stirred for 2.5 h, the mixture was cooled to −20 °C. TMSOTf (3.8 μl, 21 μmol) was then added at the same temperature. After 2.5 h, the reaction was quenched by the addition of water. The resulting mixture was filtered through a celite pad. The filtrate was washed with water, sat. NaHCO3 aq., and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 15 g, toluene:AcOEt = 50:1→30:1) afforded compound 33 as a colorless oil: Yield 103 mg (62%, 2 steps). 1H-NMR (600 MHz, CDCl3), δ 8.17 (2H, d, J = 8.1 Hz, Bz), 7.64-7.17 (40H, m aromatic), 7.04 (2H, d, J = 8.8 Hz, MPM), 6.81 (2H, d, J = 8.8 Hz, MPM), 5.30 (1H, dd, J4″,3″ = 9.5 Hz, J4″,5″ = 10.2 Hz, H-4″), 5.20 (1H, s, H-1′), 5.10 (1H, s, H-2′), 4.98 (1H, s, H-5′), 4.91 (1H, d, Jgem = 10.8 Hz, PhCH2), 4.91 (1H, d, Jgem = 10.2 Hz, PhCH2), 4.91 (1H, d, Jgem = 10.8 Hz, PhCH2), 4.79 (1H, d, Jgem = 11.5 Hz, PhCH2), 4.77 (1H, d, Jgem = 10.2 Hz, PhCH2), 4.74 (1H, d, Jgem = 12.2 Hz, PhCH2), 4.73 (1H, d, Jgem = 10.8 Hz, PhCH2), 4.72 (1H, d, J1″,2″ = 4.0 Hz, H-1″), 4.68 (1H, d, Jgem = 10.8 Hz, PhCH2), 4.56 (1H, d, Jgem = 11.5 Hz, PhCH2), 4.50 (1H, d, Jgem = 12.2 Hz, PhCH2), 4.44 (1H, d, J1,2 = 7.4 Hz, H-1), 4.16 (1H, s, H-3′), 4.03 (1H, s, H-4′), 4.03 (1H, dd, J6a,6b = 11.5 Hz, J6a,5 = 1.3 Hz, H-6a), 4.01 (1H, d, Jgem = 10.2 Hz, PhCH2), 3.92 (1H, d, Jgem = 10.2 Hz, PhCH2), 3.86 (1H, dd, J6b,6a = 11.5 Hz, J6b,5 = 4.7 Hz, H-6b), 3.79-3.77 (4H, m, H-5″, OCH3), 3.71 (1H, dd, J6a”,6b” = 10.2 Hz, J6a”,5″ = 4.0 Hz, H-6a”), 3.67 (1H, dd, J6b”,6a” = 10.2 Hz, J6b”,5″ = 2.7 Hz, H-6b”), 3.61-3.56 (4H, m, H-3, H-4, H-5, H-3″), 3.42 (1H, dd, J2,1 = 7.4 Hz, J2,3 = 8.1 Hz, H-2), 3.37 (3H, s, OCH3), 3.30 (1H, dd, J2″,1″ = 4.0 Hz, J2″,3″ = 9.5 Hz, H-2″), 2.64-2.61 (2H, m, CH3C(=O)C2H4C(=O)-), 2.41-2.35 (2H, m, CH3C(=O)C2H4C(=O)-), 2.16 (3H, s, CH3C(=O)C2H4C(=O)-), 0.99 (9H, s, C(CH3)3); 13C-NMR (150 MHz, CDCl3), δ 206.0, 170.7, 159.2, 138.0, 137.3, 135.7, 135.7, 129.9, 129.6, 129.5, 128.5, 128.5, 128.5, 128.4, 128.3,128.3, 128.3, 128.2, 128.1, 127.9, 127.8, 127.8, 127.7, 127.6, 127.6, 127.5, 127.4, 113.6, 102.3, 99.2, 98.8, 84.6, 82.2, 78.0, 77.5, 75.7, 75.2, 74.9, 74.9, 74.1, 72.4, 72.3, 72.3, 71.0, 70.7, 69.7, 67.8, 67.3, 67.1, 63.3, 61.5, 55.2, 51.8, 37.7, 29.8, 27.8, 26.6; HRMS (positive mode); Found: m/z 1590.6357 [M+Na]+, Calcd. for C90H97N3O20SiNa: 1590.6332.
Benzyl (2-O-azido-6-O-tert-butyldiphenylsilyl-2-O-deoxy-3-O-(4-methoxybenzyl) -α-d-glucopyranosyl)-(1→4)-(methyl 2-O-benzoyl-3-O-benzyl-β-d-glucopyranosyluronate)-(1→6)-2,3,4-tetra-O-benzyl-β-d-glucopyranoside
Compound 30 (649 mg, 0.413 mmol) was dissolved in pyridine/AcOH (3:2, 10 ml) and hydrazine monohydrate (30.2 μl, 0.620 mmol) was added at room temperature. After 3 h, the reaction was quenched by addition of acetone and the mixture was diluted with AcOEt. The organic layer was washed with 1 M HCl aq., sat. NaHCO3, aq., and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 30 g, toluene:EtOAc = 20:1→10:1) afforded benzyl (2-O-azido-6-O-tert-butyldiphenylsilyl-2-O-deoxy-3-O-(4-methoxybenzyl)-α-d-glucop yranosyl)-(1→4)-(methyl 2-O-benzoyl-3-O-benzyl-β-d-glucopyranosyluronate)-(1→6)-2,3,4-tetra-O-benzyl-β-d-glucopyranoside as a white solid: Yield 540 mg (89%). 1H-NMR (600 MHz, CDCl3), δ 7.96 (2H, d, J = 8.1 Hz, Bz), 7.69-7.13 (40H, m, aromatic), 6.91 (2H, d, J = 8.1 Hz, MPM), 5.40-5.38 (2H, m, H-1″, H-2′), 4.88 and 4.79 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.84 and 4.80 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.79 and 4.64 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.72 and 4.71 (each 1H, d, Jgem = 12.2 Hz, PhCH2), 4.68 and 4.45 (each 1H, d, Jgem = 11.5 Hz, PhCH2), 4.67 (1H, d, J1′,2′ = 8.1 Hz, H-1′), 4.66 and 4.41 (each 1H, d, Jgem = 12.2 Hz, PhCH2), 4.36 (1H, d, J1,2 = 7.4 Hz, H-1), 4.25 (1H, dd, J4′,5′ = 8.8 Hz, J4′,3′ = 8.8 Hz, H-4′), 4.11 (1H, d, J6a”,6b” = 10.8 Hz, H-6a”), 4.01 (1H, d, J5′,4′ = 8.8 Hz, H-5′), 3.94-3.90 (2H, m, H-6a, H-3′), 3.79-3.72 (6H, m, H-6b, H-3″, H-4″, OCH3), 3.60-3.53 (5H, m, H-3, H-6b”, OCH3), 3.47-3.40 (3H, m, H-2, H-5, H-5″), 3.35 (1H, dd, J4,5 = 9.5 Hz, J4,3 = 9.5 Hz, H-4), 3.22 (1H, dd, J2″,3″ = 9.5 Hz, J2″,1″ = 3.3 Hz, H-2″), 2.78 (1H, d, JOH,4″ = 1.3 Hz, OH), 1.05 (9H, s, C(CH3)3); 13C-NMR (150 MHz, CDCl3), δ 168.3, 164.8, 159.3, 137.8, 137.2, 135.6, 129.9, 129.9, 129.8, 129.6, 128.4, 128.3, 128.3, 128.3, 128.2, 128.1, 127.9, 127.9, 127.8, 127.7, 127.7, 127.6, 113.9, 102.1, 101.1, 97.5, 84.4, 82.2, 82.0, 78.8, 77.7, 75.6, 74.8, 74.8, 74.7, 74.5, 74.4, 74.3, 73.1, 73.0, 70.9, 70.7, 68.2, 64.0, 62.5, 55.2, 52.5, 26.8; HRMS (positive mode); Found: m/z 1492.5978 [M+Na]+, Calcd. for C85H91N3O18SiNa: 1492.5965.
Benzyl [2-O-azido-6-O-tert-butyldiphenylsilyl-2-O-deoxy-3-O-(4-methoxybenzyl)-4-O-methyl-α-d-glucopyranosyl]-(1→4)-(methyl 2-O-benzoyl-3-O-benzyl-β-d-glucopyranosyluronate)-(1→6)-2,3,4-tetra-O-benzyl-β-d-glucopyranoside (34)
To a solution of benzyl (2-O-azido-6-O-tert-butyldiphenylsilyl-2-O-deoxy-3-O-(4-methoxybenzyl)-α-d-glucop yranosyl)-(1→4)-(methyl 2-O-benzoyl-3-O-benzyl-β-d-glucopyranosyluronate)-(1→6)-2,3,4-tetra-O-benzyl-β-d-glucopyranoside (11.0 mg, 7.48 μmol) in anhydrous DMF (2 ml) were added MeI (0.90 μl, 15 μmol) and MS4AP (200 mg) at room temperature under Ar and the mixture was stirred for 2 h. After cooling to −20 °C, lithium hexamethyldisilazide (LHMDS) in THF (7.0 μl, 1.6 M) was added at the same temperature. The reaction mixture was further stirred and gradually warmed to 0 °C. After 4 h, the reaction was quenched by the addition of methanol and the reaction mixture was filtered through a celite pad. The filtrate was washed with 10% Na2S2O3 aq. and brine, dried over Na2SO4, filtered, and concentrated in vacuo. Flash chromatography (silica gel: 5 g, toluene:EtOAc = 30:1→10:1) afforded compound 34 as a white solid: Yield 9.44 mg (85%). 1H-NMR (600 MHz, CDCl3), δ 7.97 (2H, d, J = 6.8 Hz, Bz), 7.70-7.12 (40H, m, aromatic), 6.91 (2H, d, J = 8.8 Hz, MPM), 5.42 (1H, dd, J2′,1′ = 8.1 Hz, J2′,3′ = 8.1 Hz, H-2′), 5.37 (1H, d, J1″,2″ = 4.0 Hz, H-1″), 4.87 and 4.81 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.85 and 4.69 (each 1H, d, Jgem = 10.2 Hz, PhCH2), 4.82 and 4.78 (each 1H, d, Jgem = 13.6 Hz, PhCH2), 4.79 and 4.39 (each 1H, d, Jgem = 12.2 Hz, PhCH2), 4.70 (1H, d, J1′,2′ = 8.1 Hz, H-1′), 4.68 and 4.63 (each 1H, d, Jgem = 10.2 Hz, PhCH2), 4.67 and 4.42 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.34 (1H, d, J1,2 = 7.4 Hz, H-1), 4.20 (1H, dd, J4′,3′ = 8.8 Hz, J4′,5′ = 8.8 Hz, H-4′), 4.09 (1H, d, J6a,6b = 10.8 Hz, H-6a), 4.01 (1H, d, J5′,4′ = 8.8 Hz, H-5′), 3.94-3.89 (2H, m, H-3′, H-6a”), 3.81-3.78 (5H, m, H-3″, H-6b”, OCH3), 3.62-3.50 (9H, m, H-3, H-6b, H-4″, OCH3 × 2), 3.41-3.36 (2H, m, H-2, H-5), 3.32-3.28 (2H, m, H-4, H-5″), 3.25 (1H, dd, J2″,1″ = 4.0 Hz, J2″,3″ = 10.8 Hz, H-2″), 1.02 (9H, s, C(CH3)3); 13C-NMR (150 MHz, CDCl3), δ 168.3, 164.8, 159.4, 138.2, 137.2, 137.2, 135.8, 135.5, 130.0, 129.9, 129.6, 129.6, 128.4, 128.3, 128.3, 128.2, 128.1, 127.9, 127.8, 127.8, 127.8, 127.7, 127.7, 127.6, 127.6, 127.5, 113.9, 102.0, 101.0, 97.6, 84.4, 82.2, 82.0, 79.6, 79.2, 77.7, 75.6, 75.2, 74.7, 74.6, 74.5, 74.4, 74.2, 74.2, 73.0, 72.3, 70.7, 68.3, 63.1, 61.6, 60.7, 55.2, 52.5, 26.8; HRMS (positive mode); Found: m/z 1506.6133 [M+Na]+, Calcd. for C86H93N3O18SiNa: 1506.6121.
Benzyl (2-O-azido-2-O-deoxy-3-O-(4-methoxybenzyl)-4-O-methyl-α-d-glucopyranosyl)-(1→4)-(methyl 2-O-benzoyl-3-O-benzyl-β-d-glucopyranosyluronate)-(1→6)-2,3,4-tetra-O-benzyl-β-d-glucopyranoside (35)
To a solution of compound 34 (19.9 mg, 13.4 mmol) in pyridine (4 ml) was added HF•pyridine (174 μl, 6.70 mmol) at room temperature. The mixture was stirred for 2 days. The volatiles were removed in vacuo. Flash chromatography (silica gel: 5 g, toluene:EtOAc = 10:1→4:1) afforded compound 35 as a colorless oil: Yield 15.9 mg (95%). 1H-NMR (600 MHz, CDCl3), δ 7.97 (2H, d, J = 6.8 Hz, Bz), 7.47-7.13 (30H, m, aromatic), 6.90 (2H, d, J = 8.8 Hz, MPM), 5.41 (1H, d, J1″,2″ = 3.3 Hz, H-1″), 5.41 (1H, dd, J2′,1′ = 7.4 Hz, J2′,3′ = 8.8 Hz, H-2′), 4.88 and 4.86 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.82 and 4.69 (each 1H, d, Jgem = 10.2 Hz, PhCH2), 4.77 and 4.75 (each 1H, d, Jgem = 9.5 Hz, PhCH2), 4.73 (1H, d, J1′,2′ = 7.4 Hz, H-1′), 4.70 and 4.65 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.70 and 4.45 (each 1H, d, Jgem = 10.8 Hz, PhCH2), 4.69 and 4.42 (each 1H, d, Jgem = 10.2 Hz, PhCH2), 4.37 (1H, d, J1,2 = 8.1 Hz, H-1), 4.31 (1H, dd, J4′,3′ = 8.1 Hz, J4′,5′ = 8.8 Hz, H-4′), 4.12 (1H, dd, J6a,5 = 1.3 Hz, J6a,6b = 10.8 Hz, H-6a), 4.06 (1H, d, J5′,4′ = 8.8 Hz, H-5′), 3.94 (1H, dd, J3′,2′ = 8.8 Hz, J3′,4′ = 8.1 Hz, H-3′), 3.87-3.75 (8H, m, H-3″, H-6a”, OCH3 × 2), 3.69 (1H, dd, J6b”,5″ = 3.4 Hz, J6b”,6a” = 11.5 Hz, H-6b”), 3.60-3.54 (5H, m, H-3, H-6b, OCH3), 3.45-3.38 (3H, m, H-2, H-5, H-5″), 3.34 (1H, dd, J4,3 = 8.8 Hz, J4,5 = 10.2 Hz, H-4), 3.24-3.21 (2H, m, H-2″, H-4″); 13C-NMR (150 MHz, CDCl3), δ 168.6, 164.8, 138.4, 138.2, 133.2, 129.8, 129.6, 128.4, 128.3, 128.3, 128.3, 128.1, 127.9, 127.8, 127.8, 127.7, 127.7, 127.6, 113.8, 102.1, 101.0, 97.5, 84.4, 82.2, 82.0, 80.0, 79.3, 77.7, 75.6, 75.0, 75.0, 74.7, 74.6, 74.4, 74.4, 73.3, 72.1, 70.7, 68.3, 63.0, 61.2, 60.8, 55.2, 52.8; HRMS (positive mode); Found: m/z 1172.4349 [M+Na]+, Calcd. for C70H75N3O18Na: 1172.4344.
2-Deoxy-4-O-methyl-2-sulfoamino-α-d-glucopyranosyl-(1→4)-(β-d-glucopyranosyl uronic acid)-(1→6)-d-glucopyranose disodium salt (38)
To a solution of compound 35 (70.6 mg, 60.9 μmol) in methanol/THF (1:1, 1.6 ml) was added 1 M NaOH aq. (400 μl) at room temperature. After 24 h, the reaction was quenched by addition of Dowex 50W (H+). After removal of insoluble materials by filtration, the filtrate was concentrated in vacuo. To a solution of the residue in methanol/THF (1:1, 3.2 ml) was added 0.1 M NaOH aq. (974 μl) and 1 M PMe3 in THF (487 μl, 0.487 mmol) at room temperature. After 2 days, the reaction was quenched by addition of 0.1 M HCl aq. (974 μl). After the volatiles were removed in vacuo, the residue was dissolved in H2O (10 ml). SO3•pyridine (970 mg, 6.09 mmol) was added. The pH of the reaction mixture was maintained at 9.5 by the addition of 5 M NaOH aq. during the reaction. After 5 h, the volatiles were removed in vacuo. The residue was then suspended in MeOH. Insoluble materials were removed by filtration and the filtrate was concentrated in vacuo. Reversed phase chromatography (ODS: 6 g, H2O:MeOH = 8:1→0:1) was afforded compound 37. Obtained 37 was dissolved in H2O/MeOH/AcOH (5:5:1, 16 ml). Pd/C (10%, 51.2 mg) was added to the mixture. The reaction mixture was stirred for 6 days at room temperature under H2 (7 kg/cm2). After filtration by membrane filter, the filtrate was concentrated in vacuo. Gel filtration (Sephadex G-25 fine: φ1.5 × 1000 mm, H2O) afforded compound 38 as a white solid: Yield 4.52 mg (11%, α:β = 1:2, 4 steps). 1H-NMR (600 MHz, D2O), δ for β-anomer 5.40 (1H, d, J1″,2″ = 3.4 Hz, H-1″), 4.45 (1H, d, J1,2 = 8.1 Hz, H-1), 4.39 (1H, d, J1′,2′ = 8.1 Hz, H-1′), 3.97 (1H, d, J6a,6b = 11.5 Hz, H-6a), 3.92 (1H, d, J6a”,6b” = 11.5 Hz, H-6a”), 3.82 (1H, d, J5′,4′ = 8.8 Hz, H-5′), 3.67-3.60 (6H, m, H-5, H-6b, H-3′, H-4′, H-5″, H-6b”), 3.48 (1H, dd, J3″,2″ = 10.2 Hz, J3″,4″ = 9.5 Hz, H-3″), 3.41 (1H, dd, J4,3 = 8.8 Hz, J4,5 = 8.8 Hz, H-4), 3.36 (3H, s, OCH3), 3.28 (1H, dd, J3,2 = 8.8 Hz, J3,4 = 8.8 Hz, H-3), 3.22 (1H, dd, J2′,1′ = 8.1 Hz, J2′,3′ = 8.1 Hz, H-2′), 3.13 (1H, dd, J4″,3″ = 9.5 Hz, J4″,5″ = 10.2 Hz, H-4″), 3.07 (1H, dd, J2″,1″ = 3.4 Hz, J2″,3″ = 10.2 Hz, H-2″), 3.60 (1H, dd, J2,1 = 8.1 Hz, J2,3 = 8.8 Hz, H-2); 13C-NMR (150 MHz, D2O), δ 172.9, 102.6, 97.5, 95.8, 78.7, 76.4, 75.7, 75.5, 74.7, 73.9, 72.5, 70.8, 70.6, 70.2, 69.3, 68.9, 68.7, 59.6, 57.9, HRMS (negative mode); Found: m/z 632.1133 [M-Na]−, Calcd. for C19H31NO19SNa: 632.1114.
Scheme 6.
Synthesis of sulfated trisaccharide containing HP/HS disaccharide partial structure. (a) H2NNH2•H2O in pyridine/AcOH (3/2), 89%; (b) MeI, LHMDS in DMF, 85%; (c) HF•pyridine in pyridine, 95%; (d) 1 M NaOH aq. in MeOH/THF (1:1); (e) 1 M PMe3, 0.1 M NaOH aq. in THF/MeOH (1:1); (f) SO3•pyridine, pH 9.5 in H2O; (g) 10% Pd/C, H2 (7 kg/cm2) in H2O/MeOH/AcOH (5:5:1), 11% (4 steps).
Acknowledgements
The present work was financially supported in part by grants from Japan Science and Technology Agency (Evolutionally-venture program V07-05 to Y.S., CREST to Y.S.), and the Japan Ministry of Health, Labour and Welfare (MHLW) (Nanomedicine program to Y.S.), and the National Institutes of Health, USA (RO1 HL079182 to M.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Garg HG, Linhardt RJ, Hales CA, editors. Chemistry and Biology of Heparin and Heparan Sulfate. Elsevier; Amsterdam, The Netherlands: 2005. [Google Scholar]
- 2(a).Conrad HE. Heparin-Binding Proteins. Academic Press; San Diego, USA: 1998. [Google Scholar]; (b) Imberty A, Lortat-Jacob H, Pérez S. Carbohydr. Res. 2007;342:430–439. doi: 10.1016/j.carres.2006.12.019. [DOI] [PubMed] [Google Scholar]; (c) Raman R, Sasisekharan V, Sasisekharan R. Chem. Biol. 2005;12:267–277. doi: 10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]; (d) Coombe DR, Kett WC. Cell. Mol. Life Sci. 2005;62:410–424. doi: 10.1007/s00018-004-4293-7. [DOI] [PubMed] [Google Scholar]; (e) Petitou M, van Boeckel CAA. Angew. Chem. Int. Ed. 2004;43:3118–3133. doi: 10.1002/anie.200300640. [DOI] [PubMed] [Google Scholar]; (f) Rabenstein DA. Nat. Prod. Rep. 2002;19:312–331. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]; (g) Capila I, Linhardt RJ. Angew. Chem., Int. Ed. 2002;41:390–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; (h) Turnbull J, Powell A, Guimond S. Trends Cell Biol. 2001;11:75–82. doi: 10.1016/s0962-8924(00)01897-3. [DOI] [PubMed] [Google Scholar]; (i) Casu B, Lindahl U. Adv. Carbohydr. Chem. Biochem. 2001;57:159–206. doi: 10.1016/s0065-2318(01)57017-1. [DOI] [PubMed] [Google Scholar]; (j) Esko JD, Lindahl U. J. Clin. Invest. 2001;108:169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Annu. Rev. Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 3.For comprehensive reviews on the synthesis of GAGs, see: Noti C, Seeberger PH. Chem. Biol. 2005;12:731–756. doi: 10.1016/j.chembiol.2005.05.013. Karst NA, Linhardt RJ. Curr. Med. Chem. 2003;10:1993–2031. doi: 10.2174/0929867033456891. Poletti L, Lay L. Eur. J. Org. Chem. 2003:2999–3024. Yeung BKS, Chong PYC, Petillo PA. J. Carbohydr. Chem. 2002;21:799–865. Tamura J. Trends Glycosci. Glycotechnol. 2001;13:65–88.
- 4.For Recent articles on the synthesis of GAGs see: Polat T, Wong CH. J. Am. Chem. Soc. 2007;129:12795–12800. doi: 10.1021/ja073098r. Zhou Y, Lin F, Chen J, Yu B. Carbohydr. Res. 2006;341:1619–1629. doi: 10.1016/j.carres.2006.02.020. Noti C, de Paz JL, Polito L, Seeberger PH. Chem.-Eur. J. 2006;12:8664–8686. doi: 10.1002/chem.200601103. de Paz JL, Noti C, Seeberger PH. J. Am. Chem. Soc. 2006;128:2766–2767. doi: 10.1021/ja057584v. Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, Goddard WA, Nishi A, Hsieh-Wilson LC. Nat. Chem. Biol. 2006;2:467–473. doi: 10.1038/nchembio810. Codée JDC, Stubba B, Schiattarella M, Overkleeft HS, van Boeckel CAA, van Boom JH, van der Marel GA. J. Am, Chem. Soc. 2005;127:3767–3773. doi: 10.1021/ja045613g. Lee JC, Lu XA, Kulkarni SS, Wen YS, Hung SC. J. Am. Chem. Soc. 2004;126:476–477. doi: 10.1021/ja038244h. Orgueira HA, Bartolozzi A, Schell P, Litjens RE, Palmacci ER, Seeberger PH. Chem.–Eur. J. 2003;9:140–169. doi: 10.1002/chem.200390009. de Paz JL, Ojeda R. Reichadt, Martín-Lomas M. Eur. J. Org. Chem. 2003:3308–3324. Kovensky J, Mallet JM, Esnault J, Driguez PA, Sizun P, Hérault JP, Herbert JM, Petitou M, Sinaÿ P. Eur. J. Org. Chem. 2002:3595–3603. de Paz JL, Angulo J, Lassaletta JM, Nieto PM, Redondo-Horcajo M, Lozano RM, Gimenez-Gallego G, Martin-Lomas M. ChemBioChem. 2001;2:673–685. doi: 10.1002/1439-7633(20010903)2:9<673::AID-CBIC673>3.0.CO;2-7. And references cited therein.
- 5.For recent articles on HP/HS oligosaccharide library, see: Hecht ML, Rosental B, Horlacher T, Hershkovitz O, de Paz JL, Noti C, Schauer S, Porgador A, Seeberger P. J. Proteome Res. 2009;8:712–720. doi: 10.1021/pr800747c. Herczeg M, Lázár L, Borbás A, Lipták A, Antus S. Org. Lett. 2009;11:2619–2622. doi: 10.1021/ol900952d. Dilhas A, Lucas R, Loureiro-Morais L, Hersant Y, Bonnaffé D. J. Comb. Chem. 2008;10:166–169. doi: 10.1021/cc8000019. de Paz JL, Moseman EA, Noti C, Polito L, von Andrian UH, Seeberger PJ. ACS Chem. Biol. 2007;2:735–744. doi: 10.1021/cb700159m. Noti C, de Paz JL, Polito L, Seeberger PH. Chem.–Eur. J. 2006;12:8664–8686. doi: 10.1002/chem.200601103. Lu L-D, Shie C-R, Kulkarni SS, Pan G-R, Lu X-A, Hung S-C. Org. Lett. 2006;8:5995–5998. doi: 10.1021/ol062464t. Fan R-H, Achkar J, Hernándes-Torres JM, Wei A. Org. Lett. 2005;7:5095–5098. doi: 10.1021/ol052130o.
- 6.For our previous studies, see: Wakao M, Saito A, Ohishi K, Kishimoto Y, Nishimura T, Sobel M, Suda Y. Bioorg. Med. Chem. Lett. 2008;18:2499–2504. doi: 10.1016/j.bmcl.2008.01.069. Suda Y, Arano A, Fukui Y, Koshida S, Wakao M, Nishimura T, Kusumoto S, Sobel M. Bioconjugate Chem. 2006;17:1125–1135. doi: 10.1021/bc0600620. Koshida S, Suda Y, Sobel M, Kusumoto S. Tetrahedron Lett. 2001;42:1289–1292. Koshida S, Suda Y, Fukui Y, Ormsby J, Sobel M, Kusumoto S. Tetrahedron Lett. 1999;40:5725–5728. Suda Y, Bird K, Shiyama T, Koshida S, Marques D, Fukase K, Sobel M, Kusumoto S. Tetrahedron Lett. 1996;37:1053–1056.
- 7.For articles on the synthesis of uronic acids moieties, see: Hansen SU, Baráth M, Salameh BAB, Pritchard RG, Stimpson WT, Gardiner JM, Jayson GC. Org. Lett. 2009;11:4528–4531. doi: 10.1021/ol901723m. Tatai J, Osztrovszky G, Kajtár-Peredy M, Fügedi P. Carbohydr. Res. 2008;343:596–606. doi: 10.1016/j.carres.2007.12.015. Hassan HHAM. Mini-Rev. Org. Chem. 2007;4:61–74. Lohman GJS, Hunt DK, Högermeier JA, Seeberger PH. J. Org. Chem. 2003;68:7559–7561. doi: 10.1021/jo0340760. Ke W, Whitfield DM, Gill M, Larocque S, Yu S-H. Tetrahedron Lett. 2003;44:7767–7770. Barroca N, Jacquinet J-C. Carbohydr. Res. 2000;329:667–679. doi: 10.1016/s0008-6215(00)00234-2. Lubineau A, Alais GJ, Bonnaffé D. Tetrahedron Lett. 2000;41:307–311. Ojeda R, de Paz JL, Martin-Lomas M, Lassaletta JM. Synlett. 1999;8:1316–1318. Hinou H, Kurosawa H, Matsuoka K, Terunuma D, Kuzuhara H. Tetrahedron Lett. 1999;40:1501–1504. Rochepeau-Jobron L, Jacquinet J-C. Carbohydr. Res. 1997;303:395–406. doi: 10.1016/s0008-6215(98)00298-5. Medakovic D. Carbohydr. Res. 1994;253:299–300. Jacquinet J-C, Petitou M, Duchaussoy P, Lederman I, Choay J, Torri G, Sinaÿ P. Carbohydr. Res. 1984;130:221–241. doi: 10.1016/s0008-6215(00)90633-5. Czuk R, Hoenig H, Nimpf J, Weidmann H. Tetrahedron Lett. 1980;21:2135–2136.
- 8.Greene TW, Wuts PGM. Protective Groups in Organic Synthesis. 3rd Ed John Wiley & Sons, Inc; New York: 1999. [Google Scholar]
- 9.A trace amount of furanose form was observed by NMR analysis.
- 10.Alper PB, Hung S-C, Wong C-H. Tetrahedron Lett. 1996;37:6029–6032. [Google Scholar]
- 11.α-Selective glycosylation with 2-azido imidate in the presence of TBDMSOTf was previously reported, see: Kovensky J, Duchaussoy P, Petitou M, Sinaÿ P. Tetrahedron: Asymmetry. 1996;7:3119–3128.