Abstract
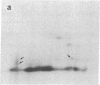
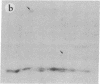
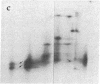
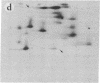
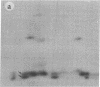
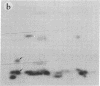
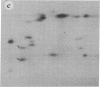
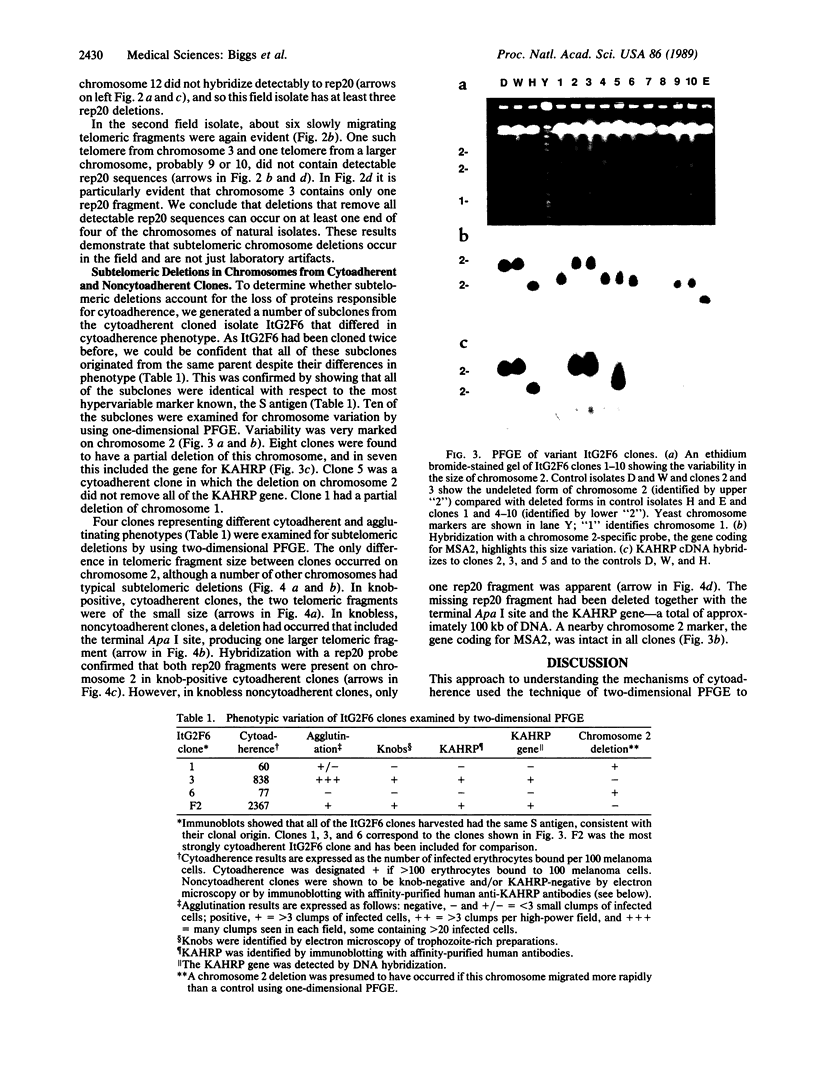
Subtelomeric deletions are responsible for the loss of expression of several Plasmodium falciparum antigens, including the knob-associated histidine-rich protein (KAHRP). Such deletions are detectable by two-dimensional pulsed-field gradient electrophoresis (PFGE) in which the chromosomes separated in dimension 1 are cleaved with Apa I, and the sizes of telomeric fragments are determined in dimension 2. This sensitive technique has enabled us to examine the role of subtelomeric deletions in two aspects of the biology of Plasmodium falciparum. First, we show that similar subtelomeric deletions to those that occur in vitro also occur in field isolates. Second, we demonstrate a correlation between subtelomeric deletions and loss of the phenotype of "cytoadherence" in cultured isolates. Subclones were generated from the cytoadherent cloned isolate ItG2F6, and their phenotypes were examined with respect to cytoadherence, the expression of "knobs," and agglutination of infected erythrocytes with rabbit antiserum. The only chromosomal change detectable by two-dimensional PFGE among subclones that differ from wild type in each of these three characteristics is a deletion of approximately 100 kilobases at one end of chromosome 2. This deletion includes the gene coding for KAHRP and the subtelomeric repeat designated rep20.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aley S. B., Sherwood J. A., Howard R. J. Knob-positive and knob-negative Plasmodium falciparum differ in expression of a strain-specific malarial antigen on the surface of infected erythrocytes. J Exp Med. 1984 Nov 1;160(5):1585–1590. doi: 10.1084/jem.160.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders R. F., Brown G. V., Edwards A. Characterization of an S antigen synthesized by several isolates of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6652–6656. doi: 10.1073/pnas.80.21.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Forsyth K. P., Bianco A. E., Brown G. V., Kemp D. J. Chromosome size polymorphisms in Plasmodium falciparum can involve deletions and are frequent in natural parasite populations. Cell. 1986 Jan 17;44(1):87–95. doi: 10.1016/0092-8674(86)90487-3. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Thompson J. K., Walliker D., Kemp D. J. Homologous recombination within subtelomeric repeat sequences generates chromosome size polymorphisms in P. falciparum. Cell. 1988 Jun 3;53(5):807–813. doi: 10.1016/0092-8674(88)90097-9. [DOI] [PubMed] [Google Scholar]
- Crewther P. E., Bianco A. E., Brown G. V., Coppel R. L., Stahl H. D., Kemp D. J., Anders R. F. Affinity purification of human antibodies directed against cloned antigens of Plasmodium falciparum. J Immunol Methods. 1986 Feb 12;86(2):257–264. doi: 10.1016/0022-1759(86)90462-x. [DOI] [PubMed] [Google Scholar]
- Culvenor J. G., Langford C. J., Crewther P. E., Saint R. B., Coppel R. L., Kemp D. J., Anders R. F., Brown G. V. Plasmodium falciparum: identification and localization of a knob protein antigen expressed by a cDNA clone. Exp Parasitol. 1987 Feb;63(1):58–67. doi: 10.1016/0014-4894(87)90078-6. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Barnwell J. W., Rock E. P., Neequaye J., Ofori-Adjei D., Maloy W. L., Lyon J. A., Saul A. Two approximately 300 kilodalton Plasmodium falciparum proteins at the surface membrane of infected erythrocytes. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):207–223. doi: 10.1016/0166-6851(88)90040-0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Corcoran L. M., Coppel R. L., Stahl H. D., Bianco A. E., Brown G. V., Anders R. F. Size variation in chromosomes from independent cultured isolates of Plasmodium falciparum. Nature. 1985 May 23;315(6017):347–350. doi: 10.1038/315347a0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Thompson J. K., Walliker D., Corcoran L. M. Molecular karyotype of Plasmodium falciparum: conserved linkage groups and expendable histidine-rich protein genes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7672–7676. doi: 10.1073/pnas.84.21.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech J. H., Barnwell J. W., Miller L. H., Howard R. J. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984 Jun 1;159(6):1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson G. G., Warrell M. J., White N. J., Looareesuwan S., Warrell D. A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985 Jun;119(3):385–401. [PMC free article] [PubMed] [Google Scholar]
- Patarapotikul J., Langsley G. Chromosome size polymorphism in Plasmodium falciparum can involve deletions of the subtelomeric pPFrep20 sequence. Nucleic Acids Res. 1988 May 25;16(10):4331–4340. doi: 10.1093/nar/16.10.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. 1986 Jul 31-Aug 6Nature. 322(6078):474–477. doi: 10.1038/322474a0. [DOI] [PubMed] [Google Scholar]
- Rosario V. Cloning of naturally occurring mixed infections of malaria parasites. Science. 1981 May 29;212(4498):1037–1038. doi: 10.1126/science.7015505. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Sherwood J. A., Marsh K., Howard R. J., Barnwell J. W. Antibody mediated strain-specific agglutination of Plasmodium falciparum--parasitized erythrocytes visualized by ethidium bromide staining. Parasite Immunol. 1985 Nov;7(6):659–663. doi: 10.1111/j.1365-3024.1985.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Smythe J. A., Coppel R. L., Brown G. V., Ramasamy R., Kemp D. J., Anders R. F. Identification of two integral membrane proteins of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5195–5199. doi: 10.1073/pnas.85.14.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Triglia T., Stahl H. D., Crewther P. E., Scanlon D., Brown G. V., Anders R. F., Kemp D. J. The complete sequence of the gene for the knob-associated histidine-rich protein from Plasmodium falciparum. EMBO J. 1987 May;6(5):1413–1419. doi: 10.1002/j.1460-2075.1987.tb02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udeinya I. J., Graves P. M., Carter R., Aikawa M., Miller L. H. Plasmodium falciparum: effect of time in continuous culture on binding to human endothelial cells and amelanotic melanoma cells. Exp Parasitol. 1983 Oct;56(2):207–214. doi: 10.1016/0014-4894(83)90064-4. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Leech J., Aikawa M., Miller L. H. An in vitro assay for sequestration: binding of Plasmodium falciparum-infected erythrocytes to formalin-fixed endothelial cells and amelanotic melanoma cells. J Protozool. 1985 Feb;32(1):88–90. doi: 10.1111/j.1550-7408.1985.tb03019.x. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Schmidt J. A., Aikawa M., Miller L. H., Green I. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981 Jul 31;213(4507):555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]
- Vernick K. D., McCutchan T. F. Sequence and structure of a Plasmodium falciparum telomere. Mol Biochem Parasitol. 1988 Mar;28(2):85–94. doi: 10.1016/0166-6851(88)90055-2. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Walliker D., Smith C. L., do Rosario V. E., Maloy W. L., Howard R. J., Carter R., McCutchan T. F. A histidine-rich protein gene marks a linkage group favored strongly in a genetic cross of Plasmodium falciparum. Cell. 1987 Jun 5;49(5):633–642. doi: 10.1016/0092-8674(87)90539-3. [DOI] [PubMed] [Google Scholar]