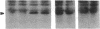Abstract
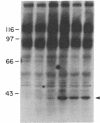
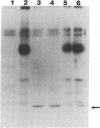
We have used the hippocampal slice preparation to investigate the regulation of protein tyrosine phosphorylation in brain. After pharmacological treatment of intact slices, proteins were separated by electrophoresis, and levels of protein tyrosine phosphorylation were assessed by immunoblotting with specific anti-phosphotyrosine antibodies. Phorbol esters, activators of the serine- and threonine-phosphorylating enzyme protein kinase C, selectively increase tyrosine phosphorylation of a soluble protein with an apparent molecular mass of approximately 40 kilodaltons. Muscarinic agonists such as carbachol and oxotremorine M that strongly activate the inositol phospholipid system also increase tyrosine phosphorylation of this protein. Neurotransmitter activation of the inositol phospholipid system and protein kinase C appears to trigger a cascade leading to increased tyrosine phosphorylation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Baraban J. M., Snyder S. H., Alger B. E. Protein kinase C regulates ionic conductance in hippocampal pyramidal neurons: electrophysiological effects of phorbol esters. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2538–2542. doi: 10.1073/pnas.82.8.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R., Martinez R., Nakamura K. D., Weber M. J. A tumor promoter stimulates phosphorylation on tyrosine. Biochem Biophys Res Commun. 1983 Sep 15;115(2):536–543. doi: 10.1016/s0006-291x(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Jaken S., König B., Sharkey N. A., Leach K. L., Jeng A. Y., Yeh E. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands. Biochem Pharmacol. 1984 Mar 15;33(6):933–940. doi: 10.1016/0006-2952(84)90448-9. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Major substrate for growth factor-activated protein-tyrosine kinases is a low-abundance protein. Mol Cell Biol. 1985 Nov;5(11):3304–3309. doi: 10.1128/mcb.5.11.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M. R., Moss S. E., Crumpton M. J. Diversity in the lipocortin/calpactin family. Cell. 1988 Oct 7;55(1):1–3. doi: 10.1016/0092-8674(88)90002-5. [DOI] [PubMed] [Google Scholar]
- Ellis P. D., Bissoon N., Gurd J. W. Synaptic protein tyrosine kinase: partial characterization and identification of endogenous substrates. J Neurochem. 1988 Aug;51(2):611–620. doi: 10.1111/j.1471-4159.1988.tb01082.x. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Bartus R. T. Regional differences in the coupling of muscarinic receptors to inositol phospholipid hydrolysis in guinea pig brain. J Neurochem. 1985 Oct;45(4):1085–1095. doi: 10.1111/j.1471-4159.1985.tb05527.x. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Figueiredo J. C., Bartus R. T. Differential stimulation of inositol phospholipid turnover in brain by analogs of oxotremorine. J Neurochem. 1984 Oct;43(4):1171–1179. doi: 10.1111/j.1471-4159.1984.tb12858.x. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Klinger P. D., Agranoff B. W. Muscarinic agonist binding and phospholipid turnover in brain. J Biol Chem. 1983 Jun 25;258(12):7358–7363. [PubMed] [Google Scholar]
- Fisher S. K., Snider R. M. Differential receptor occupancy requirements for muscarinic cholinergic stimulation of inositol lipid hydrolysis in brain and in neuroblastomas. Mol Pharmacol. 1987 Jul;32(1):81–90. [PubMed] [Google Scholar]
- Gilmore T., Martin G. S. Phorbol ester and diacylglycerol induce protein phosphorylation at tyrosine. Nature. 1983 Dec 1;306(5942):487–490. doi: 10.1038/306487a0. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L., Kamps M. P. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988 May 9;109(2):277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Grunberger G., Zick Y., Taylor S. I., Gorden P. Tumor-promoting phorbol ester stimulates tyrosine phosphorylation in U-937 monocytes. Proc Natl Acad Sci U S A. 1984 May;81(9):2762–2766. doi: 10.1073/pnas.81.9.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A. A., Greengard P., Huganir R. L. Protein tyrosine kinase activity and its endogenous substrates in rat brain: a subcellular and regional survey. J Neurochem. 1988 May;50(5):1447–1455. doi: 10.1111/j.1471-4159.1988.tb03029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G. Y., Hvalby O., Walaas S. I., Albert K. A., Skjeflo P., Andersen P., Greengard P. Protein kinase C injection into hippocampal pyramidal cells elicits features of long term potentiation. 1987 Jul 30-Aug 5Nature. 328(6129):426–429. doi: 10.1038/328426a0. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer J. A., Malenka R. C., Nicoll R. A. NMDA application potentiates synaptic transmission in the hippocampus. Nature. 1988 Jul 21;334(6179):250–252. doi: 10.1038/334250a0. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Commun. 1984 Nov 30;125(1):258–264. doi: 10.1016/s0006-291x(84)80362-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lovinger D. M., Wong K. L., Murakami K., Routtenberg A. Protein kinase C inhibitors eliminate hippocampal long-term potentiation. Brain Res. 1987 Dec 8;436(1):177–183. doi: 10.1016/0006-8993(87)91573-3. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Andrade R., Nicoll R. A. Phorbol esters mimic some cholinergic actions in hippocampal pyramidal neurons. J Neurosci. 1986 Feb;6(2):475–480. doi: 10.1523/JNEUROSCI.06-02-00475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Nicoll R. A. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986 May 8;321(6066):175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Madison D. V., Tsien R. W. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988 Oct 27;335(6193):820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- McKinney M., Richelson E. The coupling of the neuronal muscarinic receptor to responses. Annu Rev Pharmacol Toxicol. 1984;24:121–146. doi: 10.1146/annurev.pa.24.040184.001005. [DOI] [PubMed] [Google Scholar]
- McKinney M., Stenstrom S., Richelson E. Muscarinic responses and binding in a murine neuroblastoma clone (N1E-115). Mediation of separate responses by high affinity and low affinity agonist-receptor conformations. Mol Pharmacol. 1985 Feb;27(2):223–235. [PubMed] [Google Scholar]
- Moon S. O., Palfrey H. C., King A. C. Phorbol esters potentiate tyrosine phosphorylation of epidermal growth factor receptors in A431 membranes by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2298–2302. doi: 10.1073/pnas.81.8.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987 Jul 10;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Nathanson N. M. Molecular properties of the muscarinic acetylcholine receptor. Annu Rev Neurosci. 1987;10:195–236. doi: 10.1146/annurev.ne.10.030187.001211. [DOI] [PubMed] [Google Scholar]
- Ohtsuka M., Ihara S., Ogawa R., Watanabe T., Watanabe Y. Preparation and characterization of antibodies to O-phosphotyrosine and their use for identification of phosphotyrosine-containing proteins. Int J Cancer. 1984 Dec 15;34(6):855–861. doi: 10.1002/ijc.2910340618. [DOI] [PubMed] [Google Scholar]
- Pang D. T., Wang J. K., Valtorta F., Benfenati F., Greengard P. Protein tyrosine phosphorylation in synaptic vesicles. Proc Natl Acad Sci U S A. 1988 Feb;85(3):762–766. doi: 10.1073/pnas.85.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Tizard R., Mattaliano R. J., Sinclair L. K., Miller G. T., Browning J. L., Chow E. P., Burne C., Huang K. S., Pratt D. Five distinct calcium and phospholipid binding proteins share homology with lipocortin I. J Biol Chem. 1988 Aug 5;263(22):10799–10811. [PubMed] [Google Scholar]
- Saffen D. W., Cole A. J., Worley P. F., Christy B. A., Ryder K., Baraban J. M. Convulsant-induced increase in transcription factor messenger RNAs in rat brain. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7795–7799. doi: 10.1073/pnas.85.20.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K. R., Baraban J. M., Worley P. F. Norepinephrine stimulation of adenylate cyclase potentiates protein kinase C action: electrophysiological studies in the dentate gyrus. Synapse. 1988;2(6):614–618. doi: 10.1002/syn.890020606. [DOI] [PubMed] [Google Scholar]
- Sudol M., Hanafusa H. Cellular proteins homologous to the viral yes gene product. Mol Cell Biol. 1986 Aug;6(8):2839–2846. doi: 10.1128/mcb.6.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas S. I., Lustig A., Greengard P., Brugge J. S. Widespread distribution of the c-src gene product in nerve cells and axon terminals in the adult rat brain. Brain Res. 1988 Jun;427(3):215–222. doi: 10.1016/0169-328x(88)90044-7. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., McCarren M., Snyder S. H., Alger B. E. Cholinergic phosphatidylinositol modulation of inhibitory, G protein-linked neurotransmitter actions: electrophysiological studies in rat hippocampus. Proc Natl Acad Sci U S A. 1987 May;84(10):3467–3471. doi: 10.1073/pnas.84.10.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]