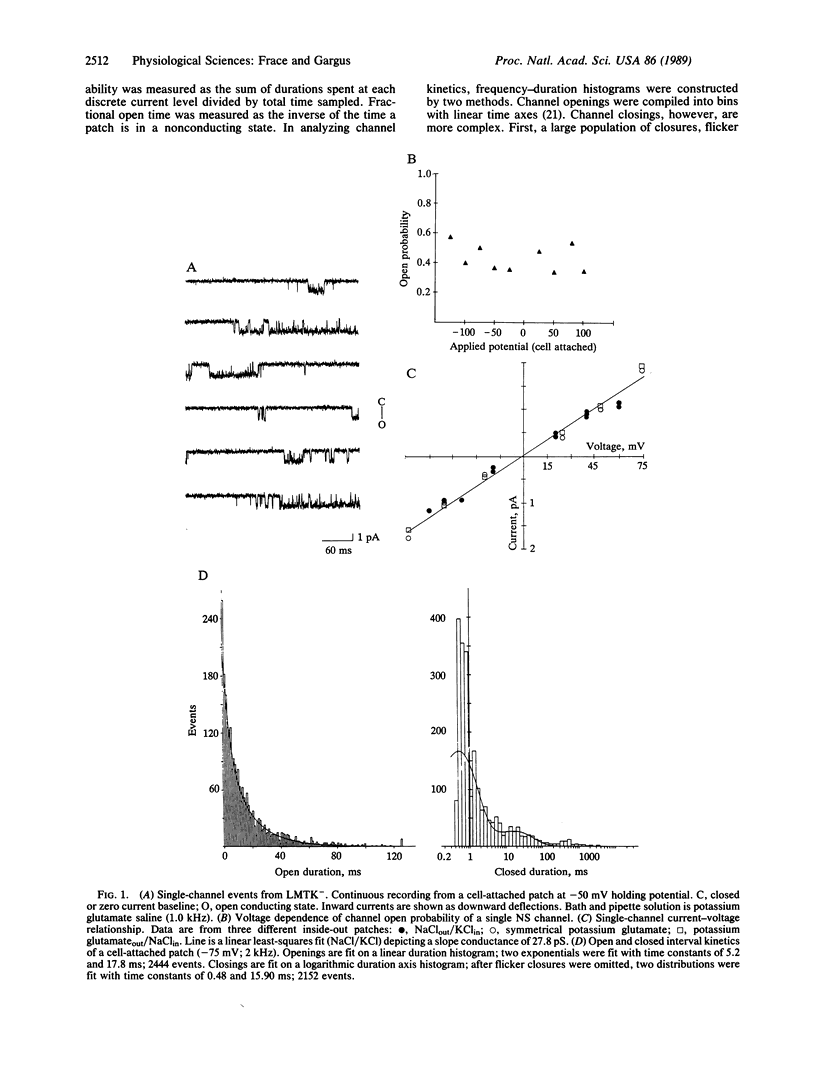
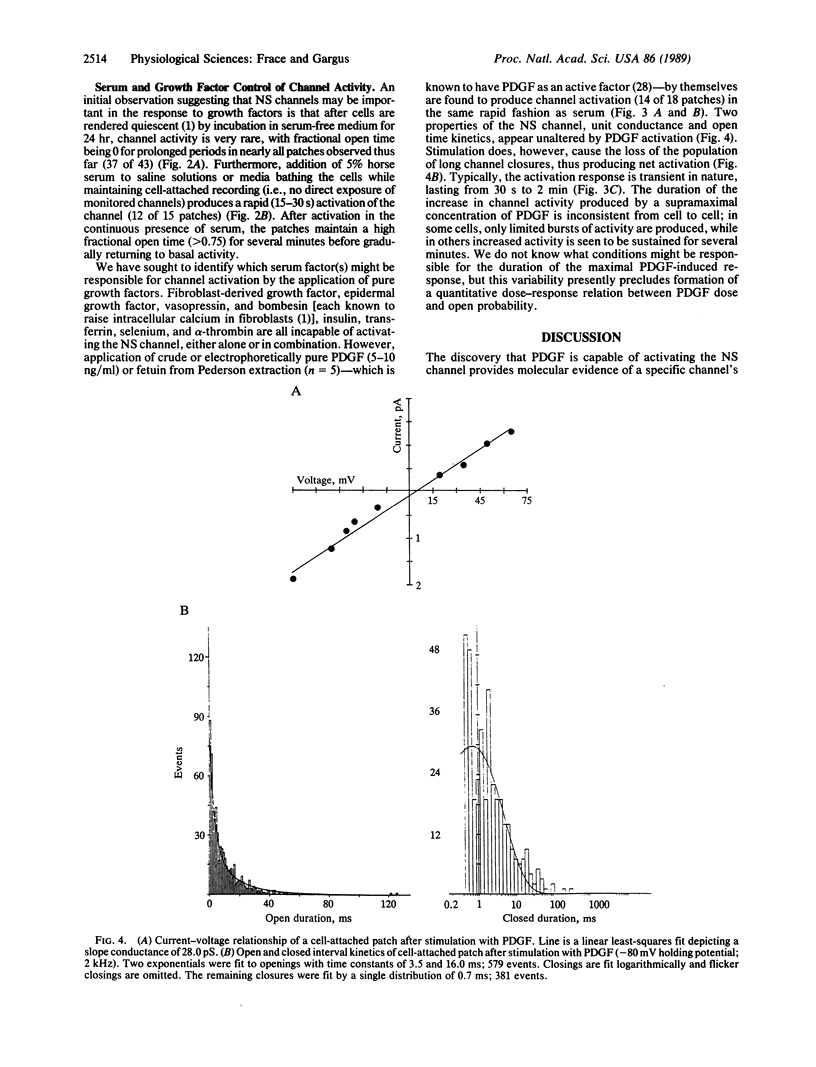
Abstract
A nonselective cation channel that we characterized in the mouse L-cell membrane becomes quiescent with serum deprivation (arrested cell growth) and rapidly active upon readdition of serum or, specifically, platelet-derived growth factor (PDGF). Using the patch-clamp technique, we find that the predominant channel in the LMTK- cell line is a bursting nonselective cation channel (the NS channel). In cell-attached and inside-out patches, the channel has a conductance of 28 pS; equal selectivity for Na+, K+, and Cs+; and no anion or divalent cation permeability. The channel open probability is voltage insensitive and in inside-out patches does not correlate with intracellular calcium (0.5 nM to 50 microM). When cultures are rendered quiescent by incubation in serum-free medium, channel open probability is virtually 0 as compared to 0.26 (+/- 0.17) in exponentially growing cultures. If mitogenesis is initiated by readdition of serum to quiescent cells while maintaining cell-attached recording, there is a rapid (15-30 s) activation of the channel (n = 12). The open probability of the patch increases (greater than 0.75) for 2-3 min and then decreases. We have attempted applications of several growth factors (fibroblast-derived growth factor, epidermal growth factor, insulin, bombesin, alpha-thrombin, and vasopressin, individually or in combination) but find that only PDGF (5-100 ng/ml; n = 9) produces channel activation. This activation should provide a Na+ entry pathway parallel to that of the Na/H exchanger.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Regulation of ion channels by inositol trisphosphate and diacylglycerol. J Exp Biol. 1986 Sep;124:323–335. doi: 10.1242/jeb.124.1.323. [DOI] [PubMed] [Google Scholar]
- Cassel D., Rothenberg P., Zhuang Y. X., Deuel T. F., Glaser L. Platelet-derived growth factor stimulates Na+/H+ exchange and induces cytoplasmic alkalinization in NR6 cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6224–6228. doi: 10.1073/pnas.80.20.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Ikeuchi M., Fujimoto W. Y. Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- DeCoursey T. E., Chandy K. G., Gupta S., Cahalan M. D. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984 Feb 2;307(5950):465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- Fichtner H., Fröbe U., Busse R., Kohlhardt M. Single nonselective cation channels and Ca2+-activated K+ channels in aortic endothelial cells. J Membr Biol. 1987;98(2):125–133. doi: 10.1007/BF01872125. [DOI] [PubMed] [Google Scholar]
- Gargus J. J., Miller I. L., Slayman C. W., Adelberg E. A. Genetic alterations in potassium transport in L cells. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5589–5593. doi: 10.1073/pnas.75.11.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargus J. J., Slayman C. W. Mechanism and role of furosemide-sensitive K+ transport in L cells: a genetic approach. J Membr Biol. 1980;52(3):245–256. doi: 10.1007/BF01869193. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. K. Phorbol esters, protein phosphorylation and the regulation of neuronal ion channels. J Exp Biol. 1986 Sep;124:375–392. doi: 10.1242/jeb.124.1.375. [DOI] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- Libby P., Raines E. W., Cullinane P. M., Ross R. Analysis of the mitogenic effect of fetuin preparations on arterial smooth muscle cells: the role of contaminant platelet-derived growth factor. J Cell Physiol. 1985 Dec;125(3):357–366. doi: 10.1002/jcp.1041250302. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivas A., Adelberg E. A., Rozengurt E. Intracellular K+ and the mitogenic response of 3T3 cells to peptide factors in serum-free medium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6275–6279. doi: 10.1073/pnas.79.20.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rivas A., Adelberg E. A., Rozengurt E. Intracellular K+ and the mitogenic response of 3T3 cells to peptide factors in serum-free medium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6275–6279. doi: 10.1073/pnas.79.20.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H. Cholecystokinin activation of single-channel currents is mediated by internal messenger in pancreatic acinar cells. Nature. 1982 Nov 4;300(5887):61–63. doi: 10.1038/300061a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Peterson O. H. Single-channel currents in isolated patches of plasma membrane from basal surface of pancreatic acini. Nature. 1982 Sep 9;299(5879):159–161. doi: 10.1038/299159a0. [DOI] [PubMed] [Google Scholar]
- Mitas M., Coogan C., Gargus J. J. Gene transfer of a putative mammalian K+ channel gene from genomic and cosmid DNA. Am J Physiol. 1988 Jul;255(1 Pt 1):C12–C18. doi: 10.1152/ajpcell.1988.255.1.C12. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Defize L. H., De Laat S. W. Ionic signalling by growth factor receptors. J Exp Biol. 1986 Sep;124:359–373. doi: 10.1242/jeb.124.1.359. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H. Effects of growth factors on intracellular pH regulation. Annu Rev Physiol. 1986;48:363–376. doi: 10.1146/annurev.ph.48.030186.002051. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Growth factors immediately raise cytoplasmic free Ca2+ in human fibroblasts. J Biol Chem. 1984 Jul 10;259(13):8066–8069. [PubMed] [Google Scholar]
- Moolenaar W. H., de Laat S. W., van der Saag P. T. Serum triggers a sequence of rapid ionic conductance changes in quiescent neuroblastoma cells. Nature. 1979 Jun 21;279(5715):721–723. doi: 10.1038/279721a0. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Peacock J., Minna J. An active electrical response in fibroblasts. J Gen Physiol. 1972 Jul;60(1):58–71. doi: 10.1085/jgp.60.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Findlay I., Suzuki K., Dunne M. J. Messenger-mediated control of potassium channels in secretory cells. J Exp Biol. 1986 Sep;124:33–52. doi: 10.1242/jeb.124.1.33. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Sardet C., Franchi A., L'Allemain G., Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg P., Reuss L., Glaser L. Serum and epidermal growth factor transiently depolarize quiescent BSC-1 epithelial cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7783–7787. doi: 10.1073/pnas.79.24.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Neher E. Patch clamp techniques for studying ionic channels in excitable membranes. Annu Rev Physiol. 1984;46:455–472. doi: 10.1146/annurev.ph.46.030184.002323. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Transducin and the cyclic GMP phosphodiesterase: amplifier proteins in vision. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):841–852. doi: 10.1101/sqb.1983.048.01.087. [DOI] [PubMed] [Google Scholar]
- Tertoolen L. G., Tilly B. C., Irvine R. F., Moolenaar W. H. Electrophysiological responses to bradykinin and microinjected inositol polyphosphates in neuroblastoma cells. Possible role of inositol 1,3,4-trisphosphate in altering membrane potential. FEBS Lett. 1987 Apr 20;214(2):365–369. doi: 10.1016/0014-5793(87)80089-3. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Aelvoet I., Erlij D. Oxytocin and cAMP stimulate monovalent cation movements through a Ca2+-sensitive, amiloride-insensitive channel in the apical membrane of toad urinary bladder. Proc Natl Acad Sci U S A. 1987 Jan;84(1):313–317. doi: 10.1073/pnas.84.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villereal M. L. Sodium fluxes in human fibroblasts: effect of serum, Ca+2, and amiloride. J Cell Physiol. 1981 Jun;107(3):359–369. doi: 10.1002/jcp.1041070307. [DOI] [PubMed] [Google Scholar]
- Villereal M. L. Sodium fluxes in human fibroblasts: kinetics of serum-dependent and serum-independent pathways. J Cell Physiol. 1981 Aug;108(2):251–259. doi: 10.1002/jcp.1041080215. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]


