Abstract
To investigate the intestinal absorption of a fibrinolytic and proteolytic lumbrokinase extracted from Eisenia andrei, we used rat everted gut sacs and an in situ closed-loop recirculation method. We extracted lumbrokinase from Eisenia andrei, and then raised polyclonal antibody against lumbrokinase. Fibrinolytic activity and proteolytic activity in the serosal side of rat everted gut sacs incubated with lumbrokinase showed dose- and time-dependent patterns. Immunological results obtained by western blotting serosal side solution using rat everted gut sacs method showed that lumbrokinase proteins between 33.6 and 54.7 kDa are absorbed mostly by the intestinal epithelium. Furthermore, MALDI-TOF mass spectrometric analysis of plasma fractions obtained by in situ recirculation method confirmed that lumbrokinase F1 is absorbed into blood. These results support the notion that lumbrokinase can be absorbed from mucosal lumen into blood by oral administration.
Keywords: Eisenia andrei, Fibrinolytic enzymes, Intestinal proteinase absorption, Gut sac, Recirculation intestinal perfusion
INTRODUCTION
The fibrinolytic enzymes, such as, urokinase, tissue type plasminogen activator (t-PA), and streptokinase, are widely used for thrombosis therapy. However, these enzymes have a low specificity for fibrin and are expensive. Mihara et al. reported that extracts of earthworm, Lumbericus rubellus, contain six different fibrinolytic and thrombolytic isoenzymes [1]. Furthermore, these enzymes have strong fibrinolytic activities, broad pH optima and resist thermal denaturation [2]. These properties are useful for extracts used to treat some clotting disorders, ischemic stroke, or myocardial infarction. The intestinal absorption of intact macromolecules remains a controversial issue. However, according to some previous reports, several macromolecules can penetrate the intestinal membrane [3-6]. In addition, it has been reported that orally administrated enzymes can be absorbed through intestinal epithelium. In particular, it has been reported that extracts of lumbrokinases from Eisenia andrei were effective at treating venous thrombosis in a rat model when orally administered.
Thus, we consider that it was worth investigating whether intestinal epithelium can absorb intact and active lumbrokinase. Accordingly, in the present study, we examined the absorption of active and full-sized lumbrokinase through intestinal membrane.
METHODS
Materials
Rabbit antisera for lumbrokinase were provided by Cytosys (Seoul). Goat anti-rabbit IgG-conjugated with horseradish peroxidase (HRP) was purchased from Amersham (Buckinghamshire, UK). L-Leucine-p-nitroanilide was purchased from Sigma (St Louis, MO). Standard marker proteins were purchased from BIO-RAD (Hercules, CA, USA). All other chemicals were of analytical grade.
Animals
Male Sprague-Dawley rats weighing 250 to 300 g were purchased from SLC Inc. (Shizuoka, Japan). Rats were housed under standard condition (23±2℃, RH 50±10%, under a 12 h light/dark cycle). Food and water were available ad libitum. To ensure adaptation to the new environment, rats were housed in a holding room for 1 week before experiments.
Extraction of lumbrokinase
The sample lumbrokinase used in these experiments was extracted using a modification of the method devised by Lee et al. [7]. Briefly, washed Eisenia andrei were homogenized in H2O, and stored for 4 hrs at 45℃ to allow self-autolysis to occur. Homogenates were the centrifugated at 4,500 g for 30 min. Supernatants were collected, filtered using a celite, refiltered using a 0.45 µM membrane filter, and lyophilized. Lyophilized crude extract powders were then suspended in 20 mM phosphate buffer (pH 7.4) and proteins were then salted out using 30~60% ammonium sulfate. Precipitates were then suspended in 20 mM phosphate buffer (pH 7.4) and passed through a 0.45 µM membrane filter. Filtrates were concentrated using an ultrafiltration system, desalted, concentrated, and lyophilized to obtain lyophilized powder (referred to as lumbrokinase filtrate). Lumbrokinase filtrate was resolved in 500 ml of 20 mM phosphate buffer (pH 7.4), filtered through a 0.45 µM membrane filter, and loaded on a DEAE-toyopearl 650 resin column (4.5×35 cm). After loading, adsorbed proteins were eluted with a linear gradient of 0~0.5 M NaCl in the same buffer. The three enzyme observed peaks were harvested. The peak I or II samples of the DEAE fractions were loaded onto a phenyltoyopearl resin column (2.0×15 cm) that was equilibrated with an ammonium sulfate buffer and washed with the same buffer. The adsorbed proteins were eluted with a linear gradient of 0~1 M ammonium sulfate at a flow rate of 1.0 ml per min. A 10 ml sample of the two fractions (referred to as lumbrokinase F1 and lumbrokinase F2) was collected. The active peak II fraction (referred to as lumbrokinase F3) was collected separately. The peak III samples of the DEAE fractions were loaded onto a benzamidine sepharose 6B column (2.0×15 cm) that was equilibrated with a 20 mM phosphate buffer. The absorbed enzymes were eluted with a linear gradient of 0~0.5 M arginine in the same buffer at a flow rate of 0.8 ml per min. A 10 ml sample of the fractions (referred to as lumbrokinase F4, lumbrokinase F5 and lumbrokinase F6) was collected. Finally, to remove contaminates, all fractions were passed through a Sephacryl S-200 column, and then collected for the further study.
Measurement of molecular weights
SDS-PAGE was run as previously described by Choi et al. [8] using 12% polyacrylamide gel. Samples were stained with Coommassie brilliant blue R-250, and molecular weights were determined using low molecular weight standards.
Intestinal preparation
The preparation of ileum sacs was performed as previously described by Vilhardt et al. [6]. Rats were fasted for 24 h before experiments, but with free access to tap water. Animals were anesthetized with ketamine (10 mg/kg, i.p.). After opening the abdominal cavity, rats were sacrificed by exsanguination. Ileums were excised above the cecum, washed with isotonic saline, and everted. They were then cut into 8 cm long segments, ligated at one end, and seroal sides were filled with 1.5 ml of the lumbrokinase filtrate solutions (0.5, 1.0, or 2.0 mg/ml), and securely ligated to create a gut sac. Immediately, the everted sacs were placed into organ baths in 70 ml of Krebs-Henseleit buffer (118 mM NaCl, 27.3 mM NaHCO3, 4.8 mM KCl, 1.2 mM MgCl2, 1.0 mM KH2PO4, 1.25 mM CaCl2, 11.1 mM glucose, pH 7.4). Solutions were gassed with 95% O2/5% CO2 and maintained at 37℃ throughout the experiments. Transport of lumbrokinase from mucosal to serosal sides was measured by sampling 200 µl of the external medium at 30, 60, and 120 min after placing sacs in organ baths. Samples of the external medium were centrifuged at 10,000 g for 10 min at 4℃, and supernatants were removed subjected to analysis.
Measurement of fibrinolytic and proteolytic activities
The fibrinolytic activities of lumbrokinases transported to serosal sides were determined by measuring areas of clear zones on fibrin plates (0.5% human fibrinogen in PBS, pH 7.5). Samples (10 µl) were put in fibrin plates and incubated at 37℃ for 19 h. Areas of clear zones were measured using an image analysis program. The proteolytic activities of samples were assayed using L-leucine-p-nitroanilide as substrate. Briefly, samples were preincubated at 37℃ for 5 min, and then substrate (final concentration, 0.283 mM) was added, and incubated for 10 min. Absorbances were read at 405 nm using a spectrophotometer (Thermo Max, Molecular Devices, USA) and a microplate reader. One unit of proteolytic activity was defined as the amount of enzyme that produced 1 µmol of p-nitroanilide [9].
Polyacrylamide gel electrophoresis (PAGE) and Western blotting
Samples were subjected to SDS-PAGE using a 10% polyacrylamide gel and electrotransferred to nitrocellulose transfer membrane (Hybond-ECL, Amersham, UK). Membranes were incubated in PBS containing 5% skim milk to block nonspecific binding and sequentially reacted with antilumbrokinase (1:500) and secondary antibody (1:1,000, goat anti-rabbit IgG-conjugated with horseradish peroxidase; HRP). Samples were visualized with DAB in PBS containing 0.015% hydrogen peroxide.
Fibrin zymography
To determine fibrinolytic activities on gel, fibrin zymography was performed [10] using a 10% polyacrylamide gel that had been prepared in the presence of fibrinogen (0.12%, wt/vol) and thrombin (10 NIH unit/ml). After electrophoresis, gels were soaked in 50 mM Tris-HCl (pH 7.4) containing 2.5% Triton X-100 solution for 30 min at room temperature. Gels were washed with distilled water and then incubated in PBS (pH 7.5) at 37℃ for 15 h.
In situ rat intestinal perfusion and MALDI-TOF
In situ recirculation method. Rats were fasted overnight and anesthetized with ketamine (50 mg/kg, i.p.). Intestinal absorption was studied using an in situ perfusion technique [11]. Briefly, a 15-cm segment of intestine was cannulated at the duodenum and jejunum. Initially, the intestine segment was rinsed with isotonic saline until the outlet solution was clear. The cannula was then connected to a plastic tube connected to a peristaltic pump (Gilson Minipuls 2, Villiers Le Bel, France). When the experiments were started, the perfused intestinal segment was repositioned in the abdominal cavity, and the abdominal incision was closed with wound clips. Rectal temperature was maintained at 37±1℃ with a warming blanket (Harvard Apparatus, Boston, MA). 30 ml lumbrokinase F1 solution (1 mg/ml) or a drug-free solution was perfused at a flow rate of 5 ml/min for 60 min. All solutions were maintained at 37℃. Blood samples were collected from the abdominal aorta. Plasma was separated by centrifugation, and replaced with 0.02 M acetate buffer (pH 4.7) containing 1 M ammonium sulfate using an ultrafiltration device (Microcon YM-30, 30,000 MWCO, Millipore).
Hydrophobic interaction column (HIC) chromatography. Ultrafiltrated plasma was loaded onto a phenyl toyopearl 650 M resin column (1.7×100 cm) and then eluted with 0.02 M acetate buffer (pH 4.7) containing 1 M ammonium sulfate at a flow rate of 0.7 ml/min. The nine plasma protein peak were separately harvested and applied to the next step.
SDS-PAGE and MALDI-TOF. Plasma protein fractions were concentrated using an ultrafiltration device (Microcon YM-10, 10,000 MWCO, Millipore), and subjected to SDS-PAGE (15% acrylamide gel). Gels in duplicate were stained with Colloidal Coomassie blue to detect protein spots, and the band with the RF value of lumbrokinase F1 was excised, and then eluted using an electro-eluter (Bio-Rad Model 422, USA). Silver staining was carried out as described by Mortz et al. [12]. The band with the RF value of lumbrokinase F1 on gels stained with silver nitrate was excised and in-gel tryptic digestion was performed using a MassPrep Robotic Workstation (MicroMass, UK). Tryptic digests were analyzed using MALDI-TOF (matrix-assisted laser desorption/ionization-time of flight) mass spectrometry (PerSeptive Biosystems, MA, USA) using α-cyano-4-hydroxy cinnamic acid as the carrier matrix [13,14]. Peptide masses were submitted to the Matrix Science-Mascot program (http://matrixscience.com) and the National Center for Biotechnology Information (NCBI) databases (http://ncbi.nlm.nih.gov) for peptide mass fingerprinting (PMF) and protein identification. Peptides were assumed to be carbamidomethylated at cysteine residues, and up to 1 missed tryptic cleavage and a 50-ppm mass tolerance were allowed.
Statistical analysis
Statistical analysis was performed using the Student's t-test or ANOVA.
RESULTS
Measurements of the molecular weights of Lumbrokinase
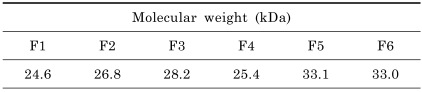
As shown in Table 1, the molecular weights of the six fractions (F1 to F6) were 24.6, 26.8, 28.2, 25.4, 33.1, and 33.0 kDa, respectively.
Table 1.
Molecular weights of the purified LK fractions
Molecular weights of the purified LK fractions were measured by SDS-PAGE as described by Choi et al. [8].
Measurement of fibrinolytic and proteolytic activities
The everted gut sac model for studying intestinal transport of proteins has been previously validated. Lumbrokinase filtrates solutions (0.5, 1.0, and 2.0 mg/ml) were added in Krebs solution at the mucosal side of rat small intestinal segments. To detect mucosal-to-serosal transport of lumbrokinase, aliquots of serosal medium were taken after different incubation times (30, 60, and 120 min), and fibrinolytic and proteolytic activities were measured.
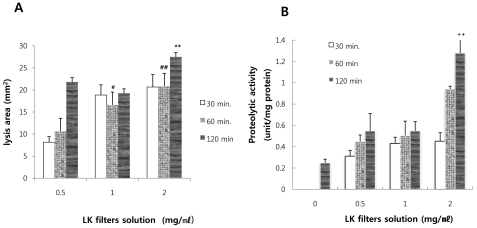
As shown in Fig. 1, serosal side solution incubated with only Krebs-Henseleit buffer solution without lumbrokinase showed no clear zones on fibrin plates. On the other hand, the serosal side solution incubated with lumbrokinase did and the areas of these zones increased in a time- and dose-dependent manner. The area of clear zones was 21.798±11.0, 22.118±0.4, 31.977±11.8 mm2 after incubation for 2 h. When lumbrokinase filtrate solutions (0.5, 1.0, and 2.0 mg/ml) were directly dropped onto a fibrin plate and incubated for 2 h, The area of clear zone was 148.3, 253.5, and 276.2, respectively. These results show that 14.7, 8.7, and 11.5% of lumbrokinase was transferred from mucosal side to serosal side after 2 h at these respective doses.
Fig. 1.
Measurement of fibrinolytic (A) and proteolytic activities (B). The lumbrokinase solutions (0.5, 1.0, and 2.0 mg/ml) were added to Krebs solution on the mucosal sides of small intestine segments. To detect mucosal-to-serosal lumbrokinase transport, aliquots of serosal medium were taken at different times (30, 60, and 120 min, respectively) during incubation, and their fibrinolytic and proteolytic activities were measured. Data are means±SEs from 5 rats. #p<0.01, ##p<0.001 vs. the value obtained with lumbrokinase solution (0.5 mg/ml) at 60 min, **p<0.001 vs. the value obtained with lumbrokinase solution (0.5 mg/ml) at 120 min, ++p<0.001 vs. control.
The proteolytic activities of serosal side solutions incubated with lumbrokinase were higher than those of serosal side solutions incubated without lumbrokinase. The proteolytic activity was found to be dependent on lumbrokinase filtrates dose and incubation time. The proteolytic activity of 1 mg of lumbrokinase was 49.48 units. On the other hand, the proteolytic activities of serosal side solutions incubated with lumbrokinase ranged from 0.06 to 1.03.
Western blotting analysis and fibrin zymography
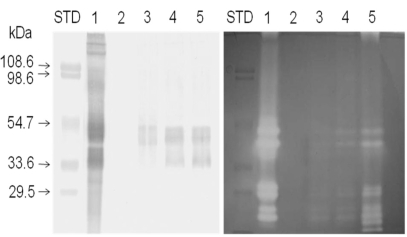
To detect the transport of lumbrokinase across the intestinal tract, Western blotting was performed using anti-lumbrokinase and fibrin zymography to analyze directly fibrinolytic enzymes on gel. Distinguishable band was not found on nitrocellulose membrane in immunoreactive color development in serosal side solutions without lumbrokinase (Fig. 2, left panel, lane2). However, immunoreactive bands (especially between 33.6 and 54.7 kDa) were found in serosal side solutions incubated with lumbrokinase (Fig. 2, left panel, lane3, 4, 5) and these bands became thicker with incubation time. These results showed that lumbrokinase was transported to the serosal side of the intestinal tract during incubation.
Fig. 2.
Immunoblot and zymogram analyses. Western blotting (left panel) using lumbrokinase polyclonal antibody and fibrin zymography (right panel) using fibrin incorporated in gel were carried out (lane 1: 20 µg of lumbrokinase, lane 2: serosal medium incubated without lumbrokinase for 120 min, lanes 3~5: serosal media incubated with 2 mg/ml lumbrokinase for 30, 60, and 120 min, respectively). Molecular weight standard markers were applied to the left of lane1 in each panel. The blots shown are representative of experiments performed in triplicate.
Fibrin zymograhy (Fig. 2, right panel) revealed that lumbrokinase transported to serosal side could be a lysis product of fibrin. Clear bands were not observed in serosal side solutions incubated without lumbrokinase. However, Clear bands were observed in lumbrokinase standard solution (Fig. 2, right panel, lane 1) and serosal side solutions incubated with lumbrokinase (Fig. 2, left panel, lane 3 to 5). Furthermore, the intensities of clear bands increased in an incubation time dependent manner.
Gel electrophoresis and MALFI-TOF mass spectrometry of lumbrokinase F1
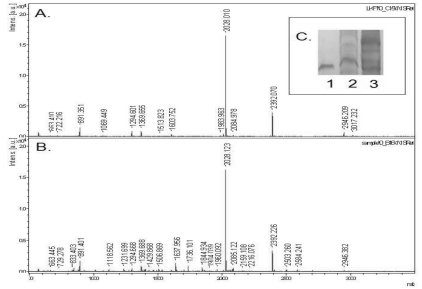
To understand the intestinal absorption of lumbrokinase, plasma protein fractions were resolved by gel electrophoresis and stained with silver. The band with the RF of lumbrokinase F1 (as shown in Fig. 3C) was excised, in-gel tryptic digestion, and MALDI-TOF. A typical ms/ms spectra of the protein (Fig. 3B), which was identified as lumbrokinase F1 is shown in Fig. 3A.
Fig. 3.
MALDI-TOF Mass Spectrometry. Lumbrokinase F1 was separated from plasma obtained by in situ recirculation method, and subjected to 15% SDS-PAGE (Fig. 3C, lane 1-lumbrokinase F1 standard, lane 2: blank plasma without lumbrokinase F1, lane 3: plasma with lumbrokinase F1 for 1 h). The band corresponding to the molecular weight of lumbrokinase F1 was excised and eluted, and the concentrated protein was treated with trypsin. Tryptic digests were analyzed by MALDI-TOF mass spectrometry (Fig. 3A: the spectrum of lumbrokinase F1 (lane 1 of Fig. 3C); Fig. 3B: the spectrum of the plasma obtained from rats with lumbrokinase F1 (lane 3 of Fig. 3C).
DISCUSSION
Fibrinolytic enzymes, which dissolve fibrin, are the main proteinaceous components of blood clots. Therapeutic fibrinolytic enzymes, such as, urokinase and tissue plasminogen activator, have been extensively investigated and are used clinically as chemotherapeutic agents. However, these enzymes are expensive and are administered only via the intravenous route [15]. Mihara et al. [1], purified strong fibrinolytic enzymes from the earthworm Lumbericus rubellus. These enzymes are cheaper than other therapeutic fibrinolytic enzymes such as urokinase and tissue plasminogen activator. Furthermore, they have the advantage of storage stability, and can be administrated orally to treat clotting disorder [2,7].
Prior to 1980, it was commonly assumed either that dietary proteins were digested completely to free amino acids within the lumen of the gastrointestinal tract before absorption occurs [16]. Furthermore, the intestinal mucosa has been described as a barrier to the passage of protein in adult mammals, because intestinal proteases rapidly hydrolyze proteins. Thus, only peptides resistant to hydrolysis by digestive enzymes of the gastrointestinal tract might have a chance of being absorbed by the body when administered orally [3]. According to some reports, macromolecules can be absorbed in intact and active forms [4,5]. However, research in this area is made difficult by the rapid clearance of absorbed proteins from blood, limited absorption of an active substance or complexation between active substances and blood components [4].
In the present study, we used the incubation chamber method, MALDI-TOF, and immunostaining, and we obtained reproducible results. When everted gut sacs were incubated with lumbrokinase, fibrinolytic activities and proteolytic activities on serosal sides increased in an incubation time-dependent manner. Our findings showed that lumbrokinase was moved from mucosal to serosal sides. The amount of fibrinolytic activity of lumbrokinase absorbed by the intestinal epithelium was maximally 14.7% as compared with that of mucosal sides. Also, according to our fibrin zymography and immunoblotting results, intact lumbrokinase penetrated the intestinal tract.
Gastrointestinal absorption of macromolecules, such as, proteins can be explained by three mechanisms as follows [16,17]. First, proteins are absorbed via receptor mediated endocytosis are as some growth factors. Second, proteins are absorbed by pinocytic vesicles, which are formed by the brush border membrane of the intestine, and normally fuse with lysosomes to form phagolysomes. Only if a protein escapes hydrolysis can it conceivably enter the extracellular space and reach the blood stream. Third, protein absorption by trans-epithelial transport via the M cells of Peyer's patches could be involved. Foreign proteins, such as, lumbrokinase are unlikely to be absorbed via receptor-mediated endocytosis, and thus, the intestinal absorption of lumbrokinase is more likely to involve the second and third mechanisms. Because it is a complex of strong serine proteases, lumbrokinase is resistant to degradation by some cellular enzymes, and thus, it could be transferred intact and across the cell membrane by pinocytic vesicles or epithelial cells (M cells).
ABBREVIATIONS
- LK
lumbrokinase
- LKF1
lumbrokinase fraction 1
- PAGE
polyacrylamide gel electrophoresis
- HIC
hydrophobic interaction column
References
- 1.Mihara H, Sumi H, Yoneta T, Mizumoto H, Ikeda R, Seiki M, Maruyama M. A novel fibrinolytic enzyme extracted from the earthworm, lumbricus rubellus. Jpn J Physiol. 1991;41:461–472. doi: 10.2170/jjphysiol.41.461. [DOI] [PubMed] [Google Scholar]
- 2.Cho IH, Choi ES, Lim HG, Lee HH. Purification and characterization of six fibrinolytic serine-proteases from earthworm lumbricus rubellus. J Biochem Mol Biol. 2004;37:199–205. doi: 10.5483/bmbrep.2004.37.2.199. [DOI] [PubMed] [Google Scholar]
- 3.Castell JV, Friedrich G, Kuhn CS, Poppe GE. Intestinal absorption of undegraded proteins in men: Presence of bromelain in plasma after oral intake. Am J Physiol. 1997;273:G139–G146. doi: 10.1152/ajpgi.1997.273.1.G139. [DOI] [PubMed] [Google Scholar]
- 4.Fan Q, Wu C, Li L, Fan R, Hou Q, He R. Some features of intestinal absorption of intact fibrinolytic enzyme iii-1 from lumbricus rubellus. Biochim Biophys Acta. 2001;1526:286–292. doi: 10.1016/s0304-4165(01)00140-4. [DOI] [PubMed] [Google Scholar]
- 5.Fujita M, Hong K, Ito Y, Misawa S, Takeuchi N, Kariya K, Nishimuro S. Transport of nattokinase across the rat intestinal tract. Biol Pharm Bull. 1995;18:1194–1196. doi: 10.1248/bpb.18.1194. [DOI] [PubMed] [Google Scholar]
- 6.Vilhardt H, Lundin S. In vitro intestinal transport of vasopressin and its analogues. Acta Physiol Scand. 1986;126:601–607. doi: 10.1111/j.1748-1716.1986.tb07861.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee CK, Shin JS, Kim BS, Cho IH, Kim YS, Lee EB. Antithrombotic effects by oral administration of novel proteinase fraction from earthworm eisenia andrei on venous thrombosis model in rats. Arch Pharm Res. 2007;30:475–480. doi: 10.1007/BF02980222. [DOI] [PubMed] [Google Scholar]
- 8.Choi NS, Kim BY, Lee JY, Yoon KS, Han KY, Kim SH. Relationship between acrylamide concentration and enzymatic activity in an improve single fibrin zymogram gel system. J Biochem Mol Biol. 2002;35:236–238. doi: 10.5483/bmbrep.2002.35.2.236. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Fan R, Wu C, He RQ. Assay of lumbrokinase with a chromophoric substrate. Protein Pept Lett. 1997;4:409–414. [Google Scholar]
- 10.Kim SH, Choi NS, Lee WY. Fibrin zymography: a direct analysis of fibrinolytic enzymes on gels. Anal Biochem. 1998;263:115–116. doi: 10.1006/abio.1998.2816. [DOI] [PubMed] [Google Scholar]
- 11.Schanker LS, Tocco DJ, Brodie BB, Hogben CA. Absorption of drugs from the rat small intestine. J Pharmacol Exp Ther. 1958;123:81–88. [PubMed] [Google Scholar]
- 12.Mortz E, Krogh TN, Vorum H, Gorg A. Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionization-time of flight analysis. Proteomics. 2001;1:1359–1363. doi: 10.1002/1615-9861(200111)1:11<1359::AID-PROT1359>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Landry F, Lombardo CR, Smith JW. A method for application of samples to matrix-assisted laser desorption ionization time-of-flight targets that enhances peptide detection. Anal Biochem. 2000;279:1–8. doi: 10.1006/abio.1999.4468. [DOI] [PubMed] [Google Scholar]
- 14.Shevchenko A, Jensen ON, Podtelejnikov AV, Sagliocco F, Wilm M, Vorm O, Mortensen P, Boucherie H, Mann M. Linking genome and proteome by mass spectrometry: Large-scale identification of yeast proteins from two dimensional gels. Proc Natl Acad Sci USA. 1996;93:14440–14445. doi: 10.1073/pnas.93.25.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakajima N, Ishihara K, Sugimoto M, Sumi H, Mikuni K, Hamada H. Chemical modification of earthworm fibrinolytic enzyme with human serum albumin fragment and characterization of the protease as a therapeutic enzyme. Biosci Biotechnol Biochem. 1996;60:293–300. doi: 10.1271/bbb.60.293. [DOI] [PubMed] [Google Scholar]
- 16.Gardner ML. Gastrointestinal absorption of intact proteins. Annu Rev Nutr. 1988;8:329–350. doi: 10.1146/annurev.nu.08.070188.001553. [DOI] [PubMed] [Google Scholar]
- 17.Sanderson IR, Walker WA. Uptake and transport of macromolecules by the intestine: Possible role in clinical disorders (an update) Gastroenterology. 1993;104:622–639. doi: 10.1016/0016-5085(93)90436-g. [DOI] [PubMed] [Google Scholar]