Abstract
Signal transduction by the T-cell antigen receptor (TCR) is initiated by phosphorylation of conserved motifs (ITAMs) contained within the cytoplasmic domains of the invariant subunits. TCR complexes contain a total of 10 ITAMs and this unusual configuration has prompted studies of the role of specific ITAMs, or of ITAM multiplicity, in regulating TCR-directed developmental and effector responses. Here, we summarize data generated during the past two decades and discuss how these findings have in some cases resolved, and in others complicated, outstanding questions relating to the function of TCR ITAMs.
Phosphorylation of conserved ITAM motifs in T-cell receptor tails allows them to bind the downstream kinase ZAP-70. The presence of multiple ITAMs produces the signal amplification needed for T-cell development.
Signal transduction in the immune system is regulated by a highly diverse set of cell surface receptors that are coupled by signaling intermediates to common downstream pathways. A large number of these receptors either contain within their cytoplasmic domains, or associate with subunits that contain, a conserved sequence (the Immune-receptor-Tyrosine-based-Activation-Motif; ITAM) that is critical for the initiation of signaling following ligand engagement (reviewed in Underhill and Goodridge 2007). ITAMs, which were identified 20 years ago on the basis of their sequence homology (Reth 1989), consist of paired YxxL/I motifs separated by a defined interval (YxxL/I-X6-8-YXXL/I). In addition, most ITAMs contain a negatively charged amino acid (D/E) in the +2 position relative to the first ITAM tyrosine (Fig. 1). ITAM containing receptors are widely expressed in hematopoietic cells including T and B lymphocytes, natural killer (NK) cells, macrophages, dendritic cells, and platelets (Fig. 2). Since their discovery, considerable progress has been made in determining how ITAMs initiate signaling cascades and thereby couple their associated receptors to distal effector responses. In this review, we focus on the TCR as a paradigm for ITAM-mediated signal initiation. In addition to being one of the most well characterized receptor complexes, the TCR exhibits several intriguing properties, such as the inclusion of an especially large number of ITAM containing subunits, which have fueled intensive study into the role of ITAMs in the TCR signaling response.
Figure 1.
ITAM-containing proteins. Murine ITAM sequences were obtained from GenBank. Amino acids that align with the consensus ITAM sequence are shown in blue.
Figure 2.
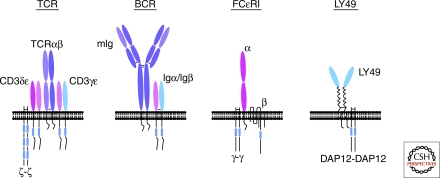
Comparison of the ITAM-containing T- and B-cell antigen receptors, Fc receptors and NK receptors. Subunit composition of the T-cell antigen receptor (TCR), B-cell antigen receptor (BCR), FcεR1 (an example of an activating Fc receptor) and Ly49 (an example of an activating NK receptor). Blue rectangles represent ITAMs.
ITAMS WITHIN THE TCR SUBUNITS
TCRs are multimeric complexes composed of subunits whose function is exclusively limited to either ligand recognition or signal transduction. The predominant TCR isoform, the αβTCR, which is expressed on mature CD4+ and CD8+ T cells, contains clonotypic TCRα and TCRβ chains that form a heterodimer conferring peptide-major histocompatibility complex (MHC) binding specificity to the TCR. The TCRα/β heterodimer lacks inherent signal transducing activity but associates noncovalently with multiple signal transducing subunits: the CD3γ,-δ and -ε chains, and, in most cases, a ζ chain homodimer. The currently accepted model of αβTCR stoichiometry proposes the following subunit composition: TCRαβ, CD3γε, CD3δε, ζζ. A distinct lineage of T cells (γδ T cells) expresses a TCR complex containing a different antigen recognition heterodimer composed of chains encoded by the TCRγ and TCRδ genes. Although the invariant subunit composition of the αβTCR and the γδTCR had long been assumed to be identical, it was recently shown that the γδTCR lacks CD3δ (Hayes and Love 2002), and therefore most likely has the following stoichiometry: TCRγδ, CD3γε, CD3γε, ζζ (Hayes and Love 2006a; Siegers et al. 2007). A third complex, the pre-TCR, is expressed only during early thymocyte development at a stage that precedes rearrangement of the TCRα gene. In lieu of TCRα, TCRβ associates with an invariant chain (pre-Tα) encoded by a gene that does not undergo V-(D)-J rearrangement (Saint-Ruf et al. 1994). Although the subunit composition of the pre-TCR has not been unequivocally established, the phenotype of knockout mice lacking individual TCR subunits suggests that it likely contains at least CD3γε and ζζ dimers in addition to the TCRβ and pre-Tα chains (Hayes et al. 2003).
A striking feature of the TCR (and pre-TCR) structures is the presence of multiple distinct ITAM bearing subunits within the receptor complex. The CD3 (−γ, −δ, −ε) chains each contain a single ITAM whereas the ζ chain contains three tandem ITAMs (Fig. 1 and Fig. 2). Consequently, the predicted octameric αβTCR and γδTCR complexes contain a total of 10 ITAMs. Moreover, although all TCR ITAMs share the conserved YxxL/I-X6-8-YXXL/I motif, the amino acid sequence of each ITAM is distinctive (Fig. 1). The question that naturally arises is whether the individual TCR ITAMs have specific signaling responses during T-cell development and T-cell activation or instead perform equivalent but additive functions.
INITIATION OF TCR SIGNALING BY ITAMS
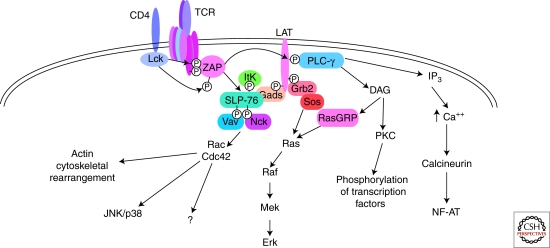
The essential role of ITAMs in the initiation of TCR signaling was first established by experiments demonstrating that the cytoplasmic domains of the CD3 or ζ chains, or the individual ITAM sequences from these proteins, were capable of activating T cells when fused to the extracellular domain of unrelated molecules (Irving and Weiss 1991; Letourneur and Klausner 1992; Romeo et al. 1992; Straus and Weiss 1993; Koyasu et al. 1994; Sturmhofel et al. 1995). It had been shown previously that tyrosine phosphorylation of TCR subunits, which represents one of the earliest events following TCR engagement, was mediated by the Src family tyrosine kinases Lck and Fyn (Samelson et al. 1986; June et al. 1990; Samelson et al. 1990) and that Lck associates constitutively with the CD4 and CD8 coreceptors (Veillette et al. 1988; Barber et al. 1989). An important breakthrough was the subsequent identification of ZAP-70, a Syk family tyrosine kinase that is rapidly recruited to phosphorylated ITAMs (Chan et al. 1992). Both ZAP-70 and Syk contain tandem SH2 domains that direct their specific and selective interaction with doubly phosphorylated ITAMs. The combinatorial action of Src kinases and ZAP-70 is sufficient for the full activation of downstream signaling pathways and for eliciting T-cell effector responses (Fig. 3).
Figure 3.
Proximal signaling events in the TCR-coupled signaling pathway. Interactions between the TCR and peptide-MHC results in the activation of Lck, a member of the Src family of tyrosine kinases. Lck then phosphorylates the two-tyrosine residues within the ITAMs of the CD3 and ζ chains. A second tyrosine kinase, ZAP-70, is specifically recruited to biphosphorylated ITAMs. Phosphorylation of ZAP-70 by Lck results in its activation. ZAP-70 and Lck phosphorylate and activate several downstream target proteins eventually leading to Ras activation, calcium mobilization, and actin cytoskeleton rearrangements, and ultimately to the activation of transcription factors.
A series of experiments performed over the past few years have served to clarify and refine the proximal events that take place following TCR engagement. A fundamental question has been how ligand binding promotes ITAM phosphorylation and thereby leads to the initiation of TCR signaling. One explanation, relevant to cells expressing the αβTCR, is that cobinding of the peptide-MHC complex by the TCR and by CD4 or CD8 results in the juxtaposition of Lck and the cytoplasmic tails of the invariant TCR subunits resulting in the phosphorylation of ITAMs by Lck (Chu and Littman 1994). However, this mechanism cannot explain how ITAMs within the pre-TCR and γδTCR are phosphorylated as these receptors are expressed on cells that do not express either CD4 or CD8. This paradox has led to the proposal of several new models [reviewed in (Smith-Garvin et al. 2009)] that invoke mechanisms including ligand-induced conformational changes in the TCR, receptor re-distribution on the cell surface, or subunit movement relative to the plasma membrane to explain how TCR engagement could render ITAMs more accessible to phosphorylation. One especially attractive model stems from the observation that the cytoplasmic tails of CD3ε and ζ interact with acidic phospholipids that are enriched in the inner leaflet of the plasma membrane (Aivazian and Stern 2000; Sigalov et al. 2006; Xu et al. 2008). Such interactions, which would be predicted to result in the insertion of ITAM tyrosines into the hydrophobic core of the lipid bilayer, could explain the inaccessibility of ITAMs as targets for phosphorylation in resting T cells. According to this model, ligand induced deformation of the TCR leads to dissociation of the CD3 and ζζ dimers from the plasma membrane rendering them accessible to phosphorylation by Lck and/or Fyn.
The binding of ZAP-70 to phosphorylated ITAMs and its subsequent activation has been clarified by experiments that systematically evaluated the importance of key domains and amino-acid residues within ZAP-70 (reviewed in Au-Yeung et al. 2009). Specifically, these studies have shown that ITAM binding by ZAP-70 relieves an auto-inhibitory conformation of the kinase and that phosphorylation of specific tyrosine residues within the interdomain separating the C-terminal SH2 domain and the kinase domain (interdomain B) by Lck/Fyn are important for regulating the activity of ZAP-70 (Au-Yeung et al. 2009).
FUNCTION OF INDIVIDUAL TCR CHAINS AND ITAMS
The discovery that the TCR complex contains several different ITAM-bearing subunits immediately prompted a series of studies aimed at determining whether individual ITAMs were important for particular signaling or effector responses. Experiments performed in T-cell lines indicated that the cytoplasmic domains of the CD3ε and ζ subunits were independently capable of providing activating signals similar to that of the intact TCR complex (Irving and Weiss 1991; Letourneur and Klausner 1992; Wegener et al. 1992) and suggested that TCR ITAMs are functionally redundant but additive with respect to signaling responses (Irving et al. 1993). In other studies, the binding of potential effector proteins including ZAP-70, Shc, PI3K(p85), Grb2, Fyn, and Ras-GAP to the TCR ITAMs was analyzed using synthetic phosphorylated peptides containing individual ITAM sequences (Isakov et al. 1995; Osman et al. 1996; Zenner et al. 1996). Only one of the three studies compared binding to all 6 TCR ITAM sequences (ζ1, ζ2, ζ3, CD3−γ, −δ, and −ε) (Osman et al. 1996). Data from these experiments provided evidence for preferential binding of effector molecules to individual TCR ITAMs, and in some cases (Shc, p85, Grb2, and Fyn) selective binding to a subset of TCR ITAMs (Table 1); however, additional experiments to follow up on these initial observations have not be performed. It also remains unclear if any exclusive ITAM-effector associations can be inferred from these studies because in vitro analysis of binding to isolated ITAMs may not reflect binding in the context of the intact TCR where cooperative interactions or ordered binding of effectors may take place.
Table 1.
Differences in the binding affinities of major signaling molecules in the TCR-coupled signaling pathway for the ITAMs within the invariant TCR subunits
| Signaling Molecule | ITAM Binding Hierarchy | References |
|---|---|---|
| ZAP-70 | ζ1 ≥ ζ2 > ε ≥ ζ3 | Isakov et al. 1995a |
| ζ1 = γ = δ > ζ3 > ζ2 = ε | Osman et al. 1996b | |
| ζ1 > ζ3 > ζ2 | Zenner et al. 1996c | |
| Shc | γ = δ > ζ3 = ζ1 | Osman et al. 1996 |
| ζ1> ζ3 | Zenner et al. 1996 | |
| p85 regulatory subunit of PI3K | ζ3 = γ = δ>ζ1 = ζ2 | Osman et al. 1996 |
| ζ1 ≥ ζ2 > ζ3 | Zenner et al. 1996 | |
| Grb2 | ζ1 = γ = δ | Osman et al. 1996 |
| ζ2 | Zenner et al. 1996 | |
| Fyn | ζ2 = γ = δ > ζ1 | Osman et al. 1996 |
| Ras-GTPase activating protein (GAP) | ζ2 ≥ ζ1 > ζ3 | Zenner et al. 1996 |
aAll 3 TCRζ ITAMs and the CD3ε ITAM were tested in this study.
bAll 6 ITAMs (ζ1, ζ2, ζ3, ε, δ, and γ) were tested in this study. Only the specific ITAMs that physically interacted with the signaling molecule are listed.
cAll 3 TCRζ ITAMs were tested in this study. Only the specific ζ ITAMs that physically interacted with the signaling molecule are listed.
The notion that the extent of TCR ITAM phosphorylation, or the order in which the individual TCR ITAMs are phosphorylated after ligand engagement, may play a role in regulating the TCR signaling response also formed the basis of intensive research over the past decade. Early experiments revealed that two prominent tyrosine phosphorylated forms of ζ-chain, p21-ζ, and p23-ζ, are detected in T cells (reviewed in Pitcher et al. 2003b). It was subsequently shown that p21-ζ, which is detected in unstimulated ex vivo thymocytes and T cells or in cells activated with partial agonist/antagonist ligands, contains partially phosphorylated ITAMs that are either not associated with ZAP-70 or that bind inactivated ZAP-70, whereas p23-ζ, which is generated only after TCR cross-linking or stimulation with agonist peptides, contains fully phosphorylated ITAMs that bind activated ZAP-70 (Sloan-Lancaster et al. 1994; van Oers et al. 1994; Madrenas et al. 1995). These findings led to the suggestion that partial phosphorylation of ζ-chain may be important for the induction of T-cell anergy and may in fact be inhibitory (Kersh et al. 1999). One mechanism proposed to explain ITAM-mediated inhibition is that partial phosphorylation of individual ITAMs could result in the generation of an “ITIM-like” sequence (YXXL/I/V) which then leads to the recruitment of tyrosine phosphatases including SHP-1 and SHIP to the TCR (Barrow and Trowsdale 2006; Pinheiro da Silva et al. 2008). However, other studies implied that p21-ζ may instead represent a “primed” state that facilitates rapid T-cell activation and that formation of p21-ζ through self-MHC interactions may be important for T-cell survival (Witherden et al. 2000). To address these questions, van Oers and colleagues engineered transgenic mouse lines that are capable of generating p21-ζ but not p23-ζ, or that are incapable of generating either p21-ζ or p23-ζ (Pitcher et al. 2003a). Analysis of these mice demonstrated that the ability to generate p21-ζ was not essential for T-cell antagonism. In addition, they found that the ability to generate p23-ζ was not required for agonist-induced T-cell proliferation (Pitcher et al. 2003a). Similar results were obtained by other investigators using an identical but independently constructed experimental system (Ardouin et al. 1999). Whether these results indicate that partially phosphorylated TCR ITAMs (or partial phosphorylation of individual TCR ITAMs) have no inhibitory function remains unclear as the potential for redundancy with other ITAM containing subunits still exists in this model system.
To evaluate the importance of TCR signaling for T-cell development, in vivo studies were performed with gene targeted mice in which specific ITAM containing subunits were mutated. In an initial series of experiments, mice lacking expression of the CD3γ, −δ, or −ε chains (Malissen et al. 1995; Dave et al. 1997; DeJarnette et al. 1998; Haks et al. 1998) or the ζ chain (Liu et al. 1993; Love et al. 1993; Malissen et al. 1993; Ohno et al. 1993) were generated. Analysis of T-cell development in these mice revealed an important function for CD3γ, CD3ε, and ζ in regulating the transition of thymocytes from the CD4−CD8− (Double Negative, DN) to the CD4+CD8+ (Double Positive, DP) stage, an event known to be controlled by pre-TCR signaling (von Boehmer 2005). Moreover, each of the invariant TCR subunits was found to be required for efficient transition from the DP to the mature CD4+CD8− or CD4−CD8+ (Single Positive, SP) stage, which is dependent upon αβTCR signaling (Sebzda et al. 1999; Starr et al. 2003). Importantly, αβTCR surface expression was found to be adversely affected in each of the knockout mouse lines, results that were in agreement with in vitro studies showing that all of the TCR subunits are required for proper assembly and surface expression of the TCR (Klausner et al. 1990). Although these studies clearly demonstrated a critical role for CD3γ, −δ, −ε, and ζ in T-cell development, the effects of the gene deletions on TCR surface expression meant that knockout mice could not be used to directly evaluate the importance of ITAMs and ITAM-mediated signaling.
To specifically address the role of TCR ITAMs in thymocyte development, three groups reconstituted TCRζ knockout mice with transgenes encoding wild-type or mutant forms of ζ chain lacking one or more ITAMs, either by deleting individual ITAMs (Shores et al. 1994) or by mutation of ITAM tyrosines to phenylalanine (Ardouin et al. 1999; Pitcher et al. 2005b) (Table 2). Significantly, all three studies found that none of the TCRζ ITAMs was specifically required for thymocyte maturation. However, each study noted that the efficiency of thymocyte development was decreased in the absence of one or more ζ ITAMs, and the severity of the developmental block correlated with the number of inactivated ITAMs but not with the loss of any specific ITAM. A non-essential requirement was also demonstrated for the CD3γ ITAM (Haks et al. 2002) and CD3ε ITAM (Sommers et al. 2000) (Table 2). Together, these results strongly suggested that TCR ITAMs function collectively to amplify TCR signals (Love and Shores 2000).
Table 2.
Roles of ITAMs within the CD3ε, CD3γ, CD3δ, and TCRζ chains in T-cell development and function
| Subunit | Experimental approach | Effects on development/function | References |
|---|---|---|---|
| CD3ε | Reconstitution of CD3ε−/− mice with a transgene encoding a mutant form of CD3ε containing a Y→F substitution in the distal tyrosine residue of the CD3ε ITAM | ·Restores T-cell development to CD3ε−/− mice | Sommers et al. 2000 |
| ·Impaired proliferative responses and cytokine responses when low doses of stimulating antibody are used | |||
| ·Defects in survival of T cells expressing TCRs with low affinity to self-ligands | |||
| CD3γ | Knock-in lacking the CD3γ ITAM | ·Impaired efficiency of positive but not negative selection | Haks et al. 2001; Haks et al. 2002 |
| ·Defects in proximal TCR signaling events | |||
| ·Proliferative responses and cytokine responses are equivalent to those of wild-type mice | |||
| TCRζ | Reconstitution of TCRζ−/− mice with transgene encoding truncated form of TCRζ containing no ITAMs | ·Restores T-cell development to TCRζ−/− mice | Shores et al. 1994; Love et al 2000; Ardouin et al. 1999; Pitcher et al. 2003; Pitcher et al. 2005 |
| ·Defects in selection of thymocytes expressing TCRs with low affinity to self-ligands | |||
| ·Impaired proliferative responses and cytokine responses when low concentrations of peptide are used | |||
| Reconstitution of TCRζ−/− mice with transgene encoding mutated form of TCRζ containing Y→F substitutions in all ITAMs. | ·Restores T-cell development to TCRζ−/− mice | ||
| ·Defects in selection of thymocytes expressing TCRs with low affinity to self-ligands | |||
| All | RAG1−/− mice reconstituted with CD3- and TCRζ-deficient bone marrow transduced with retroviral vectors encoding ITAM-mutant CD3 and TCRζ chains | ·Restoration of T-cell development requires the expression of at least 7 ITAMs | Holst et al. 2008 |
| ·Impaired proliferative responses | |||
| ·Autoimmunity is observed when two to six ITAMs are expressed |
POTENTIAL FUNCTIONS FOR ITAM-MEDIATED SIGNAL AMPLIFICATION DURING T-CELL DEVELOPMENT
Recent data indicate that ITAM-mediated signal amplification may have an important role in regulating αβ/γδ T lineage choice during thymocyte development. These experiments were based on two observations: 1) that αβ and γδ T cells originate from a common DN thymocyte progenitor prior to rearrangement of the TCRα locus (Petrie et al. 1992), and 2) that direct comparison of the signaling responses of the γδTCR and the αβTCR revealed that under equivalent stimulation conditions, the γδTCR delivers a stronger signal than the αβTCR (Hayes and Love 2002). Since the pre-TCR is expressed at much lower levels than the γδTCR on DN thymocytes (Hayes and Love 2006b), this raised the possibility that the relatively strong signals transmitted by the γδTCR promote commitment to the γδ lineage whereas relatively weak pre-TCR signals promote αβ lineage commitment. This differed from a model proposing that αβ/γδ lineage choice is determined before expression of the γδTCR or the pre-TCR (Kang and Raulet 1997). The importance of TCR signal strength in αβ/γδ lineage choice was tested in two studies that used an experimental system where all immature DN thymocytes express a single transgenic γδTCR (Haks et al. 2005; Hayes et al. 2005). Attenuation of γδTCR signaling potential by reducing the number of ζ ITAMs (Hayes et al. 2005) or by changing the affinity of the γδTCR-MHC interaction (Haks et al. 2005) resulted in a diversion from the γδ to the αβ lineage supporting the signal strength model of αβ/γδ lineage commitment. Consistent with these results, single cell lineage tracing experiments also demonstrated that αβ/γδ lineage choice does not take place prior to pre-TCR/γδTCR expression (Kreslavsky et al. 2008).
A large body of work derived from several independent laboratories supports the idea that ITAM-mediated signal amplification plays a critical role in selection of the mature T-cell repertoire. In DP thymocytes, productive rearrangement of the TCRα locus results in surface expression of clonally distinct αβTCR complexes. To ensure that only those cells that express TCRs with the appropriate specificities are allowed to mature, DP thymocytes are subjected to a selection process on the basis of their TCR specificity for peptide+MHC that promotes the survival and differentiation of functionally competent cells (positive selection) and triggers the deletion of overtly autoreactive cells (negative selection) (Sebzda et al. 1999; Starr et al. 2003). Positive selection is thought to be mediated by relatively weak non-agonist peptides (Hogquist et al. 1994; Santori et al. 2002) indicating that amplification of TCR signals generated from such interactions may be especially important for positive selection. Consistent with this notion, positive selection was found to be markedly impaired in ζ-deficient mice reconstituted with transgenes encoding ITAM mutant ζ chains (Shores et al. 1994; Ardouin et al. 1999; Pitcher et al. 2005b). Moreover, in each of these model systems, the extent to which positive selection was compromised was directly related to the number of ζ ITAMs that had been deleted or inactivated. Interestingly, analysis of positive selection using different αβTCR transgenes demonstrated the number of TCR ITAMs that were required for positive selection was dependent upon the presumed affinity of the TCR for its selecting self ligand. DP thymocytes expressing TCRs that are thought to bind to self peptide+MHC with relatively high affinity could be positively selected even if several ζ ITAMs were deleted, whereas positive selection of thymocytes expressing relatively low affinity TCRs required all 10 TCR ITAMs (Love et al. 2000). Thus, the requirement for ITAM-mediated signal amplification was most clearly evident under conditions where positive selection was induced by weak TCR-ligand interactions.
ITAM-MEDIATED SIGNAL AMPLIFICATION AND CENTRAL TOLERANCE
The discovery that TCR ITAM multiplicity was important for positive selection raised the question of whether negative selection is also compromised when the number of TCR ITAMs is reduced. The effect on negative selection of mutating or removing one or more of the ζ ITAMs was examined in two of the three experimental models described above. In both cases, negative selection was found to be compromised when the number of ζ ITAMs was reduced (Shores et al. 1997; Love et al. 2000; Pitcher et al. 2005a). Similar to the effects on positive selection, the impact on negative selection was directly related to the number of ITAMs that were eliminated. Importantly, although potentially auto-reactive T cells were generated in these mice, no overt signs of autoimmune disease were observed. The absence of autoimmune disease in ζ ITAM mutant mice is consistent with the hypothesis that the reactivity of T cells is “tuned” during thymocyte selection through the regulated expression of compensatory molecules such as CD5 which function to modulate the integrated TCR signaling response (Grossman and Singer 1996; Azzam et al. 2001; Wong et al. 2001; Saibil et al. 2003). However, a recent report has challenged this concept of thymocyte selection (Holst et al. 2008). In this study, retrovirus encoded wild-type or ITAM mutant CD3γ, −δ, −ε, and ζ chains (termed retrogenics) were used to reconstitute bone marrow cells from CD3ε−/−, ζ−/−, or CD3ε−/− x ζ−/− mice. In these experiments, mice in which T-cell development was reconstituted with constructs that yielded fewer than seven functional ITAMs per TCR developed a lethal, multi-organ autoimmune disease that was attributed to failure of central tolerance (Table 2). The reason for the striking discrepancy between these results and those of prior studies are presently unclear but may relate to the different experimental systems employed. A potential drawback of all three studies is that transgenic or retrogenic reconstitution does not replicate endogenous gene expression. Notwithstanding, these new results, which imply that genetic or environmental changes that attenuate TCR signaling in preselection thymocytes can result in failure of central tolerance, call for additional studies to resolve this important issue.
CONCLUDING REMARKS
Research over the past two decades since the discovery and initial characterization of ITAMs has served to substantially advance our understanding of the mechanisms by which ITAMs function to initiate signaling by the TCR. Currently, the preponderance of data support the idea that the multiple TCR ITAMs function mainly to amplify TCR signals and suggest that this capacity for signal amplification is especially critical for selection of the T-cell repertoire. Data generated from experiments in which the number of ITAMs per TCR are reduced have yielded conflicting results regarding the effects of these alterations on central tolerance and the risk for autoimmune disease. Because these findings impact our basic understanding of thymocyte selection, it will be both important and worthwhile to establish more physiologically appropriate models, such as “knockin” mutations to resolve this issue. Experiments revisiting the question of whether individual TCR ITAMs selectively bind to potential downsteam effectors are also overdue and are especially promising as they may yield new insights into the “raison d’être” for the multiple ITAM configuration of the TCR complex.
Footnotes
Editors: Lawrence E. Samelson and Andrey Shaw
Additional Perspectives on Immunoreceptor Signaling available at www.cshperspectives.org
REFERENCES
- Aivazian D, Stern LJ 2000. Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nat Structural Biol 7:1023–1026 [DOI] [PubMed] [Google Scholar]
- Ardouin L, Boyer C, Gillet A, Trucy J, Bernard AM, Nunes J, Delon J, Trautmann A, He HT, Malissen B, et al. 1999. Crippling of CD3-ζ ITAMs does not impair T cell receptor signaling. Immunity 10:409–420 [DOI] [PubMed] [Google Scholar]
- Au-Yeung BB, Deindl S, Hsu LY, Palacios EH, Levin SE, Kuriyan J, Weiss A 2009. The structure, regulation, and function of ZAP-70. Immunol Rev 228:41–57 [DOI] [PubMed] [Google Scholar]
- Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE 2001. Fine tuning of TCR signaling by CD5. J Immunol 166:5464–5472 [DOI] [PubMed] [Google Scholar]
- Barber EK, Dasgupta JD, Schlossman SF, Trevillyan JM, Rudd CE 1989. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci 86:3277–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow AD, Trowsdale J 2006. You say ITAM and I say ITIM, let's call the whole thing off: the ambiguity of immunoreceptor signalling. Eur J Immunol 36:1646–1653 [DOI] [PubMed] [Google Scholar]
- Chan AC, Iwashima M, Turck CW, Weiss A 1992. ZAP-70: A 70 kd protein-tyrosine kinase that associates with the TCR ζ chain. Cell 71:649–662 [DOI] [PubMed] [Google Scholar]
- Chu K, Littman DR 1994. Requirement for kinase activity of CD4-associated p56lck in antibody-triggered T cell signal transduction. J Biol Chem 269:24095–24101 [PubMed] [Google Scholar]
- Dave VP, Cao Z, Browne C, Alarcon B, Fernandez-Miguel G, Lafaille J, de la Hera A, Tonegawa S, Kappes DJ 1997. CD3 δ deficiency arrests development of the αβ but not the γδ T cell lineage. EMBO J 16:1360–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJarnette JB, Sommers CL, Huang K, Woodside KJ, Emmons R, Katz K, Shores EW, Love PE 1998. Specific requirement for CD3έ in T cell development. ProcNatl Acad Sci 95:14909–14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Z, Singer A 1996. Tuning of activation thresholds explains flexibility in the selection and development of T cells in the thymus. ProcNatl Acad Sci 93:14747–14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haks MC, Krimpenfort P, Borst J, Kruisbeek AM 1998. The CD3gamma chain is essential for development of both the TCRαβ and TCRγδ lineages. EMBO J 17:1871–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, Kappes DJ, Wiest DL 2005. Attenuation of γδTCR signaling efficiently diverts thymocytes to the αβ lineage. Immunity 22:595–606 [DOI] [PubMed] [Google Scholar]
- Haks MC, Pepin E, van den Brakel JH, Smeele SA, Belkowski SM, Kessels HW, Krimpenfort P, Kruisbeek AM 2002. Contributions of the T cell receptor-associated CD3γ-ITAM to thymocyte selection. J Exp Med 196:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Li L, Love PE 2005. TCR signal strength influences αβ/γδ lineage fate. Immunity 22:583–593 [DOI] [PubMed] [Google Scholar]
- Hayes SM, Love PE 2002. Distinct structure and signaling potential of the γδ TCR complex. Immunity 16:827–838 [DOI] [PubMed] [Google Scholar]
- Hayes SM, Love PE 2006a. Stoichiometry of the murine γδ T cell receptor. J Exp Med 203:47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Love PE 2006b. Strength of signal: a fundamental mechanism for cell fate specification. Immunol Rev 209:170–175 [DOI] [PubMed] [Google Scholar]
- Hayes SM, Shores EW, Love PE 2003. An architectural perspective on signaling by the pre-, αβ and γδ T cell receptors. Immunol Rev 191:28–37 [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR 1994. T cell receptor antagonist peptides induce positive selection. Cell 76:17–27 [DOI] [PubMed] [Google Scholar]
- Holst J, Wang H, Eder KD, Workman CJ, Boyd KL, Baquet Z, Singh H, Forbes K, Chruscinski A, Smeyne R, et al. 2008. Scalable signaling mediated by T cell antigen receptor-CD3 ITAMs ensures effective negative selection and prevents autoimmunity. Nat Immunol 9:658–666 [DOI] [PubMed] [Google Scholar]
- Irving BA, Chan AC, Weiss A 1993. Functional characterization of a signal transducing motif present in the T cell antigen receptor ζ chain. J Exp Med 177:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving BA, Weiss A 1991. The cytoplasmic domain of the T cell receptor ζ chain is sufficient to couple to receptor-associated signal transduction pathways. Cell 64:891–901 [DOI] [PubMed] [Google Scholar]
- Isakov N, Wange RL, Burgess WH, Watts JD, Aebersold R, Samelson LE 1995. ZAP-70 binding specificity to T cell receptor tyrosine-based activation motifs: The tandem SH2 domains of ZAP-70 bind distinct tyrosine-based activation motifs with varying affinity. J Exp Med 181:375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, Fletcher MC, Ledbetter JA, Schieven GL, Siegel JN, Phillips AF, Samelson LE 1990. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. ProcNatl Acad Sci 87:7722–7726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Raulet DH 1997. Events that regulate differentiation of αβ TCR+ and γδ TCR+ T cells from a common precursor. Sem Immunol 9:171–179 [DOI] [PubMed] [Google Scholar]
- Kersh EN, Kersh GJ, Allen PM 1999. Partially phosphorylated T cell receptor ζ molecules can inhibit T cell activation. J Exp Med 190:1627–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Lippincott-Schwartz J, Bonifacino JS 1990. The T cell antigen receptor: Insights into organelle biology. Ann Rev Cell Biol 6:403–431 [DOI] [PubMed] [Google Scholar]
- Koyasu S, Tse AG, Moingeon P, Hussey RE, Mildonian A, Hannisian J, Clayton LK, Reinherz EL 1994. Delineation of a T-cell activation motif required for binding of protein tyrosine kinases containing tandem SH2 domains. Proc Natl Acad Sci 91:6693–6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslavsky T, Garbe AI, Krueger A, von Boehmer H 2008. T cell receptor-instructed αβ versus γδ lineage commitment revealed by single-cell analysis. J Exp Med 205:1173–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Klausner RD 1992. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 epsilon. Science 255:79–82 [DOI] [PubMed] [Google Scholar]
- Liu CP, Ueda R, She J, Sancho J, Wang B, Weddell G, Loring J, Kurahara C, Dudley EC, Hayday A, et al. 1993. Abnormal T cell development in CD3-ζ-/- mutant mice and identification of a novel T cell population in the intestine. EMBO J 12:4863–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love PE, Lee J, Shores EW 2000. Critical relationship between TCR signaling potential and TCR affinity during thymocyte selection. J Immunol 165:3080–3087 [DOI] [PubMed] [Google Scholar]
- Love PE, Shores EW 2000. ITAM multiplicity and thymocyte selection: how low can you go? Immunity 12:591–597 [DOI] [PubMed] [Google Scholar]
- Love PE, Shores EW, Johnson MD, Tremblay ML, Lee EJ, Grinberg A, Huang SP, Singer A, Westphal H 1993. T cell development in mice that lack the ζ chain of the T cell antigen receptor complex. Science 261:918–921 [DOI] [PubMed] [Google Scholar]
- Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN 1995. ζ phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science 267:515–518 [DOI] [PubMed] [Google Scholar]
- Malissen M, Gillet A, Ardouin L, Bouvier G, Trucy J, Ferrier P, Vivier E, Malissen B 1995. Altered T cell development in mice with a targeted mutation of the CD3-έ gene. EMBO J 14:4641–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M, Gillet A, Rocha B, Trucy J, Vivier E, Boyer C, Kontgen F, Brun N, Mazza G, Spanopoulou E, et al. 1993. T cell development in mice lacking the CD3-ζ/ή gene. The EMBO J 12:4347–4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Aoe T, Taki S, Kitamura D, Ishida Y, Rajewsky K, Saito T 1993. Developmental and functional impairment of T cells in mice lacking CD3 ζ chains. EMBO J 12:4357–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman N, Turner H, Lucas S, Reif K, Cantrell DA 1996. The protein interactions of the immunoglobulin receptor family tyrosine-based activation motifs present in the T cell receptor ζ subunits and the CD3 γ, δ, and έ chains. Eur J Immunol 26:1063–1068 [DOI] [PubMed] [Google Scholar]
- Petrie HT, Scollay R, Shortman K 1992. Commitment to the T cell receptor-αβ or -γδ lineages can occur just prior to the onset of CD4 and CD8 expression among immature thymocytes. Eur J Immunol 22:2185–2188 [DOI] [PubMed] [Google Scholar]
- Pinheiro da Silva F, Aloulou M, Benhamou M, Monteiro RC 2008. Inhibitory ITAMs: A matter of life and death. Trends Immunol 29:366–373 [DOI] [PubMed] [Google Scholar]
- Pitcher LA, Mathis MA, Subramanian S, Young JA, Wakeland EK, Love PE, van Oers NS 2005a. Selective expression of the 21-kilodalton tyrosine-phosphorylated form of TCR ζ promotes the emergence of T cells with autoreactive potential. J Immunol 174:6071–6079 [DOI] [PubMed] [Google Scholar]
- Pitcher LA, Mathis MA, Young JA, DeFord LM, Purtic B, Wulfing C, van Oers NS 2005b. The CD3 γέ/δέ signaling module provides normal T cell functions in the absence of the TCR zeta immunoreceptor tyrosine-based activation motifs. Eur J Immunol 35:3643–3654 [DOI] [PubMed] [Google Scholar]
- Pitcher LA, Ohashi PS, van Oers NS 2003a. T cell antagonism is functionally uncoupled from the 21- and 23-kDa tyrosine-phosphorylated TCR ζ subunits. J Immunol 171:845–852 [DOI] [PubMed] [Google Scholar]
- Pitcher LA, Young JA, Mathis MA, Wrage PC, Bartok B, van Oers NS 2003b. The formation and functions of the 21- and 23-kDa tyrosine-phosphorylated TCR ζ subunits. Immunol Rev 191:47–61 [DOI] [PubMed] [Google Scholar]
- Reth M 1989. Antigen receptor tail clue. Nature 338:383–384 [PubMed] [Google Scholar]
- Romeo C, Amiot M, Seed B 1992. Sequence requirements for induction of cytolysis by the T cell antigen/Fc receptor ζ chain. Cell 68:889–897 [DOI] [PubMed] [Google Scholar]
- Saibil SD, Ohteki T, White FM, Luscher M, Zakarian A, Elford A, Shabanowitz J, Nishina H, Hugo P, Penninger J, et al. 2003. Weak agonist self-peptides promote selection and tuning of virus-specific T cells. Eur J Immunol 33:685–696 [DOI] [PubMed] [Google Scholar]
- Saint-Ruf C, Ungewiss K, Groettrup M, Bruno L, Fehling HJ, von Boehmer H 1994. Analysis and expression of a cloned pre-T cell receptor gene. Science 266:1208–1212 [DOI] [PubMed] [Google Scholar]
- Samelson LE, Patel MD, Weissman AM, Harford JB, Klausner RD 1986. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell 46:1083–1090 [DOI] [PubMed] [Google Scholar]
- Samelson LE, Phillips AF, Luong ET, Klausner RD 1990. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. ProcNatl Acad Sci 87:4358–4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santori FR, Kieper WC, Brown SM, Lu Y, Neubert TA, Johnson KL, Naylor S, Vukmanovic S, Hogquist KA, Jameson SC 2002. Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity 17:131–142 [DOI] [PubMed] [Google Scholar]
- Sebzda E, Mariathasan S, Ohteki T, Jones R, Bachmann MF, Ohashi PS 1999. Selection of the T cell repertoire. Ann Rev Immunol 17:829–874 [DOI] [PubMed] [Google Scholar]
- Shores EW, Huang K, Tran T, Lee E, Grinberg A, Love PE 1994. Role of TCR ζ chain in T cell development and selection. Science 266:1047–1050 [DOI] [PubMed] [Google Scholar]
- Shores EW, Tran T, Grinberg A, Sommers CL, Shen H, Love PE 1997. Role of the multiple T cell receptor (TCR)-zeta chain signaling motifs in selection of the T cell repertoire. J Exp Med 185:893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegers GM, Swamy M, Fernandez-Malave E, Minguet S, Rathmann S, Guardo AC, Perez-Flores V, Regueiro JR, Alarcon B, Fisch P, et al. 2007. Different composition of the human and the mouse γδ T cell receptor explains different phenotypes of CD3γ and CD3δ immunodeficiencies. J Exp Med 204:2537–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov AB, Aivazian DA, Uversky VN, Stern LJ 2006. Lipid-binding activity of intrinsically unstructured cytoplasmic domains of multichain immune recognition receptor signaling subunits. Biochemistry 45:15731–15739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM 1994. Partial T cell signaling: Altered phospho-ζ and lack of zap70 recruitment in APL-induced T cell anergy. Cell 79:913–922 [DOI] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS 2009. T cell activation. Ann Rev Immunol 27:591–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers CL, Dejarnette JB, Huang K, Lee J, El-Khoury D, Shores EW, Love PE 2000. Function of CD3 έ-mediated signals in T cell development. J Exp Med 192:913–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA 2003. Positive and negative selection of T cells. Ann Rev Immunol 21:139–176 [DOI] [PubMed] [Google Scholar]
- Straus DB, Weiss A 1993. The CD3 chains of the T cell antigen receptor associate with the ZAP-70 tyrosine kinase and are tyrosine phosphorylated after receptor stimulation. J Exp Med 178:1523–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturmhofel K, Brando C, Martinon F, Shevach EM, Coligan JE 1995. Antigen-independent, integrin-mediated T cell activation. J Immunol 154:2104–2111 [PubMed] [Google Scholar]
- Underhill DM, Goodridge HS 2007. The many faces of ITAMs. Trends Immunol 28:66–73 [DOI] [PubMed] [Google Scholar]
- van Oers NS, Killeen N, Weiss A 1994. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR ζ in murine thymocytes and lymph node T cells. Immunity 1:675–685 [DOI] [PubMed] [Google Scholar]
- Veillette A, Bookman MA, Horak EM, Bolen JB 1988. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 55:301–308 [DOI] [PubMed] [Google Scholar]
- von Boehmer H 2005. Unique features of the pre-T-cell receptor α-chain: Not just a surrogate. Nat Rev 5:571–577 [DOI] [PubMed] [Google Scholar]
- Wegener AM, Letourneur F, Hoeveler A, Brocker T, Luton F, Malissen B 1992. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell 68:83–95 [DOI] [PubMed] [Google Scholar]
- Witherden D, van Oers N, Waltzinger C, Weiss A, Benoist C, Mathis D 2000. Tetracycline-controllable selection of CD4(+) T cells: Half-life and survival signals in the absence of major histocompatibility complex class II molecules. J Exp Med 191:355–364 [DOI] [PubMed] [Google Scholar]
- Wong P, Barton GM, Forbush KA, Rudensky AY 2001. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J Exp Med 193:1179–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Gagnon E, Call ME, Schnell JR, Schwieters CD, Carman CV, Chou JJ, Wucherpfennig KW 2008. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell 135:702–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenner G, Vorherr T, Mustelin T, Burn P 1996. Differential and multiple binding of signal transducing molecules to the ITAMs of the TCR-ζ chain. J Cell Biochem 63:94–103 [DOI] [PubMed] [Google Scholar]