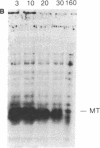
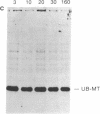
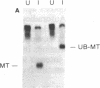
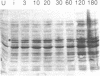
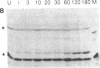
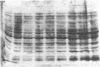
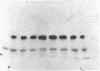
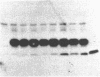
Abstract
Despite the availability of efficient transcription and translation signals, some heterologous gene products are not adequately expressed when introduced into prokaryotes and eukaryotes. An expression system has been established in Escherichia coli to increase the yield of cloned gene products, where the C terminus of ubiquitin was fused to the N terminus of unstable or poorly expressed proteins. Fusion of ubiquitin to yeast metallothionein or to the alpha subunit of the adenylate cyclase-stimulatory GTP-binding protein increased the yield from undetectable to 20% of the total cellular protein. A ubiquitin-N alpha-protein hydrolase has been partially purified from rabbit reticulocytes; this enzyme faithfully cleaves the junction peptide bound between the C-terminal Gly-76 of ubiquitin and the fusion protein. The increased yield of cloned gene products is very likely due to increased stability and/or more efficient translation of the fusion proteins. Possible mechanisms for the augmentation of ubiquitin fusion-protein expression in prokaryotes and eukaryotes are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmair A., Finley D., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986 Oct 10;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Berka T., Shatzman A., Zimmerman J., Strickler J., Rosenberg M. Efficient expression of the yeast metallothionein gene in Escherichia coli. J Bacteriol. 1988 Jan;170(1):21–26. doi: 10.1128/jb.170.1.21-26.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. Ubiquitin is a heat shock protein in chicken embryo fibroblasts. Mol Cell Biol. 1985 May;5(5):949–956. doi: 10.1128/mcb.5.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt T. R., Khan M. I., Marsh J., Ecker D. J., Crooke S. T. Ubiquitin-metallothionein fusion protein expression in yeast. A genetic approach for analysis of ubiquitin functions. J Biol Chem. 1988 Nov 5;263(31):16364–16371. [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Ecker D. J., Butt T. R., Marsh J., Sternberg E. J., Margolis N., Monia B. P., Jonnalagadda S., Khan M. I., Weber P. L., Mueller L. Gene synthesis, expression, structures, and functional activities of site-specific mutants of ubiquitin. J Biol Chem. 1987 Oct 15;262(29):14213–14221. [PubMed] [Google Scholar]
- Graziano M. P., Casey P. J., Gilman A. G. Expression of cDNAs for G proteins in Escherichia coli. Two forms of Gs alpha stimulate adenylate cyclase. J Biol Chem. 1987 Aug 15;262(23):11375–11381. [PubMed] [Google Scholar]
- Gross M., Sweet R. W., Sathe G., Yokoyama S., Fasano O., Goldfarb M., Wigler M., Rosenberg M. Purification and characterization of human H-ras proteins expressed in Escherichia coli. Mol Cell Biol. 1985 May;5(5):1015–1024. doi: 10.1128/mcb.5.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. L., Rose I. A. The mechanism of ubiquitin activating enzyme. A kinetic and equilibrium analysis. J Biol Chem. 1982 Sep 10;257(17):10329–10337. [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin pathway for the degradation of intracellular proteins. Prog Nucleic Acid Res Mol Biol. 1986;33:19-56, 301. doi: 10.1016/s0079-6603(08)60019-7. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Jonnalagadda S., Butt T. R., Marsh J., Sternberg E. J., Mirabelli C. K., Ecker D. J., Crooke S. T. Expression and accurate processing of yeast penta-ubiquitin in Escherichia coli. J Biol Chem. 1987 Dec 25;262(36):17750–17756. [PubMed] [Google Scholar]
- Monia B. P., Ecker D. J., Jonnalagadda S., Marsh J., Gotlib L., Butt T. R., Crooke S. T. Gene synthesis, expression, and processing of human ubiquitin carboxyl extension proteins. J Biol Chem. 1989 Mar 5;264(7):4093–4103. [PubMed] [Google Scholar]
- Nagai K., Perutz M. F., Poyart C. Oxygen binding properties of human mutant hemoglobins synthesized in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7252–7255. doi: 10.1073/pnas.82.21.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E., Finley D., Solomon M. J., Varshavsky A. The yeast ubiquitin genes: a family of natural gene fusions. EMBO J. 1987 May;6(5):1429–1439. doi: 10.1002/j.1460-2075.1987.tb02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. Heat-shock proteins. Coming in from the cold. Nature. 1988 Apr 28;332(6167):776–777. doi: 10.1038/332776a0. [DOI] [PubMed] [Google Scholar]
- Redman K. L., Rechsteiner M. Extended reading frame of a ubiquitin gene encodes a stable, conserved, basic protein. J Biol Chem. 1988 Apr 5;263(10):4926–4931. [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. A specific endpoint assay for ubiquitin. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1477–1481. doi: 10.1073/pnas.84.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. Ubiquitin genes as a paradigm of concerted evolution of tandem repeats. J Mol Evol. 1987;25(1):58–64. doi: 10.1007/BF02100041. [DOI] [PubMed] [Google Scholar]
- Shatzman A. R., Rosenberg M. Efficient expression of heterologous genes in Escherichia coli. The pAS vector system and its applications. Ann N Y Acad Sci. 1986;478:233–248. doi: 10.1111/j.1749-6632.1986.tb15534.x. [DOI] [PubMed] [Google Scholar]
- Siegelman M., Bond M. W., Gallatin W. M., St John T., Smith H. T., Fried V. A., Weissman I. L. Cell surface molecule associated with lymphocyte homing is a ubiquitinated branched-chain glycoprotein. Science. 1986 Feb 21;231(4740):823–829. doi: 10.1126/science.3003913. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winge D. R., Nielson K. B., Gray W. R., Hamer D. H. Yeast metallothionein. Sequence and metal-binding properties. J Biol Chem. 1985 Nov 25;260(27):14464–14470. [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]