Abstract
Background and Objective: The objective of the study was to describe bone loss rates across the transmenopause related to FSH staging and the final menstrual period (FMP).
Design and Setting: This was a population-based cohort of 629 women (baseline age 24–44 yr) with annual data points over 15 yr.
Measurements: Measures were bone mineral density (BMD), FSH to define four FSH stages, and menstrual bleeding cessation to define the FMP. Bone loss rates were reported by obesity status.
Results: Annualized rates of lumbar spine bone loss began in FSH stage 3, which occurs approximately 2 yr prior to the FMP (1.67%/yr); bone loss continued into FSH stage 4 (1.21%/yr). Mean spine BMD in FSH stage 4 was 6.4% less than spine BMD value in FSH stage 1. Annualized rates of femoral neck (FN) bone loss began in FSH stage 3 (0.55%/yr) and continued into FSH stage 4 (0.72%/yr). The FN difference between mean values in FSH stage 1 and FSH stage 4 was 5%. Annualized rates of spine bone loss in the 2 yr prior to the FMP were 1.7%/yr, 3.3%/yr in the 2 yr after the FMP, and 1.1%/yr in the 2- to 7-yr period after the FMP. Nonobese women had lower BMD levels and greater bone loss rates.
Conclusions: Spine and FN bone loss accelerates in FSH stage 3. Bone loss also began to accelerate 2 yr before the FMP with the greatest loss occurring in the 2 yr after the FMP. Bone loss rates in both spine and FN BMD were greater in nonobese women than obese women.
Rates of spine and femoral neck bone mineral density loss are marked in FSH stage 3 and 2 years before the final menstrual period (FMP), with greater bone loss in the 2 years following the FMP.
In women the decline in ovarian function around the menopause generates increased bone loss that, along with age, becomes a primary risk factor for osteoporosis and fracture (1). Whereas bone loss is expected after the menopause transition, there is minimal specific information about the amount of bone loss during the menopause transition. Recent reports identified that bone loss accelerates dramatically in the late perimenopause compared with loss in pre- or early perimenopausal women (2) and that the amount of bone loss during the menopause transition is predicted by both FSH level and the amount of FSH change (3). Further information is needed as to when major bone loss is initiated, how rapidly bone mass actually changes during the transmenopause, the degree to which endogenous hormone levels influence bone loss, and the importance of body size in bone loss patterns during the transition.
We focused on describing amount of bone loss in relation to time around the final menstrual period (FMP) and an endocrine biomarker of the stages of the menopause transition (FSH staging). Endocrine measures of ovarian aging and diminished folliculogenesis, including inhibins, activin, and FSH, have marked changes at midadulthood that may contribute to menopausal bone loss (4,5,6,7). Changing FSH levels have been proposed as a biomarker of critical thresholds in reproductive aging, which may be associated with bone loss (8).
We examined longitudinally acquired measures of bone mineral density (BMD) in relation to time intervals before and after the FMP and in relation to four FSH stages representing reproductive aging across the transmenopausal period. These FSH stages have been identified based on acceleration or deceleration in the rate of FSH change in the transmenopause (8). We hypothesized that: 1) there would be no detectable decrease in BMD level or increased rates of BMD loss, whereas FSH levels were less than 15 mIU/ml (FSH stage 1); 2) there would be increased rates of BMD loss occurring during the period of 2–7 yr before the FMP (FSH stage 2) because inhibin B levels are increasingly unable to suppress FSH; 3) there would be further evidence of increased rates of bone loss during FSH stage 3 (which is associated with that time frame approximately 2 yr before and 2 yr after the FMP), a time period representing the convergence of the demise of inhibin A, a profound increase in FSH rate of change, and a marked decline in estradiol levels (8,9); and 4) that the rate of BMD loss would lessen and stabilize at a new rate of change after the FMP during FSH stage 4. Importantly, we hypothesized that the rates of bone loss in relation to time around the FMP and during these FSH stages would be greater in nonobese women as compared with obese women.
Subjects and Methods
Population
The Michigan Bone Health and Metabolism Study is a population-based longitudinal natural history study of changing reproductive hormones and their relation to the initiation and development of musculoskeletal conditions, metabolic diseases, and functional limitations. The two sampling frames and subsequent recruitment of the 664 enrollees have been previously described (8,9,10). This report includes data collected during the 15-yr period from 1992–1993 through 2006–2007; however, no data were collected in the 18- and 14-month periods in 1997 and 2003, respectively, because of funding lapses. For this report, 629 women contributed one or more sex hormone and bone data points to the longitudinal data analyses. Sixty-four percent of women had data at every time point; 16% of women had nine to 11 data points; 16% of women had three to nine data points; and 4% of women had one to two data points. No annual data were collected when women were pregnant (0–3% in any given year). In any given year, data for 2–7% of women was limited to questionnaires because participants were ill, had relocated to more than 2.5 h from the research clinic, or refused phlebotomy (<0.5%). Data were removed from analysis at time of death for 16 participants (2%). Data from women using hormone therapy (continuous or cyclic hormones, including hormones for contraception) were excluded from statistical modeling for each year of use. Data for women having chemotherapy or consistently using glucocorticoids were removed from analysis at the year in which use was initiated. Written informed consent was obtained annually from participants and based on protocol approval by the University of Michigan Institutional Review Board.
Bone and health measures
Annual cohort health evaluations incorporated phlebotomy for metabolic measures; physical measurement of bone, body composition, and physical functioning; and interviews about health status, menstrual bleeding patterns, and health-related behaviors. Height (centimeters) and weight (kilograms) were measured and used to calculate body mass index (BMI) as weight (in kilograms) divided by height squared (meters).
Areal BMD (grams per square centimeter) was measured with dual-energy x-ray absorptiometry. Coefficients of variation for the dual-energy x-ray absorptiometry measurements of the lumbar spine (LS) and femoral neck (FN) were less than 1.0%. Because of equipment upgrade in densitometers in 2005, at that year’s annual examination, every woman had measurement of the LS and FN on the GE Lunar Prodigy with DPXIQ software (Madison, WI; used from 2005 to present). Additionally, each woman had a measurement of LS BMD or FN BMD on the DPX-L densitometer [DPX-L, software version 1.3y; Lunar (used from 1992 to 2005)]. These replicate measures were used to cross-calibrate the two instruments.
Hormone assay and classification
Single annual specimens were collected as women were fasted and in d 2–7 of the follicular phase of the menstrual cycle (usually in d 2–5 of the follicular phase of the menstrual cycle). When women became amenorrheic, specimens were collected on the annual anniversary of study enrollment ± 15 d. Biological samples were aliquoted and stored at −80 C without thaw until assay.
Serum FSH concentrations were measured with a two-site chemiluminometric immunoassay directed to different β-subunit regions. The intra- and interassay coefficients of variation were 12.0 and 6.0% with a lower limit of detection of 1.05 mIU/ml. Laboratory quality control preparations at the levels of 8.3 and 13.7 mIU/ml (n = 415 and 462) gave coefficients of variation of 9.4 and 7.2%, respectively.
We calculated rates of BMD loss in four different FSH hormone stages (8) based on FSH values (Table 1). FSH staging in an approach to describe stages of the transmenopause based on endocrine changes (8). Annual assignment to an FSH stage was based on the following assumptions: first, women could remain in their current stage or progress to the next stage, but they could not regress to a previous stage; second, stages could be skipped from one visit to the next; third, women could be assigned to an FSH stage, even if they had gynecological surgery or chemotherapy; fourth, if a woman was using exogenous hormones at a particular visit, an FSH stage was not assigned for that visit; fifth, if FSH stage could not be determined, that field was coded as nondiscernible for that study visit.
Table 1.
FSH stages (8) in the reproductive period through the menopausal transition
| FSH stages | FSH (mIU/ml) | Time relationship to the FMP (yr) | Age of FSH stages (yr) |
|---|---|---|---|
| Stage 1 | <15 | −7 before the FMP | <43.6 |
| Stage 2 | 15–33 | −7 to −2 before the FMP | 43.6 to 47.6 |
| Stage 3 | 34–54 | −2 to +2 around the FMP | 47.6 to 51.0 |
| Stage 4 | >54 | +2 to 6 after the FMP | >51.0 |
The FMP
Annually, women were asked about the number and regularity of menstrual bleeding events. FMP was defined retrospectively to the closest month after 12 months of amenorrhea with no alternative physiologically normal explanation. The FMP data analysis for this report was restricted to 183 women with an FMP not obscured by exogenous hormone use or hysterectomy.
Data analysis
Variable distributions were examined and transformations were applied as necessary to satisfy statistical modeling assumptions including normality and constant variance. Two different approaches were used to describe bone loss rates across the transmenopause: 1) bone loss rates in relation to FSH staging and 2) bone loss rates in relation to the FMP.
BMD data from 629 women were organized according to FSH stages and mean BMD (ses) values were estimated using generalized linear mixed models. Pairwise comparisons were used to assess differences in group means. Duration of time in each FSH stage was estimated using generalized linear mixed models.
The relationships between the rates of BMD loss and time to the FMP could not be appropriately modeled by using simple linear or quadratic parametric forms (11). Therefore, we used a two-step approach to evaluate the patterns of BMD changes over time in relation to FMP in the 181 women with an unobscured FMP. Specific details are described in Appendix 1. In the first step, subject-specific BMD instantaneous rates of bone loss and accelerations were identified as the first- and second-order derivatives based on the subject-specific spline curves (12,13). In the second step, nonparametric stochastic mixed modeling was used to characterize change in BMD rates of loss over time in relation to the FMP.
Analyses were stratified to compare obese and nonobese women using a BMI cut point of 30 kg/m2, defined at the time of the last observation.
Analyses were implemented in Matlab7.0 (The MathWorks, Inc., Natick, MA), SAS (version 9.1; SAS Institute, Cary, NC), SAS macro language, and SAS/IML.
Results
In the total sample of women at the initial 1992–1993 annual examination, the participants’ age range was 24–44 yr with a mean of 36.9 yr (sd 4.9 yr). The mean FN BMD ± sd was 0.994 ± 0.140 g/cm2; the mean ± sd for LS BMD was 1.293 ± 0.151 g/cm2, values within the expected BMD range (Table 2). The mean BMI (sd) was 26.2 (5.5) kg/m2. By the 2007–2008 examination, 43% of women were classified as obese (BMI ≥30 kg/m2).
Table 2.
Baseline and most recent characteristics of Michigan Bone Health and Metabolism Study enrollees and the subset with a retrospectively defined FMP
| Total sample
|
Women with FMP
|
|||
|---|---|---|---|---|
| Initial examination Mean ± sd (n = 600)a | Most recent Mean ± sd (n = 506)b | Initial examination Mean ± sd (n = 183)a | Most recent Mean ± sd (n = 183)b | |
| Age (yr) | 36.9 ± 4.9 | 52.2 ± 4.9 | 39.9 ± 3.4 | 55 ± 3.5 |
| BMI (kg/m2) | 26.2 ± 5.5 | 28.9 ± 6.3 | 27.3 ± 5.6 | 29.4 ± 6.4 |
| BMD (g/cm2) | ||||
| FN | 0.994 ± 0.140 | 0.920 ± 0.133 | 0.987 ± 0.135 | 0.884 ± 0.118 |
| LS | 1.293 ± 0.151 | 1.212 ± 0.173 | 1.289 ± 0.149 | 1.170 ± 0.180 |
| FSH (mIU/ml) | 5.3 ± 4.6 | 34.6 ± 37.1 | 6.2 ± 4.7 | 63.8 ± 34.1 |
| FSH stage (%) | ||||
| 1 | 68% | 17% | 75% | — |
| 2 | 3% | 9% | 5% | 2% |
| 3 | 2% | 10% | 1% | 11% |
| 4 | 1% | 50% | 1% | 85% |
| Exogenous hormone use | 24% | 16% | 12% | — |
| Blood sample unavailable | 2% | 1% | 4% | 1% |
| Obesity (%) | ||||
| Less than 30 kg/cm2 | 76% | 59% | 74% | 53% |
| Greater than 30 kg/cm2 | 24% | 41% | 26% | 47% |
| Parity (%) | ||||
| Nulliparous | 18% | 14% | 13% | 12% |
| Parity of 1–2 | 51% | 53% | 49% | 50% |
| Parity greater than 2 | 31% | 33% | 38% | 38% |
| Smoking status (%) | ||||
| Never | 57% | 54% | 54% | 50% |
| Former | 20% | 31% | 23% | 37% |
| Current | 23% | 15% | 23% | 13% |
| Menopausal status (%) | ||||
| Premenopause | 68% | 29% | 81% | — |
| Perimenopause | 7% | 11% | 5% | — |
| Postmenopause | 1% | 29% | 2% | 100% |
| Surgical menopause | 0% | 15% | — | — |
| Exogenous hormone use | 24% | 16% | 12% | — |
Initial examination for more than 95% of cohort (n = 600 of 629) in 1992–1993 excluding pregnant or lactating women.
Current examination in 2007–2008 for 80.4% participants (506 of 629) who are still alive and living within 2.5 h of the clinical site.
The average age at FMP was 50 yr; there was no difference in mean age at FMP in obese vs. nonobese women. Not surprisingly, the women who achieved an FMP were older than the total sample, were more likely to have achieved assignment to FSH stage 4, and had lower subsequent BMD (Table 2).
Lumbar spine BMD loss and FSH staging
Decline in LS BMD was associated with specific FSH stages. In FSH stage 1, the mean spine BMD was 1.286 g/cm2, whereas the annualized rate of bone loss during FSH stage 1 was 0.17% (se 0.13%). In FSH stage 2, the mean spine BMD was 1.294 g/cm2 and the annualized rate of bone loss was 0.44% (se 0.19%). In FSH stage 3, beginning 2–3 yr before the FMP, the mean spine BMD was 1.266 g/cm2 and the annualized rate of bone loss was 1.67% (se 0.24%). In FSH stage 4, the mean spine BMD was 1.219 g/cm2 and the annualized rate of bone in spine BMD was 1.21% (se 0.19%). The mean spine BMD in FSH stage 4 was 6.4% less than the mean spine BMD value in FSH stage 1. Statistically there was no difference in the annualized rate of bone loss between FSH stages 1 and 2 in which the difference in the rates was 0.26% (se 0.2%, P < 0.21). However, the rates of bone loss in FSH stages 3 and 4 were significantly different from the FSH stage 2 rate of bone loss (P < 0.0001 and P = 0.0001, respectively).
FN BMD loss and FSH staging
In FSH stage 1, the mean FN BMD was 0.970 g/cm2 and the annualized rate of bone loss was 0.18%. In FSH stage 2, the mean FN BMD was 0.965 g/cm2 and the annualized rate of bone loss remained at 0.18%. In FSH stage 3, the mean FN BMD was 0.947 g/cm2, and the annualized rate of bone loss was 0.55%, a rate of loss that was significantly different from the rate of loss observed in FSH stage 2. In FSH stage 4, the mean FN BMD was 0.920 g/cm2, and the annualized rate of bone loss was 0.72%, which was different from the rate of bone loss in FSH stage 2 (P < 0.008). The difference between mean BMD values in FSH stage 1 and FSH stage 4 was 5%.
Rates of bone loss in relation to the FMP
The decline in LS BMD began before the FMP (Fig. 1). The annualized rate of spine bone loss in the period more than 2 yr before the FMP was 0.2% (se 0.14%), a rate of spine bone loss that was not significantly different from no loss (P = 0.15). The annualized rate of bone loss in the 2-yr period before the FMP was 1.7%/yr (se 0.44%) and was significantly different from no loss (P = 0.0002). The annualized rate of spine BMD loss in the 2-yr period after the FMP was 3.3%/yr (se 0.50%), and the annualized rate of spine BMD in the 2- to 7-yr period after the FMP was 1.1%/yr (se 0.28%).
Figure 1.
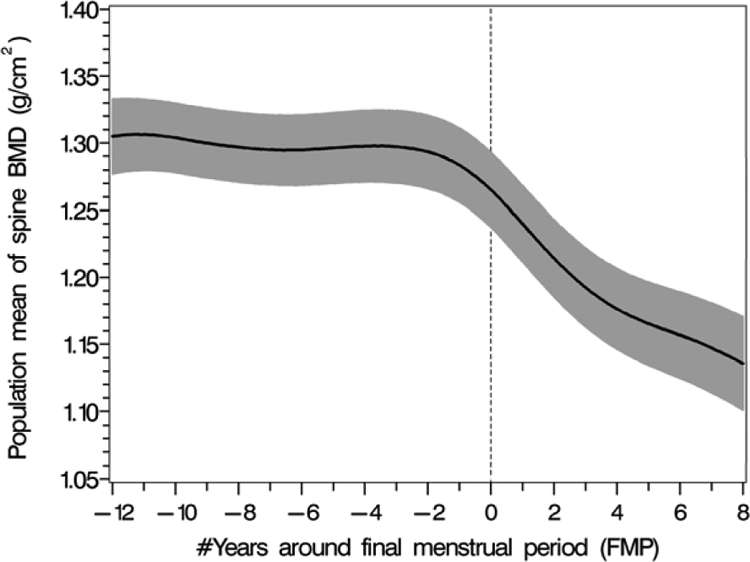
The pattern of overall population mean LS BMD values (black solid line) in relation to the FMP with 95% upper and lower confidence intervals (shaded area).
FN BMD began declining before the FMP with instantaneous rates of femoral bone loss increasing about 2–3 yr before the FMP (Fig. 2). The annualized rate of FN bone loss occurring more than 2 yr before the FMP was 0.15%/yr (se 0.07%). The annualized rate of FN bone loss in the 2-year period before the FMP increased to 0.7%/yr (se 0.23%) a rate of bone loss that was significantly different from no loss (P = 0.002). The annualized rate of FN BMD loss in the 2-year period after the FMP was 2.0% /yr (se 0.26%). However, the annualized rate of FN BMD loss in the two-to-seven year period after the FMP slowed to 1%/yr (se = 0.14%).
Figure 2.
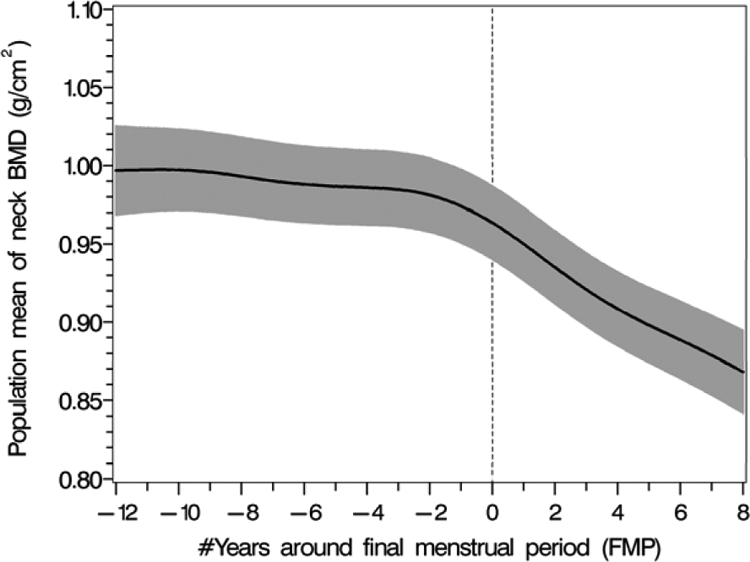
The pattern of population mean femoral neck BMD values (black solid line) in relation to the FMP with 95% upper and lower confidence intervals (shaded area).
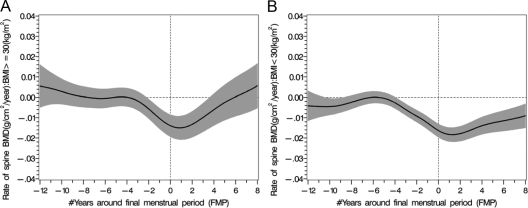
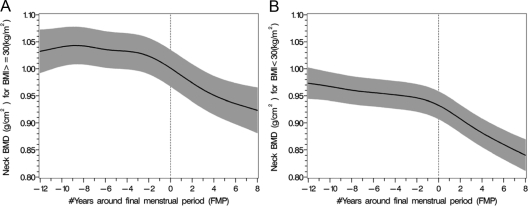
Obesity
The mean LS BMD levels and the rates of bone loss were significantly different when comparing obese and nonobese women (Figs. 3 and 4). Similarly, the rates of FN bone loss were also significantly different in obese and nonobese women (Fig. 5). There was a 5.9% rate of bone loss of spine BMD from FSH stage 1 to FSH stage 4 in nonobese women, whereas there was a 4.5% rate of spine bone loss in the obese women (P < 0.002). There was a 5.0% rate of FN BMD loss from FSH stage 1 to FSH stage 4 in nonobese women, whereas there was a 3.6% rate of FN bone loss in the obese women (P < 0.0005).
Figure 3.
The patterns of decline in population mean LS BMD values (black solid line) over the menopause transition in obese (A) vs. nonobese (B) women with 95% upper and lower confidence intervals (shaded area).
Figure 4.
The rates of LS BMD loss (black solid line) around the FMP in women (greater than 30 kg/m2 A) and less than 30 kg/m2 (B) with 95% upper and lower confidence intervals (shaded area).
Figure 5.
The patterns of decline in the mean population mean FN BMD values (black solid line) over the menopause transition in obese (A) and nonobese (B) women with 95% upper and lower confidence intervals (shaded area).
Discussion
Commonly, women ask about the amount of bone loss during the menopause transition and when it begins. We quantified BMD loss during the transmenopause in both the LS and FN using two approaches, FSH staging, and time around the FMP. We also evaluated the potential role for obesity in these relationships. The information is based on longitudinal data acquired over a 15-yr time period in women whose average age was 37 yr at study initiation, the average age at which the probability of subfertility increases and women enter the late reproductive stage.
We identified three important elements associated with greater bone loss using these two approaches, and the congruence in the findings with two approaches was notable. First, marked BMD loss occurred in FSH stage 3 (operationalized as commencing about 2–3 yr before the FMP or in which annual FSH levels were >34 mIU/ml) and loss continued into FSH stage 4. Likewise, there was greater bone loss in the 2 yr before the FMP and the 2 yr after the FMP. Second, the 2-yr period after the FMP was associated with the greatest spine and FN BMD rate of loss; thereafter the rate of bone loss slowed. Third, the rates of bone loss patterns were markedly different in obese and nonobese women, with lower BMD levels and greater rates of bone loss in the nonobese women.
We used two different approaches to describe transmenopausal bone loss rates. Timing of bone loss to the FMP is a widely applied approach; however, some women do not achieve an unobscured FMP, and assignment of an FMP, by definition, is based on 12 months of amenorrhea; therefore, an alternative or supplemental approach could be clinically useful. Our alternative was to use an endocrine biomarker linked to the reproductive aging process and ascertain whether there was congruency in the findings. Candidates for relevant biomarkers might include a panel of hormone changes associated with increasingly compromised folliculogenesis including measures of inhibins, activin, and FSH in addition to estradiol (4,5,6). FSH staging, a paradigm developed from identifying differences in FSH rates of change during the menopause transition, served as the clinical biomarker of reproductive aging (8). It was in FSH stage 3 that we observed lower levels of BMD and greater bone loss.
We have previously shown that among pre- and early perimenopausal women enrolled in the Study of Women’s Health Across the Nation, it was higher FSH levels, not lower estradiol levels, that were associated with lower BMD, both cross-sectionally (14) and longitudinally (3). In the same study, there were lower BMD levels before the FMP when women were classified as being in the late perimenopause, defined as a period of increasing amenorrhea (2). Previously levels of pre- and perimenopausal bone loss had been estimated to be 1–2% per year, whereas levels of bone loss in the immediate postmenopause were thought to be about 2% per year (15,16,17,18,19,20,21). Using a sigmoid function, one study of less than 50 women estimated that bone loss begins approximately 3 yr before the FMP (22). Thus, this report is in concert with other data but provides additional specific information about the rates of loss within the context of two frames (time period or endocrine related stage) using data that spans from the mid- to late reproductive period to the postmenopause. To place bone loss in perspective, Pouillès et al. (23) identified that women with hip fractures had a 12–21% lower femoral BMD compared with those without fracture, and a prospective study identified that each sd decline in femoral BMD was associated with a 2.0–2.7 increase in hip fracture incidence (24).
It is well documented that, on average, persons of greater body size have greater BMD and that fatness is usually considered protective for fractures, especially hip fractures (25,26). Obesity was associated with marked differences in BMD loss trajectories. In the nonobese women, the LS BMD loss rate subsided by almost 50% after the FMP to achieve a new rate of loss threshold. However, in obese women, the rate of LS bone loss appeared to recover to the baseline equilibrium levels. This difference in spine loss and recovery patterns may be related to underlying hormone status or biomechanical forces generated by fatness. Based on our evaluation of estradiol levels (9), we speculate that there may be supplementation of the estradiol pool by androstenedione and estrone (E1) from extragonadal sources, including adipose tissue when androstenedione is converted to E1 by aromatase, and then E1 and 17β-estradiol (E2) (27) are bidirectionally converted by type 1 17β-hydroxysteroid dehydrogenase. The greater E1 levels in obese women relative to the low E2 levels in all women during the postmenopause may reverse the gradient to favor the formation of a small but detectable amount of E2 from E1. Bagur et al. (28) reported that older women with endogenous E2 levels greater than 10 pg/ml had higher postmenopausal BMD and lower levels of bone-specific alkaline phosphatase than postmenopausal women younger than 65 yr of age. This information about the bone formation marker helps to support the concept that observed differences rates of BMD change among obese vs. nonobese women is not just an obesity-introduced artifact of the BMD measurements.
This report includes data from two skeletal sites (LS and FN) as representative of trabecular and cortical bone, respectively, and potentially these have different hormone responsiveness (29,30). We confirmed that there was greater decline and greater rates of change in LS BMD compared with FN, a site that includes a substantial amount of cortical bone. It has been argued that cortical bone levels will fall more slowly than the more trabecular bone levels because the thresholds for estrogen or bioestrogen are different in the two bone types (31). Notably, FN BMD and LS BMD, reflecting the two bone types, also had different rates of change when the data were dichotomized according to obesity levels.
This report is based on a large population-based cohort of premenopausal women who were followed longitudinally over a 15-yr period; however, somewhat less than half the cohort has an observable FMP, which may be associated with an unknown bias. This highlights the value of also reporting bone loss based on FSH staging in that the underlying endocrine events affiliated with bone loss could be identified and considered in all study participants, even in the absence of an unobscured FMP.
In summary, we quantified important factors associated with bone loss during the midlife. Significant BMD loss began 2–3 yr before the FMP and when average annual FSH levels exceeded 34 mIU/ml as defined in FSH stage 3. Second, bone loss was fastest in the first 2 yr of the postmenopause and then slowed to a rate of approximately 1%/yr. These rates of bone loss were greater in women with a BMI less than 30 kg/m2. Third, rates of bone loss were greater in the more trabecular LS bone compared with the FN with a larger proportion of cortical bone. Thus, marked bone loss during the transmenopause begins earlier than is typically projected whether the relationship was described in relation to the FMP or according to an FSH staging paradigm, and the marked loss extended to about 2 yr after the FMP. The actual rates of bone loss in the 4- to 5-yr period around the FMP were dependent on the type of bone and the body size of women.
Supplementary Material
Acknowledgments
The National Institutes of Health, the funding source of this work, had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants AR051384 (M.R.S., principal investigator), AR040888 (M.R.S., principal investigator), and AR020557 (M.R.S., principal investigator).
Disclosure Summary: M.R.S., B.N., and S.H. have grants pending and listed in manuscript; H.Z., M.L.J., D.M., and J.F.R. have nothing to declare.
First Published Online March 9, 2010
Abbreviations: BMD, Bone mineral density; BMI, body mass index; E1, estrone; E2, 17β-estradiol; FMP, final menstrual period; FN, femoral neck; LS, lumbar spine.
References
- Riggs BL, Khosla S, Melton 3rd LJ 2002 Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, Neer RM 2008 Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 93:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, Neer RM, Johnston J, Ettinger B 2006 Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab 91:1261–1267 [DOI] [PubMed] [Google Scholar]
- Perrien DS, Achenbach SJ, Bledsoe SE, Walser B, Suva LJ, Khosla S, Gaddy D 2006 Bone turnover across the menopause transition: correlations with inhibins and follicle-stimulating hormone. J Clin Endocrinol Metab 91:1848–1854 [DOI] [PubMed] [Google Scholar]
- Martin TJ, Gaddy D 2006 Bone loss goes beyond estrogen. Nat Med 12:612–613 [DOI] [PubMed] [Google Scholar]
- Inoue S, Nomura S, Hosoi T, Ouchi Y, Orimo H, Muramatsu M 1994 Localization of follistatin, an activin-binding protein, in bone tissues. Calcif Tissue Int 55:395–397 [DOI] [PubMed] [Google Scholar]
- Perrien DS, Akel NS, Edwards PK, Carver AA, Bendre MS, Swain FL, Skinner RA, Hogue WR, Nicks KM, Pierson TM, Suva LJ, Gaddy D 2007 Inhibin A is an endocrine stimulator of bone mass and strength. Endocrinology 148:1654–1665 [DOI] [PubMed] [Google Scholar]
- Sowers MR, Zheng H, McConnell D, Nan B, Harlow S, Randolph Jr JF 2008 Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab 93:3958–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MR, Zheng H, McConnell D, Nan B, Harlow S, Randolph Jr JF 2008 Estradiol rates of change in relation to the final menstrual period from a population-based cohort of women. J Clin Endocrinol Metab 93:3847–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MF, Kshirsagar A, Crutchfield MM, Updike S 1992 Joint influence of fat and lean body composition compartments on femoral bone mineral density in premenopausal women. Am J Epidemiol 136:257–265 [DOI] [PubMed] [Google Scholar]
- Zhang D, Lin X, Raz J, Sowers MF 1998 Semiparametric stochastic mixed models for longitudinal hormone data. J Am Stat Assoc 93:710–719 [Google Scholar]
- Ruppert D, Wand MP, Carroll RJ 2003 Semiparametric Regression. 1st ed. Cambridge, UK: Cambridge University Press [Google Scholar]
- Durbán M, Harezlak J, Wand MP, Carroll RJ 2005 Simple fitting of subject-specific curves for longitudinal data. Stat Med 24:1153–1167 [DOI] [PubMed] [Google Scholar]
- Sowers MR, Finkelstein JS, Ettinger B, Bondarenko I, Neer RM, Cauley JA, Sherman S, Greendale GA 2003 The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos Int 14:44–52 [DOI] [PubMed] [Google Scholar]
- Recker RR, Lappe JM, Davies KM, Kimmel DB 1992 Change in bone mass immediately before menopause. J Bone Miner Res 7:857–862 [DOI] [PubMed] [Google Scholar]
- Sowers MR, Clark MK, Hollis B, Wallace RB, Jannausch M 1992 Radial bone mineral density in pre- and perimenopausal women: a prospective study of rates and risk factors for loss. J Bone Miner Res 7:647–657 [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Fukunaga M, Nakamura T, Chen JT, Shiraki M, Hashimoto T, Yoh K, Nakamura T, Mizunuma H, Tomomitsu T, Kasagi F, Masunari N, Orimo H 1998 Rates of change in spinal bone density among Japanese women. Calcif Tissue Int 63:202–207 [DOI] [PubMed] [Google Scholar]
- Sowers M, Clark K, Wallace R, Jannausch M, Lemke J 1991 Prospective study of radial bone mineral density in a geographically defined population of postmenopausal Caucasian women. Calcif Tissue Int 48:232–239 [DOI] [PubMed] [Google Scholar]
- Ravn P, Hetland ML, Overgaard K, Christiansen C 1994 Premenopausal and postmenopausal changes in bone mineral density of the proximal femur measured by dual-energy X-ray absorptiometry. J Bone Miner Res 9:1975–1980 [DOI] [PubMed] [Google Scholar]
- Löfman O, Larsson L, Ross I, Toss G, Berglund K 1997 Bone mineral density in normal Swedish women. Bone 20:167–174 [DOI] [PubMed] [Google Scholar]
- Baran DT 1994 Magnitude and determinants of premenopausal bone loss. Osteoporos Int 4:S31–S34 [DOI] [PubMed] [Google Scholar]
- Recker R, Lappe J, Davies K, Heaney R 2000 Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res 15:1965–1973 [DOI] [PubMed] [Google Scholar]
- Pouillès JM, Tremollières F, Vellas B, Albarède JL, Ribot C 1992 Fracture of the upper extremity of the femur in elderly women: respective role of fall and bone demineralization. Rev Rhum Mal Osteoartic 59:241–246 [PubMed] [Google Scholar]
- Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM 1993 Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 341:72–75 [DOI] [PubMed] [Google Scholar]
- Kreiger N, Kelsey JL, Holford TR, O'Connor T 1982 An epidemiologic study of hip fracture in postmenopausal women. Am J Epidemiol 116:141–148 [DOI] [PubMed] [Google Scholar]
- Hutchinson TA, Polansky SM, Feinstein AR 1979 Post-menopausal oestrogens protect against fractures of hip and distal radius. A case-control study. Lancet 2:705–709 [DOI] [PubMed] [Google Scholar]
- Brailly S, Gougeon A, Milgrom E, Bomsel-Helmreich O, Papiernik E 1981 Androgens and progestins in the human ovarian follicle: differences in the evolution of preovulatory, healthy nonovulatory, and atretic follicles. J Clin Endocrinol Metab 53:128–134 [DOI] [PubMed] [Google Scholar]
- Bagur A, Oliveri B, Mautalen C, Belotti M, Mastaglia S, Yankelevich D, Sayegh F, Royer M 2004 Low levels of endogenous estradiol protect bone mineral density in young, postmenopausal women. Climacteric 7:181–188 [DOI] [PubMed] [Google Scholar]
- Lloyd T, Buchanan JR, Ursino GR, Myers C, Woodward G, Halbert DR 1989 Long-term oral contraceptive use does not affect trabecular bone density. Am J Obstet Gynecol 160:402–404 [DOI] [PubMed] [Google Scholar]
- Hernández ER, Seco-Durban C, Revilla M, González-Riola J, Rico H 1995 Evaluation of bone density with peripheral quantitative computed tomography in healthy premenopausal, perimenopausal, and postmenopausal women. Age Ageing 24:447–450 [DOI] [PubMed] [Google Scholar]
- Khosla S, Riggs BL, Robb RA, Camp JJ, Achenbach SJ, Oberg AL, Rouleau PA, Melton 3rd LJ 2005 Relationship of volumetric bone density and structural parameter at different skeletal sites to sex steroid levels in women. J Clin Endocrinol Metab 90:5096–5103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.