Abstract
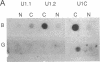
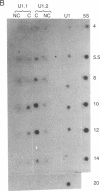
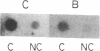
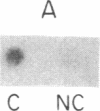
The majority of the genes for U1 RNA are organized in tandemly repeated units in the sea urchin. To assess the level of expression of these genes in the sea urchin Lytechinus variegatus, we measured the transcription of sequences 3' to the gene. The tandemly repeated U1 genes are expressed in morula and continue to be expressed at high rates until 2 hr after hatching, at which time the rate of expression of all the U1 genes and the tandemly repeated U1 genes declines sharply. By the gastrula stage the synthesis of total U1 RNA has declined by a factor of 8. The major tandemly repeated genes are inactive by this time, although other U1 genes remain active. The sequence of U1 RNA synthesized late in embryonic development differs from the sequence of U1 RNA encoded by the tandemly repeated set of U1 RNA genes, indicating that there must be other U1 RNA genes that are active late in embryonic development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benecke B. J., Ben-Ze'ev A., Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978 Aug;14(4):931–939. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- Brandhorst B. P., Humphreys T. Synthesis and decay rates of major classes of deoxyribonucleic acid a5brandhorst BP, Humphreys T: Synthesis and decay rates of major classes of deoxyribonucleic acid-like ribonucleic acid in sea urchin embryos. Biochemistry. 1971 Mar 2;10(5):877–881. doi: 10.1021/bi00781a023. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Morris G. F., Chodchoy N., Sprecher C., Marzluff W. F. Structure of the sea urchin U1 RNA repeat. Nucleic Acids Res. 1985 Jan 25;13(2):537–556. doi: 10.1093/nar/13.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card C. O., Morris G. F., Brown D. T., Marzluff W. F. Sea urchin small nuclear RNA genes are organized in distinct tandemly repeating units. Nucleic Acids Res. 1982 Dec 11;10(23):7677–7688. doi: 10.1093/nar/10.23.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodchoy N., Levine B. J., Sprecher C., Skoultchi A. I., Marzluff W. F. Expression of mouse histone genes: transcription into 3' intergenic DNA and cryptic processing sites downstream from the 3' end of the H3 gene. Mol Cell Biol. 1987 Mar;7(3):1039–1047. doi: 10.1128/mcb.7.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes D. J., Kirschner M. W., Caput D., Dahlberg J. E., Lund E. Differential expression of multiple U1 small nuclear RNAs in oocytes and embryos of Xenopus laevis. Cell. 1984 Oct;38(3):681–689. doi: 10.1016/0092-8674(84)90263-0. [DOI] [PubMed] [Google Scholar]
- Forbes D. J., Kornberg T. B., Kirschner M. W. Small nuclear RNA transcription and ribonucleoprotein assembly in early Xenopus development. J Cell Biol. 1983 Jul;97(1):62–72. doi: 10.1083/jcb.97.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieter P. A., Hendricks M. B., Hemminki K., Weinberg E. S. Histone gene switch in the sea urchin embryo. Identification of late embryonic histone messenger ribonucleic acids and the control of their synthesis. Biochemistry. 1979 Jun 26;18(13):2707–2716. doi: 10.1021/bi00580a004. [DOI] [PubMed] [Google Scholar]
- Kaumeyer J. F., Weinberg E. S. Sequence, organization and expression of late embryonic H3 and H4 histone genes from the sea urchin, Strongylocentrotus purpuratus. Nucleic Acids Res. 1986 Jun 11;14(11):4557–4576. doi: 10.1093/nar/14.11.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. A., Childs G. J. Temporal expression of late histone messenger RNA in the sea urchin Lytechinus pictus. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2411–2415. doi: 10.1073/pnas.81.8.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Calzone F. J., Britten R. J., Angerer R. C., Davidson E. H. Activation of sea urchin actin genes during embryogenesis. Measurement of transcript accumulation from five different genes in Strongylocentrotus purpuratus. J Mol Biol. 1986 Mar 20;188(2):173–183. doi: 10.1016/0022-2836(86)90302-5. [DOI] [PubMed] [Google Scholar]
- Levine B. J., Liu T. J., Marzluff W. F., Skoultchi A. I. Differential expression of individual members of the histone multigene family due to sequences in the 5' and 3' regions of the genes. Mol Cell Biol. 1988 May;8(5):1887–1895. doi: 10.1128/mcb.8.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo S. M., Marzluff W. F., Seufert A. C., Dean W. L., Schultz G. A., Simerly C., Schatten G. Localization and expression of U1 RNA in early mouse embryo development. Dev Biol. 1988 Jun;127(2):349–361. doi: 10.1016/0012-1606(88)90321-1. [DOI] [PubMed] [Google Scholar]
- Lobo S. M., Marzluff W. F. Synthesis of U1 RNA in isolated mouse cell nuclei: initiation and 3'-end formation. Mol Cell Biol. 1987 Dec;7(12):4290–4296. doi: 10.1128/mcb.7.12.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. L., Blin N., Stafford D. W. Cloning and organization of genes for 5S ribosomal RNA in the sea urchin. Lytechinus variegatus. Gene. 1981 Jun-Jul;14(1-2):51–62. doi: 10.1016/0378-1119(81)90147-5. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Differential accumulation of U1 and U4 small nuclear RNAs during Xenopus development. Genes Dev. 1987 Mar;1(1):39–46. doi: 10.1101/gad.1.1.39. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E., Forbes D. J. The two embryonic U1 small nuclear RNAs of Xenopus laevis are encoded by a major family of tandemly repeated genes. Mol Cell Biol. 1984 Dec;4(12):2580–2586. doi: 10.1128/mcb.4.12.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Kahan B., Dahlberg J. E. Differential control of U1 small nuclear RNA expression during mouse development. Science. 1985 Sep 20;229(4719):1271–1274. doi: 10.1126/science.2412294. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Maxson R. E., Jr, Wilt F. H. Accumulation of the early histone messenger RNAs during the development of Strongylocentrotus purpuratus. Dev Biol. 1982 Dec;94(2):435–440. doi: 10.1016/0012-1606(82)90360-8. [DOI] [PubMed] [Google Scholar]
- Morris G. F., Marzluff W. F. A factor in sea urchin eggs inhibits transcription in isolated nuclei by sea urchin RNA polymerase III. Biochemistry. 1983 Feb 1;22(3):645–653. doi: 10.1021/bi00272a019. [DOI] [PubMed] [Google Scholar]
- Morris G. F., Marzluff W. F. Synthesis of U1 RNA in isolated nuclei from sea urchin embryos: U1 RNA is initiated at the first nucleotide of the RNA. Mol Cell Biol. 1985 May;5(5):1143–1150. doi: 10.1128/mcb.5.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. F., Price D. H., Marzluff W. F. Synthesis of U1 RNA in a DNA-dependent system from sea urchin embryos. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3674–3678. doi: 10.1073/pnas.83.11.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash M. A., Kozak S. E., Angerer L. M., Angerer R. C., Schatten H., Schatten G., Marzluff W. F. Sea urchin maternal and embryonic U1 RNAs are spatially segregated in early embryos. J Cell Biol. 1987 May;104(5):1133–1142. doi: 10.1083/jcb.104.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash M. A., Marzluff W. F. Structure of an unusual sea urchin U1 RNA gene cluster. Gene. 1988 Apr 15;64(1):53–63. doi: 10.1016/0378-1119(88)90480-5. [DOI] [PubMed] [Google Scholar]
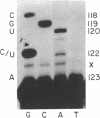
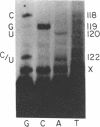
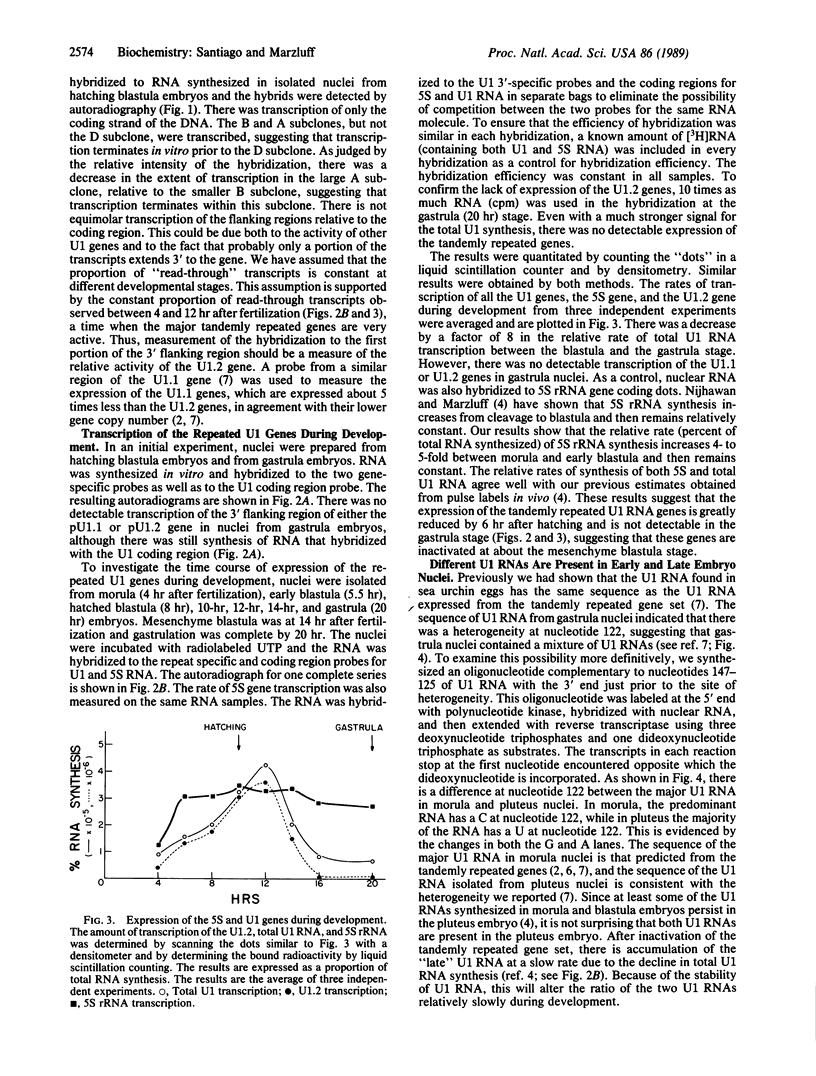
- Nijhawan P., Marzluff W. F. Metabolism of low molecular weight ribonucleic acids in early sea urchin embryos. Biochemistry. 1979 Apr 3;18(7):1353–1360. doi: 10.1021/bi00574a035. [DOI] [PubMed] [Google Scholar]
- Rowland R. D., Rill R. L. Atypical changes in chromatin structure during development in the sea urchin, Lytechinus variegatus. Biochim Biophys Acta. 1987 Feb 27;908(2):169–178. doi: 10.1016/0167-4781(87)90056-x. [DOI] [PubMed] [Google Scholar]
- Weinberg E. S., Hendricks M. B., Hemminki K., Kuwabara P. E., Farrelly L. A. Timing and rates of synthesis of early histone mRNA in the embryo of Strongylocentrotus purpuratus. Dev Biol. 1983 Jul;98(1):117–129. doi: 10.1016/0012-1606(83)90340-8. [DOI] [PubMed] [Google Scholar]
- Yu J. C., Nash M. A., Santiago C., Marzluff W. F. Structure and expression of a second sea urchin U1 RNA gene repeat. Nucleic Acids Res. 1986 Dec 22;14(24):9977–9988. doi: 10.1093/nar/14.24.9977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller R., Carri M. T., Mattaj I. W., De Robertis E. M. Xenopus laevis U1 snRNA genes: characterisation of transcriptionally active genes reveals major and minor repeated gene families. EMBO J. 1984 May;3(5):1075–1081. doi: 10.1002/j.1460-2075.1984.tb01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]