Abstract
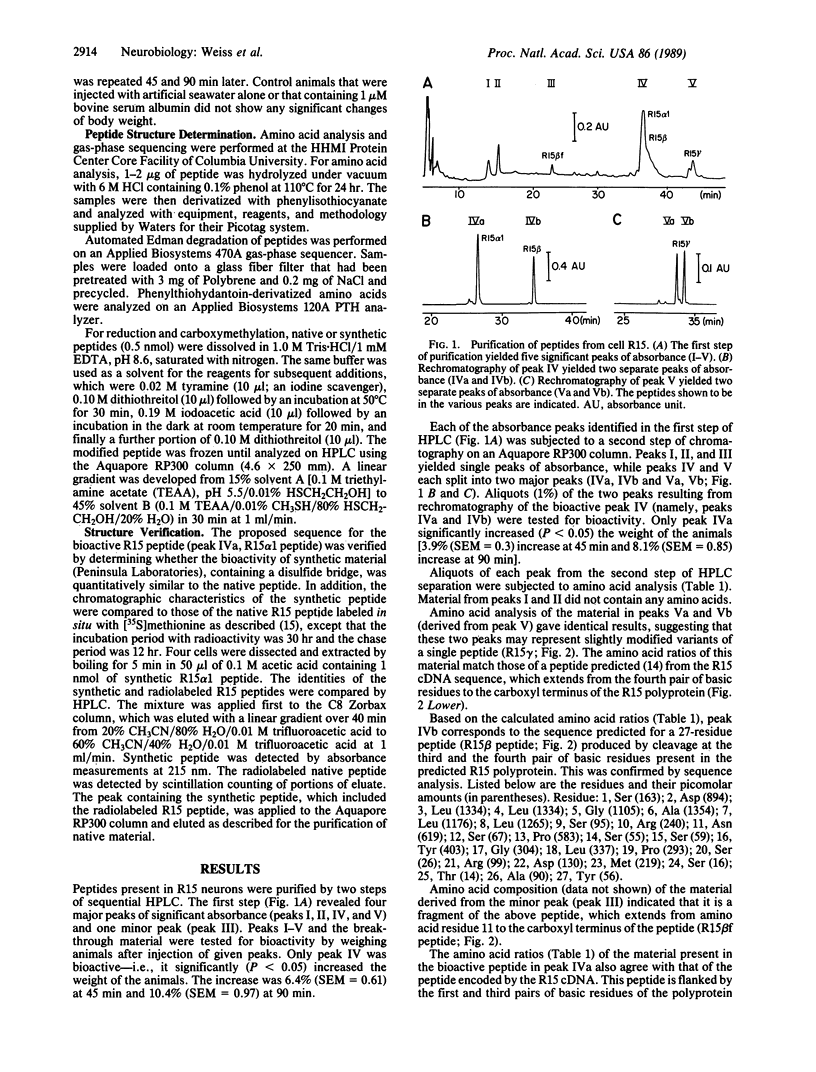
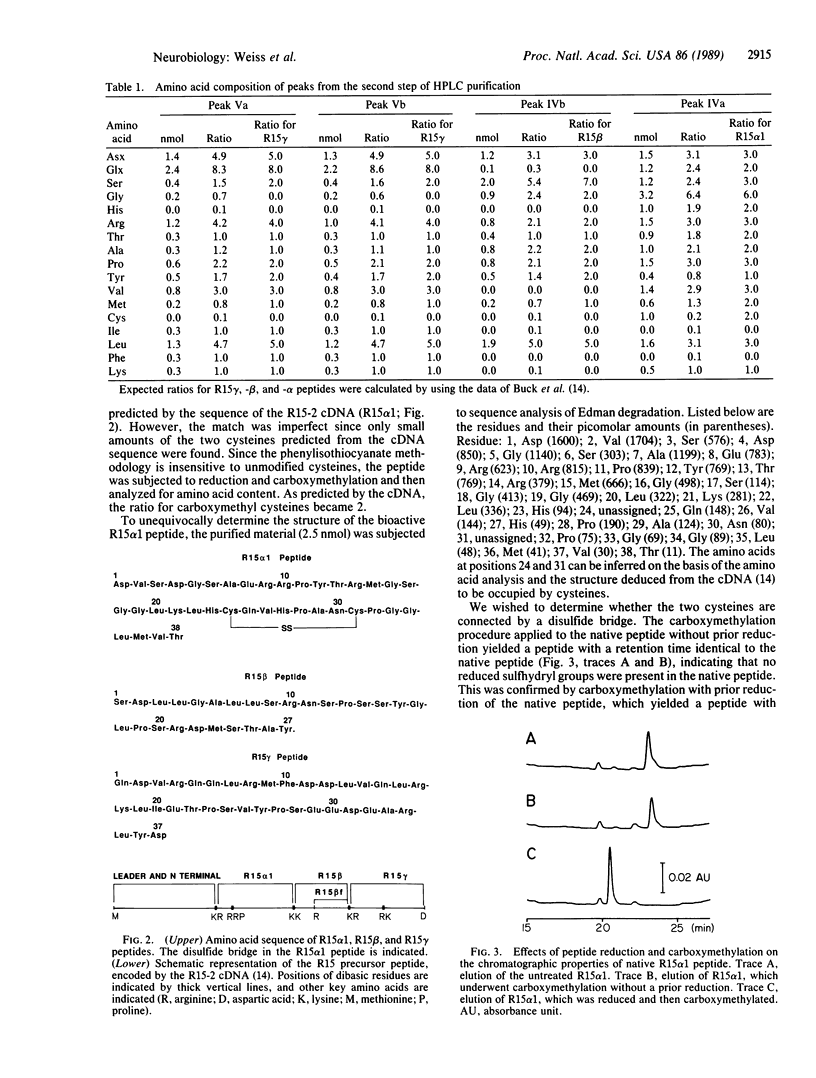
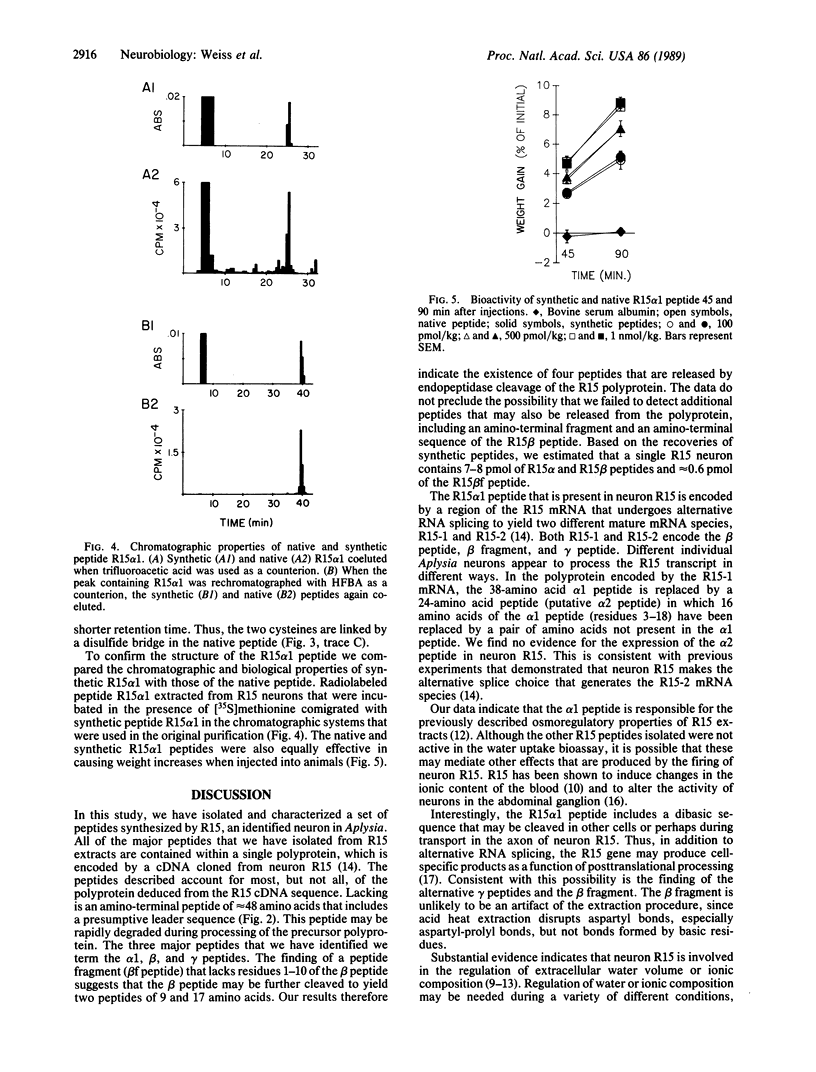
R15 is a large identified neuron present in the abdominal ganglion of the mollusc Aplysia. Previous studies have indicated that this neuron may play a role in water balance and possibly renovascular functions. A peptidic factor contained in the neuron R15 has been shown to increase the water content of Aplysia. To determine the structure of the peptides contained in R15, we purified the extracts of 820 R15 cells by means of two steps of reverse-phase HPLC. The purification yielded a number of peptides, only one of which, R15 alpha 1, resulted in water uptake when injected into animals. Determination of the amino acid content and sequence analysis of the R15 alpha 1 peptide demonstrated that this peptide contains 38 residues, including two cysteines. The peptide failed to react with iodoacetate, indicating that the two cysteines are connected by a disulfide bridge. To confirm the assigned structure, the peptide was synthesized with a disulfide bridge. The chromatographic properties and bioactivity of the synthetic material were identical to those of the native peptide. Several other R15 peptides were inactive in the bioassay for water uptake. The sequence of one of these peptides (R15 beta) was determined, and it was established that the peptide contains 28 residues. Amino acid analysis of three other peaks was performed. One of these peaks contained a peptide (R15 beta f) whose amino acid composition suggests that it is a fragment of the R15 beta peptide. The other two peaks contained peptides with identical amino acid compositions, suggesting that they are variants of a single peptide (R15 gamma). The amino acid sequences of all the peptides identified in neuron R15 correspond to stretches of a polyprotein encoded by a recently sequenced R15 cDNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. B., Benson J. A. The generation and modulation of endogenous rhythmicity in the Aplysia bursting pacemaker neurone R15. Prog Biophys Mol Biol. 1985;46(1):1–49. doi: 10.1016/0079-6107(85)90011-2. [DOI] [PubMed] [Google Scholar]
- Aswad D. W. Biosynthesis and processing of presumed neurosecretory proteins in single identified neurons of Aplysia californica. J Neurobiol. 1978 Jul;9(4):267–284. doi: 10.1002/neu.480090404. [DOI] [PubMed] [Google Scholar]
- Bablanian G. M., Treistman S. N. Seawater osmolarity influences bursting pacemaker activity in intact Aplysia californica. Brain Res. 1983 Jul 25;271(2):342–345. doi: 10.1016/0006-8993(83)90298-6. [DOI] [PubMed] [Google Scholar]
- Brown R. O., Mayeri E. Central actions of R15, a putative peptidergic neuron in Aplysia. J Neurobiol. 1987 Jan;18(1):3–13. doi: 10.1002/neu.480180103. [DOI] [PubMed] [Google Scholar]
- Buck L. B., Bigelow J. M., Axel R. Alternative splicing in individual Aplysia neurons generates neuropeptide diversity. Cell. 1987 Oct 9;51(1):127–133. doi: 10.1016/0092-8674(87)90017-1. [DOI] [PubMed] [Google Scholar]
- Gainer H., Barker J. L. Synaptic regulation of specific protein synthesis in an identified neuron. Brain Res. 1974 Sep 27;78(2):314–319. doi: 10.1016/0006-8993(74)90555-1. [DOI] [PubMed] [Google Scholar]
- Herbert E., Uhler M. Biosynthesis of polyprotein precursors to regulatory peptides. Cell. 1982 Aug;30(1):1–2. doi: 10.1016/0092-8674(82)90002-2. [DOI] [PubMed] [Google Scholar]
- Jahan-Parwar B., Smith M., Von Baumgarten R. Activation of neurosecretory cells in Aplysia by osphradial stimulation. Am J Physiol. 1969 May;216(5):1246–1257. doi: 10.1152/ajplegacy.1969.216.5.1246. [DOI] [PubMed] [Google Scholar]
- Kupfermann I., Carew T. J. Behavior patterns of Aplysia californica in its natural environment. Behav Biol. 1974 Nov;12(3):317–337. doi: 10.1016/s0091-6773(74)91503-x. [DOI] [PubMed] [Google Scholar]
- Kupfermann I., Weiss K. Water regulation by a presumptive hormone contained in identified neurosecretory cell R15 of Aplysia. J Gen Physiol. 1976 Jan;67(1):113–123. doi: 10.1085/jgp.67.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd P. E., Kupfermann I., Weiss K. R. Sequence of small cardioactive peptide A: a second member of a class of neuropeptides in Aplysia. Peptides. 1987 Jan-Feb;8(1):179–184. doi: 10.1016/0196-9781(87)90184-7. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Gainer H. Low molecular weight specific proteins in identified molluscan neurons. I. Synthesis and storage. Brain Res. 1975 Jul 11;92(2):181–192. doi: 10.1016/0006-8993(75)90268-1. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Gainer H. Low molecular weight specific proteins in identified molluscan neurons. II. Processing, turnover, and transport. Brain Res. 1975 Jul 11;92(2):193–205. doi: 10.1016/0006-8993(75)90269-3. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Jackson J. F., McAllister L. B., Schwartz J. H., Kandel E. R., Axel R. A family of genes that codes for ELH, a neuropeptide eliciting a stereotyped pattern of behavior in Aplysia. Cell. 1982 Apr;28(4):707–719. doi: 10.1016/0092-8674(82)90050-2. [DOI] [PubMed] [Google Scholar]
- Scott A. P., Ratcliffe J. G., Rees L. H., Landon J., Bennett H. P., Lowry P. J., McMartin C. Pituitary peptide. Nat New Biol. 1973 Jul 18;244(133):65–67. doi: 10.1038/newbio244065a0. [DOI] [PubMed] [Google Scholar]
- Stinnakre J., Tauc L. Central neuronal response to the activation of osmoreceptors in the osphradium of Aplysia. J Exp Biol. 1969 Nov;51(2):347–361. doi: 10.1242/jeb.51.2.347. [DOI] [PubMed] [Google Scholar]
- Strumwasser F., Wilson D. L. Patterns of proteins synthesized in the R15 neuron of Aplysia. Temporal studies and evidence for processing. J Gen Physiol. 1976 Jun;67(6):691–702. doi: 10.1085/jgp.67.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. L. Molecular weight distribution of proteins synthesized in single, identified neurons of Aplysia. J Gen Physiol. 1971 Jan;57(1):26–40. doi: 10.1085/jgp.57.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]


